cac1451bba9685ca6ec639cf5f0de130.ppt
- Количество слайдов: 65

Beyond Methylation: Thimerosal’s Impact on DNA and Sulfur. Dependent Redox in Autism A New Toxic/Sulfur/Genetic Impact Theory And Pathway of Recovery Presented by Michael Lang, M. F. A. Autism. One Conference, Chicago ©May, 2005

Introduction-Research History Autism is likely caused by the “live” actions of thimerosal mercury (Hg) in tandem with prior “envirogenetic” susceptibility on familial DNA from cumulative, multi-generational effects of metal, agricultural and other chemical toxins. The groundbreaking research of Drs. James, Neubrander, Deth, Boris, & Goldblatt cite mercuryinitiated impairments in transsulfuration & methylation processes as primary impacts in Autism Spectrum Disorders.

Introduction-Research History Drs. Amy Holmes and Boyd Haley, and Mark Blaxill, conducted a “first cut” baby hair study and found lower levels of mercury in autistic samples than controls. They postulated Autistic children may have a susceptibility to impaired mercury detoxification. A scientifically thorough follow-up study conducted at M. I. T. fully confirmed these findings. Dr. H. Vasken Aposhian theorized that Autism may be caused by a genetic compromise in “efflux” or transport phase of Hg detox, similar to Wilson’s Disease, which creates toxic accumulations of copper.
Hg, Sulfur, Redox, & DNA Damage Mercury damages brain neurons, pervasively disregulates enzyme, protein, lipid cell membrane and methylation functions. Mercury also most significantly impacts sulfur-dependent processes by binding to sulfur, severely disrupting its functions. Mercury inhibits processes like methylation due to disruption of sulfur-dependent precursor proteins via a slowing of their electron transfer/redox processes, initiating the biochemical cascade of ASD symptoms. Studies have established that Hg and other toxins cause major oxidative damage, lesions and singlestrand breakage on human DNA. Hg further inhibits sulfur-dependent DNA repair of damage it causes.

A New Origin Impact & Genetic Susceptibility Theory This presentation is an extensive review of biochemical research papers that proposes a new unifying theory of how recycling or redox of biochemical processes governed by iron-sulfur protein clusters are a likely primary impact biochemistry in the effects of mercury toxicity. This theory provides a deeper biochemical basis and connectivity the for oxidation, methylation, and Hg efflux defects cited by Drs. James, Neubrander, Deth, Boris, Goldblatt, & Aposhian.

Sulfur Chemistry As a Primary Hg Impact And Recovery Pathway Iron-sulfur proteins provide indispensable “Administrative Assistant” functions in transferring crucially needed electrons from one biochemical interaction to another. The “ Metabolic CEOs” can then complete processes they oversee to maintain the body’s collective “Gross National Product, ” keeping the biochemical “economy” robust. “Downsize” several seemingly benign biochemical “Admin. Assistants, ” then routinely overwork those who remain to see how little work “CEOs” do on their own! Without optimal reduction or recycling via unsung sulfurbased “Admin. Assistants, ” essential biochemical work slows down, processes are diminished, and metabolic functions suffer from ongoing, pervasive impairments.

Hg Damage & Diminished Redox: Compromised DNA Code & Cure Target If we close in to a more elemental point of impact, we can develop a more effective understanding of how mercury damages the body via both live and ongoing damage to the brain, metabolic, & DNA functions & why recovered children may retain lifelong detox issues & oxidative stress. For now, nutritional therapy can minimize many issues that may be eventually curable with more targeted DNA repair. A way of testing for this active DNA damage is by urinary 8 hydroxydeoxyguanosine as a marker along with elevated oxidized NADP to reduced NADPH, oxidized cobalamin, and other redox process indicators would be important tests to confirm/deny this theory, possibly leading to more natural/targeted/effective Autism recovery protocols.
RESEARCH HYPOTHESES: 1. Hg-initiated oxidative disruptions in iron-sulfur cluster-dependent redox biochemically precede impairments in glutathione synthesis via methylation pathway. 2. These sulfur disruptions are the primary reason levels of reduced or active glutathione (GSH) are low & elevated levels of oxidized or spent glutathione (GSSG) occur in Autism.

RESEARCH HYPOTHESES: 3. Hg-initiated oxidative DNA damage may facilitate ongoing oxidative stress, diminishing antioxidant & global redox/recycling processes. 4. Boosting antioxidants, using low dose/diverse sulfur & redox support, naturally & gently detoxing metals, & supporting DNA repair with iron-sulfur rich micro-algae may provide more comprehensive Autism recovery options.

