16dbb2e6325e82fa1dfcb42a405f98b4.ppt
- Количество слайдов: 16
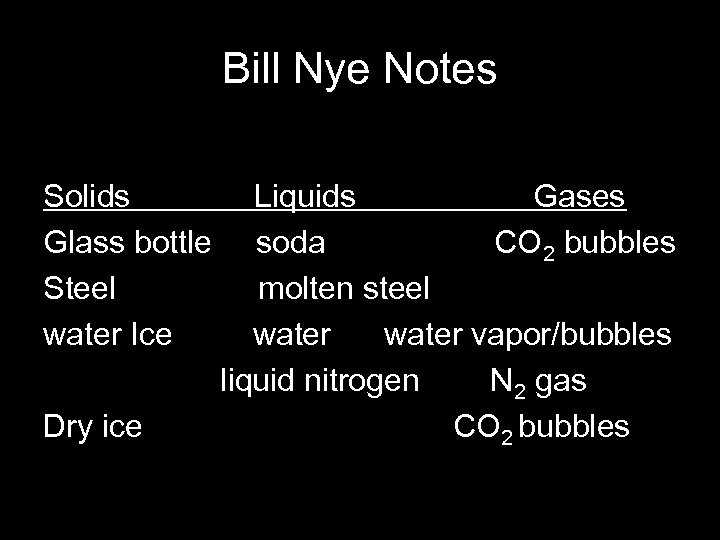
Bill Nye Notes Solids Glass bottle Steel water Ice Dry ice Liquids Gases soda CO 2 bubbles molten steel water vapor/bubbles liquid nitrogen N 2 gas CO 2 bubbles

1/6/16 warm up Please have your SLG HW ready
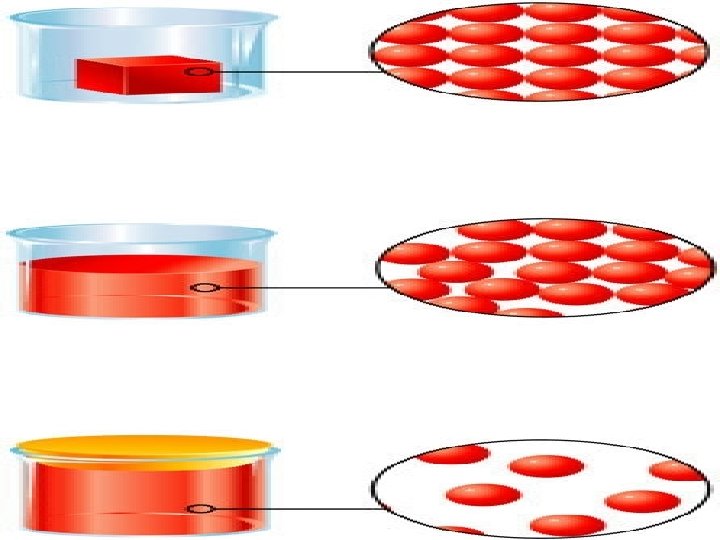
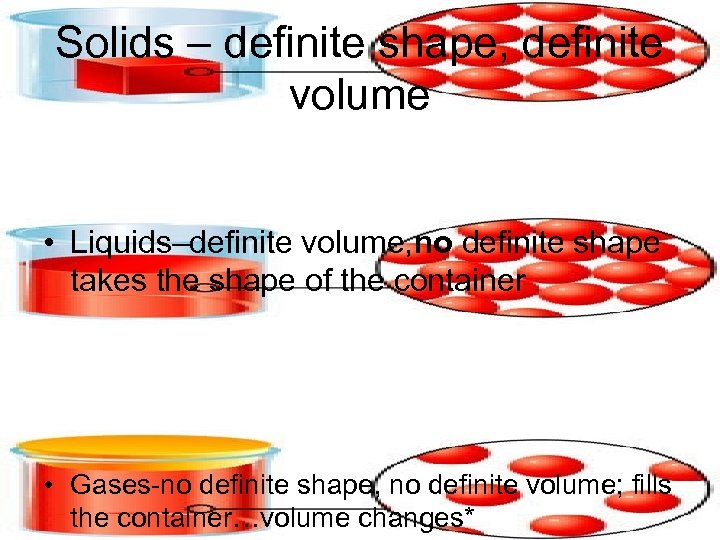
Solids – definite shape, definite volume • Liquids–definite volume, no definite shape takes the shape of the container • Gases-no definite shape, no definite volume; fills the container…volume changes*

Independent work • P 68 -70 • Draw particle view of Cu, Hg, and He • Label position of particles (atoms) • FINISH FOR HOMEWORK • EXTRA - REDO ON COMPUTER?

