e717310feaa2eb67b6a663bbd31cdf2b.ppt
- Количество слайдов: 28

Bio-Technology in Korea Current Status and Growth Strategy 2005. 9. 20 Yong-Kyung Choe
Contents Current Status of Biotechnology in Korea BT Growth Strategy KRIBB Overview 2

Current Status of Biotechnology in Korea 3

Chronological Undertaking for the Development of Biotechnology in Korea 1970 s Initiation Phase – Technology licensing & imitation research - Launched Biotechnology Research Division in KIST 1980 s Growth Phase – Establishing R&D base by fostering genetic engineering - Enacted Genetic Engineering Fostering Law (1983) - Established Genetic Engineering Center (1985) 1990 s Maturation Phase – Expanding biotechnology research base - “Biotechnology Development Master Plan” Kick-Off (1992) - Undertook 21 st Century Frontier Initiative (1999) 2000 s Renewed Jump-up Phase – Facilitating commercialization of biotechnology - Selected as one of the Next-Generation Growth Engines (2003) 4
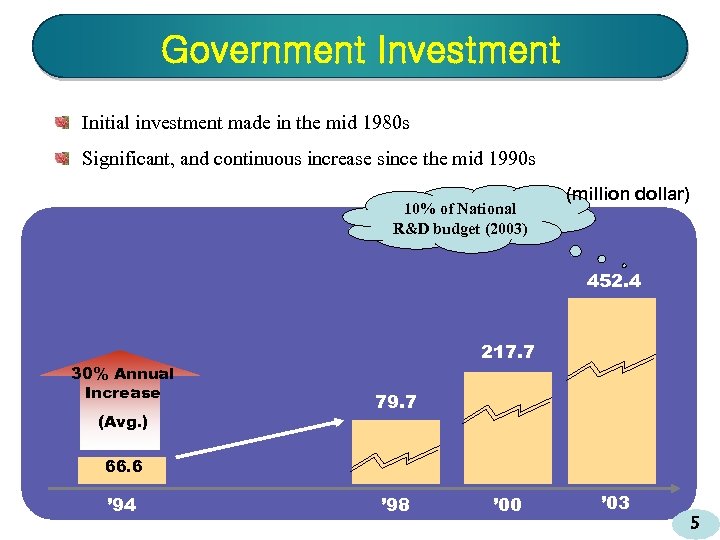
Government Investment Initial investment made in the mid 1980 s Significant, and continuous increase since the mid 1990 s 10% of National R&D budget (2003) (million dollar) 452. 4 30% Annual Increase (Avg. ) 217. 7 79. 7 66. 6 ’ 94 ’ 98 ’ 00 ’ 03 5
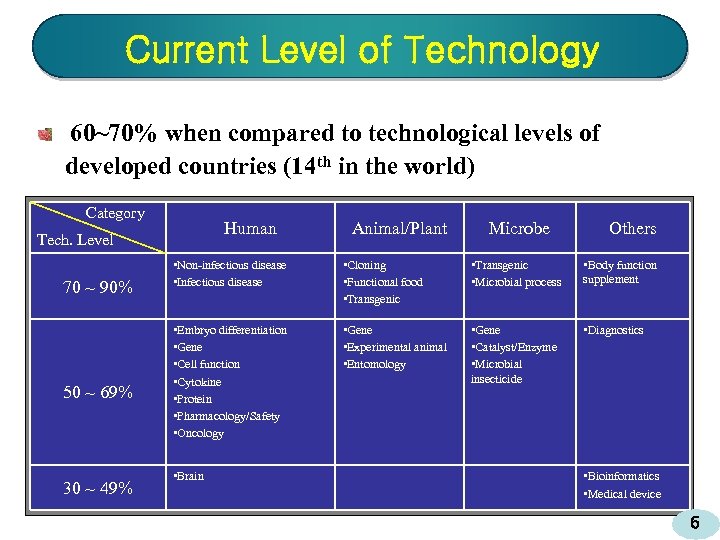
Current Level of Technology 60~70% when compared to technological levels of developed countries (14 th in the world) Category Human Tech. Level 70 ~ 90% 50 ~ 69% 30 ~ 49% Animal/Plant Microbe Others • Non-infectious disease • Infectious disease • Cloning • Functional food • Transgenic • Microbial process • Body function supplement • Embryo differentiation • Gene • Cell function • Cytokine • Protein • Pharmacology/Safety • Oncology • Gene • Experimental animal • Entomology • Gene • Catalyst/Enzyme • Microbial insecticide • Diagnostics • Brain • Bioinformatics • Medical device 6
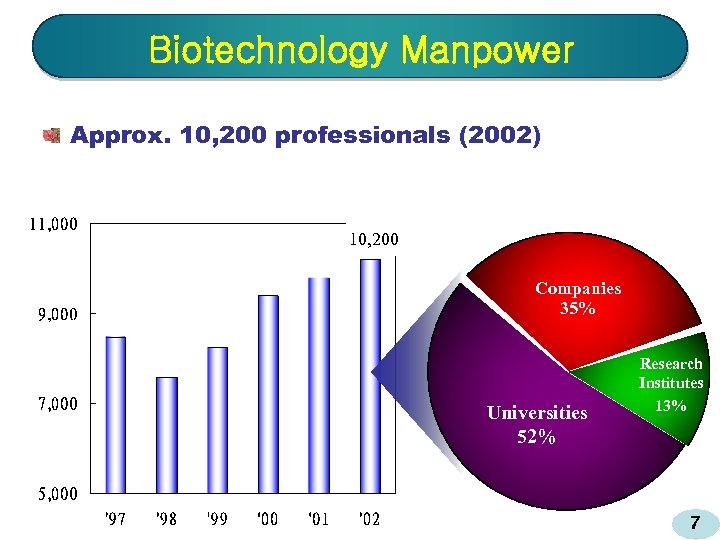
Biotechnology Manpower Approx. 10, 200 professionals (2002) 10, 200 Companies 35% Universities 52% Research Institutes 13% 7
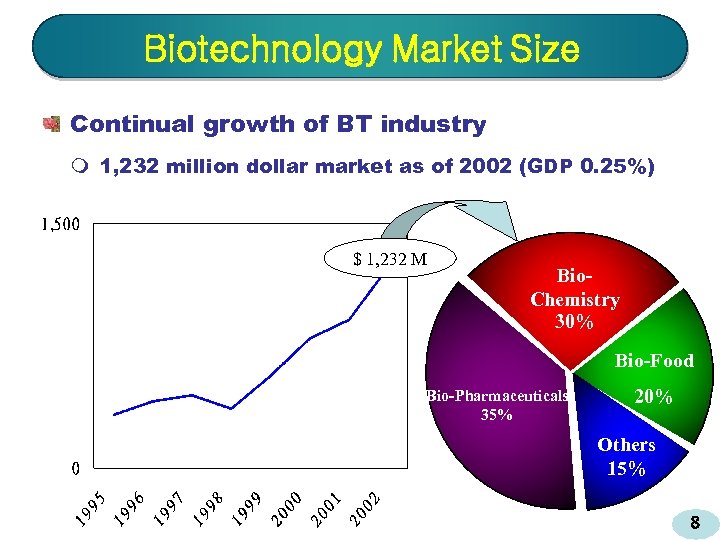
Biotechnology Market Size Continual growth of BT industry m 1, 232 million dollar market as of 2002 (GDP 0. 25%) $ 1, 232 M Bio. Chemistry 30% Bio-Food Bio-Pharmaceuticals 35% 20% Others 15% 8
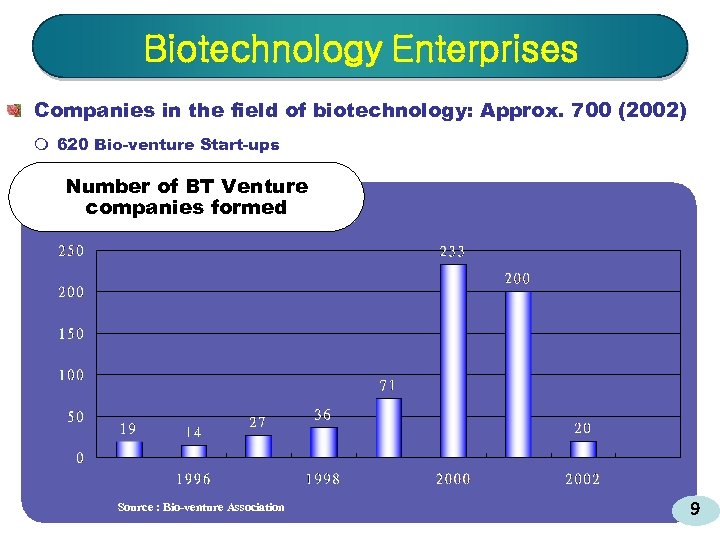
Biotechnology Enterprises Companies in the field of biotechnology: Approx. 700 (2002) m 620 Bio-venture Start-ups Number of BT Venture companies formed Source : Bio-venture Association 9