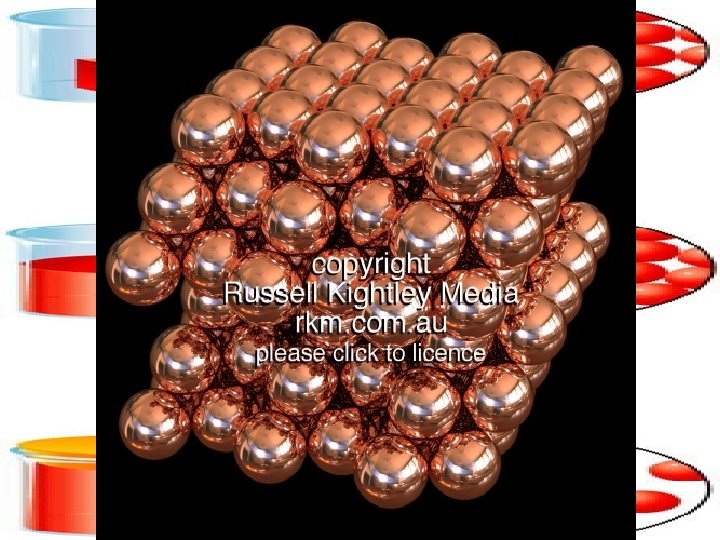
Why solid? • “Copper atoms are packed close together in an orderly arrangement”

1/7/16 warm up • Please open notebook to your nanoscopic / particle view of Cu, Hg, and He.

Why solid? • “Copper atoms are packed close together in an orderly arrangement”

Why liquid? • “The mercury atoms are close together but their arrangement is more random than the arrangement of atoms in copper. ” • Mercury atoms can slide past each other mercury atoms can flow

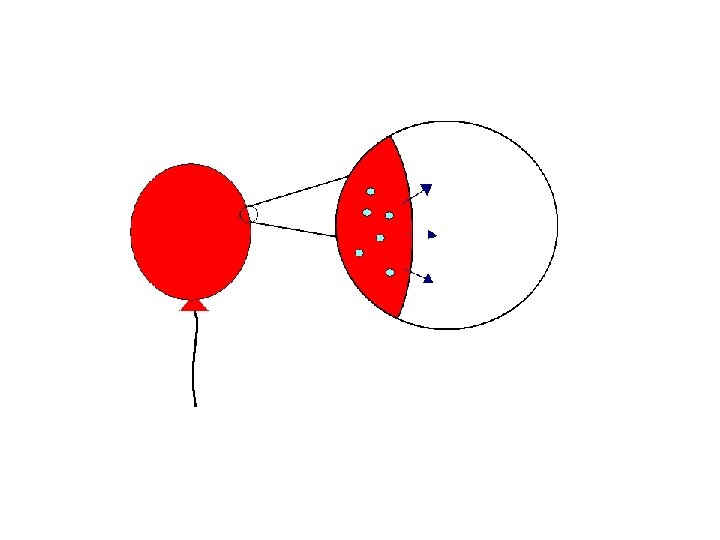
Why gas? • “The helium atoms are…at random locations throughout the balloon. There is more space between two helium atoms in the balloon…”

1/7/16 HW assignment Need : Internet, printer Assignment : Brainpop video / print quiz “States of Matter” Due: Monday 1/11/16 ( 10 points) Extra – pause video on nanoscopic / zoom / particle view. Think think…
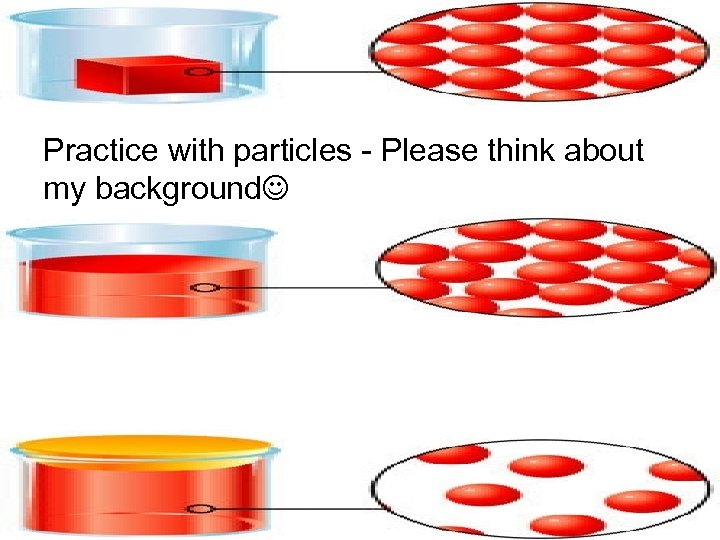
Practice with particles - Please think about my background

Particle Practice 1. What does the textbook p 68 -70 have to say about: Solid particles liquid particles gas particles? Orderly close together spaced Arrangement more random (crystals) “slide freely” “fill container”

16dbb2e6325e82fa1dfcb42a405f98b4.ppt