Publication / Patent u Papers published in the journal of IF > 20 l 21% Increase in 2003 compared to 2002 u Gene patent applications increasing by 42% in average each year Domestic applicants: 38% (1998) 43% (2001)

BT Growth Strategy 11

BT as a new breakthrough Income per capita $10, 000 $79 1960 1995 2002 § Hovered near 10, 000 $ for 9 years § Distance from developed countries Korea Approach of developing countries 12

Goal of National BT Growth Strategy No. 7 in the world’s bio-industry Vision of the year 2012 − BT portion of the government’s entire R&D investment 10% (2003) 20% (2012) $13. 25 billion Employment Added-value 4. 96 $9. 74 billion 1. 16 0. 74 ’ 03 Export 4. 43 ’ 07 ’ 12 (Unit: 10, 000) 9. 7 1. 0 ’ 03 4. 5 ’ 07 ’ 12 13

Approved Capability of BT as a Next-Generation Growth Engine Secure competitive advantage by making maximum use of existing competitiveness Global Market Size Strategic Importance Market·Tech Trends Possibility to secure competitiveness Pervasive Effects Criteria for the selection of Next-Generation Growth Engines 14
![[ Bio-pharmaceuticals] Recent active investment → fruition of R&D m Domestic companies have accumulated [ Bio-pharmaceuticals] Recent active investment → fruition of R&D m Domestic companies have accumulated](https://present5.com/presentation/e717310feaa2eb67b6a663bbd31cdf2b/image-15.jpg)
[ Bio-pharmaceuticals] Recent active investment → fruition of R&D m Domestic companies have accumulated new drug discovery know-how − 1 US FDA Approval / 8 Domestic new drugs (Cumulative sales: 13 million dollars) − 177 Domestic new drug candidates under development m Secured competitiveness in the structure-based field of new drug discovery m Obtained world-class technologies in areas such as genetic engineering, transgenic, fermentation process, purification, etc. 15
![[ Artificial organs] Infrastructure has been established for the production & transplantation technology of [ Artificial organs] Infrastructure has been established for the production & transplantation technology of](https://present5.com/presentation/e717310feaa2eb67b6a663bbd31cdf2b/image-16.jpg)
[ Artificial organs] Infrastructure has been established for the production & transplantation technology of transgenic, cloned pigs m Expected to secure global technical superiority in 5 ~ 10 years 16
![[ Bio-Chip ] Establish a research base in bio-chip field m Utilize IT strengths [ Bio-Chip ] Establish a research base in bio-chip field m Utilize IT strengths](https://present5.com/presentation/e717310feaa2eb67b6a663bbd31cdf2b/image-17.jpg)
[ Bio-Chip ] Establish a research base in bio-chip field m Utilize IT strengths in HW & SW development Challenge the global medical bio-chip market m Need to develop market-oriented products/technology to meet medical needs 17

Biotechnology Clusters Bio-cluster Nurture Plan (MOCIE, ’ 03) <Kangwon/ Kyungsang District> -Bio-environment, process (Chuncheon) -Marine resources (Kangneung) -Bio-energy (Uljin) -Bio-health industry (Andong) -Functional materials (Sangju) -Bio-chemistry (Jinju) -Marine bioresources (Busan) <Daejeon/ Chungcheong District> -Biopharmaceuticals (Daejeon) -Healthcare (Osong) -Oriental medicine (Jecheon) -Animal resources (Nonsan) -Functional food (Youngdong) <Jeongra/Jeju District> -Natural new materials (Jeonju) -Bio-food (Naju) -Bio-agriculture (Hwasun) -Plant/Marine (Jeju) 제주 강릉 춘천 오송 울진 제천 안동 대전 논산 상주 영동 전주 부산 나주 화순 진주 18

Strengths & Opportunities of Korea Strengths u Educational enthusiasm and creativity u World-class IT platform u Growth of Bio-ventures u Government’s strong commitment Opportunities v Little dependence upon natural resources v Small gap with developed countries v Abundant unexplored areas v Initial stages of industrialization v Increased public awareness about Bio-industry 19

KRIBB Overview 20

History Feb. 1985 Genetic Engineering Center (GEC) was established in KAIST Jul. 1990 Dec. 1990 GEC moved to its current location in Daedeog Science Park Mar. 1995 GERI changed its name to KRIBB May 1999 KRIBB became an independent organization under KRCF GEC changed its name to GERI 21

Overview Goal Manpower Budget Papers (’ 04) Patents (’ 04) Development of basic BT platform technology 950 in total : 299 Regular employers [232 Ph. D. ] 99 million dollars (2005) [SCI] 276 [Total] 368 [Overseas] 25 applications [Domestic] 134 applications 15 registrations 65 registrations 22
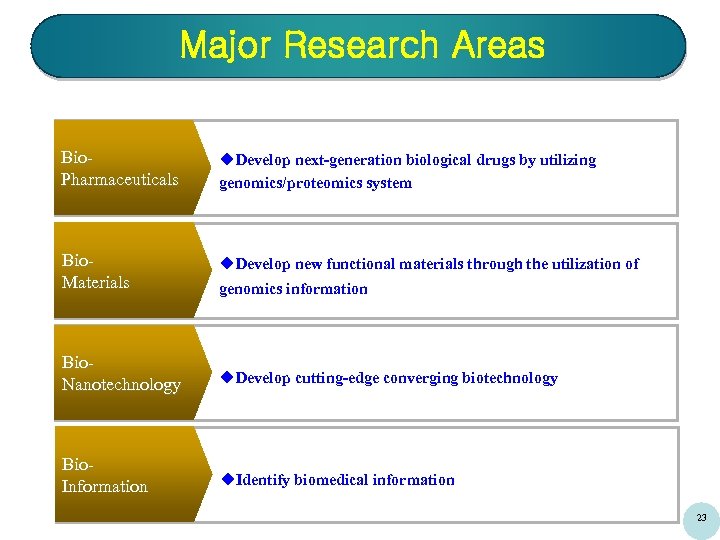
Major Research Areas Bio. Pharmaceuticals u. Develop next-generation biological drugs by utilizing genomics/proteomics system Bio. Materials u. Develop new functional materials through the utilization of Bio. Nanotechnology u. Develop cutting-edge converging biotechnology Bio. Information u. Identify biomedical information genomics information 23
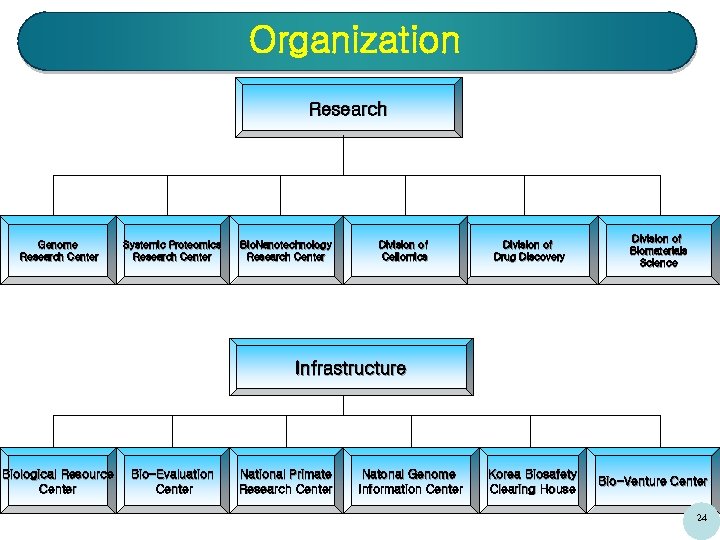
Organization Research Genome Research Center Systemic Proteomics Research Center Bio. Nanotechnology Research Center Division of Cellomics Division of Drug Discovery Division of Biomaterials Science Infrastructure Biological Resource Center Bio-Evaluation Center National Primate Research Center Natonal Genome Information Center Korea Biosafety Clearing House Bio-Venture Center 24

Development Strategy Local branches Ochang Campus q Jeonbuk ㆍ Microbial metabolic engineering & fermentation process technology KRIBB Headquarter § Application & commercialization of bio-pharmaceutical areas § Bio-infra function § Basic platform technologies of genomics and proteomics, etc. 25

Global Collaboration Network u. Expand global collaboration network in order to grow as a world-class research institute (MOU with 32 institutions in 12 countries ] l. Fred-Hutchison Cancer Research Center (Negotiating to host branch research center) l. Utilize overseas scientist, Expert exchange (Constructing overseas scientist DB) Korea-U. K. Focal Point Collaboration with Canadian universities Join research center with Hutchison Cancer Research Center Korea-China BT Collaboration Korea-Israel BT Collaboration Construct Asia BT Network 26

Big step !! The first to successfully clone a human embryo, and then cull from it master stem cells; many doctors consider this as a key to develop customized cures for diabetes, Parkinson and other diseases. 27

Biotechnology, Our Future! Thank you!
e717310feaa2eb67b6a663bbd31cdf2b.ppt