What is Oxidation & Oxidative Stress? Examples of familiar oxidation reactions: q Sliced apple browning: prevented by lemon juice q Nuts and chips: oxygen-generated rancidity/staleness q White wine: browns after oxygen enters bottle q Rust: oxidation of iron in combination with water Antioxidants prevent or halt oxidative reactions & stress

What is Oxidation & Oxidative Stress? Think of oxidation of cells like the soiling of clothes or food left on dirty dishes. Washing & drying clothes & dishes “reduces” them to a cleansed or “ready” state. Oxidative stress occurs in the body when reserves of antioxidants are in insufficient form or supply to stem the cell and DNA-damaging effects of oxidizing chemistries. Heavy oxidizers: Singlet oxygen & iron, agricultural toxins, petrochemicals, heavy metals, & radiation. Heavy casualties of heavy metal toxicity are oxidation of lipids, proteins and DNA.
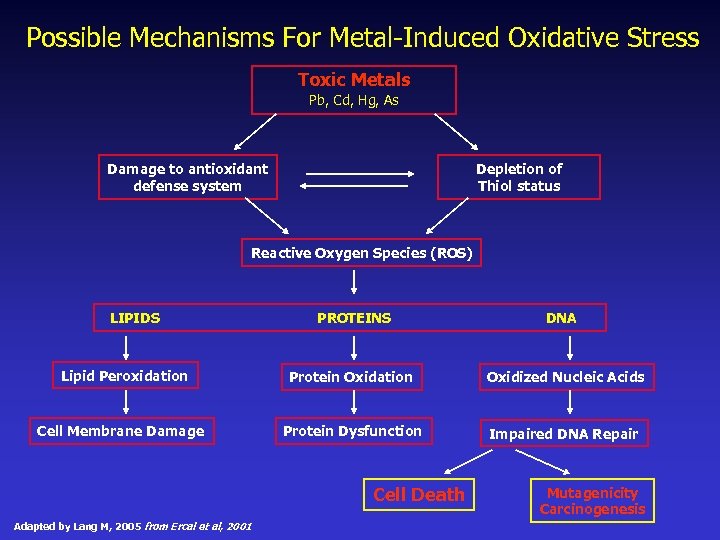
Possible Mechanisms For Metal-Induced Oxidative Stress Toxic Metals Pb, Cd, Hg, As Damage to antioxidant defense system Depletion of Thiol status Reactive Oxygen Species (ROS) LIPIDS PROTEINS DNA Lipid Peroxidation Protein Oxidation Oxidized Nucleic Acids Cell Membrane Damage Protein Dysfunction Impaired DNA Repair Cell Death Adapted by Lang M, 2005 from Ercal et al, 2001 Mutagenicity Carcinogenesis
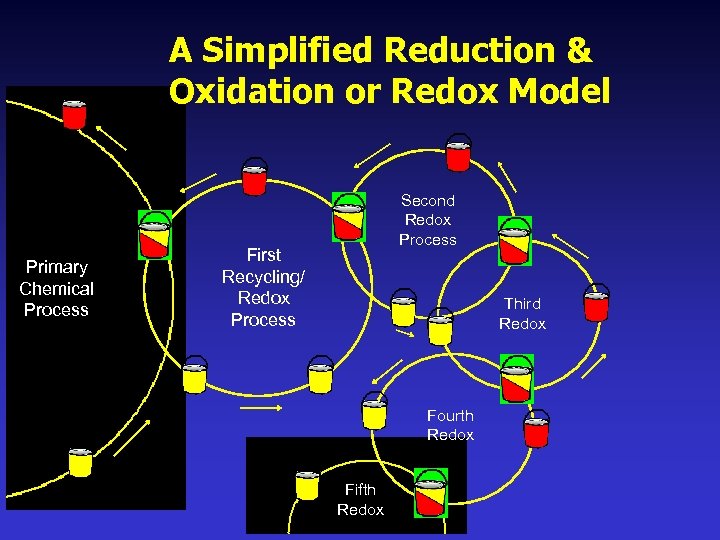
What is Redox & Why is it Important? Redox reactions always occur in pairs; as one substance is oxidized, another is reduced. The molecule that loses electrons in a biochemical reaction, is Oxidized; the molecule that gains those electrons is Reduced. These electron transfers are called Reduction/Oxidation or Redox reactions. A way to remember this relationship: Redox Definition: Loss of Electron = Oxidation. Gain of Electrons = Reduction or remember: (LEO the lion says GER)

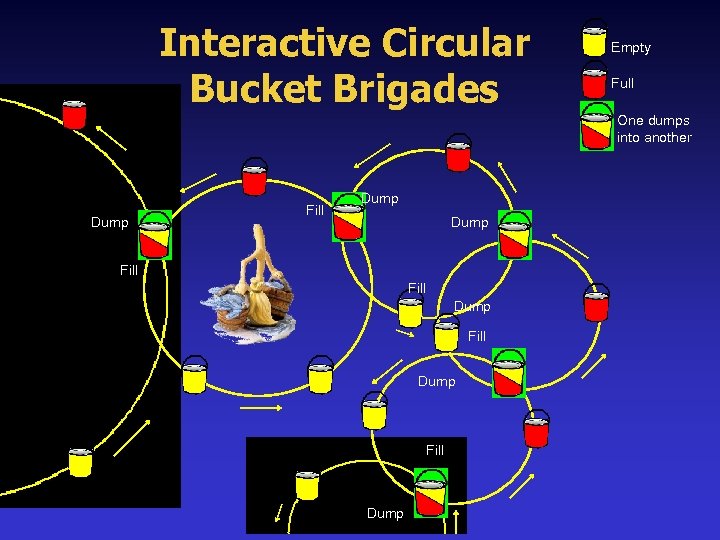
What is Redox & Why is it Important? Redox moves electrons of oxygen, nitrogen, hydrogen, and other elements from one process where they are reacted or will be disruptive if they remain and also delivers them to other processes where they can be used, converted, or eliminated from the body. Redox processes work like interactive circular bucket brigades in biochemistry, transferring vital electrons from one cycle to another to maintain the thousands of biological processes occurring simultaneously within living organisms.
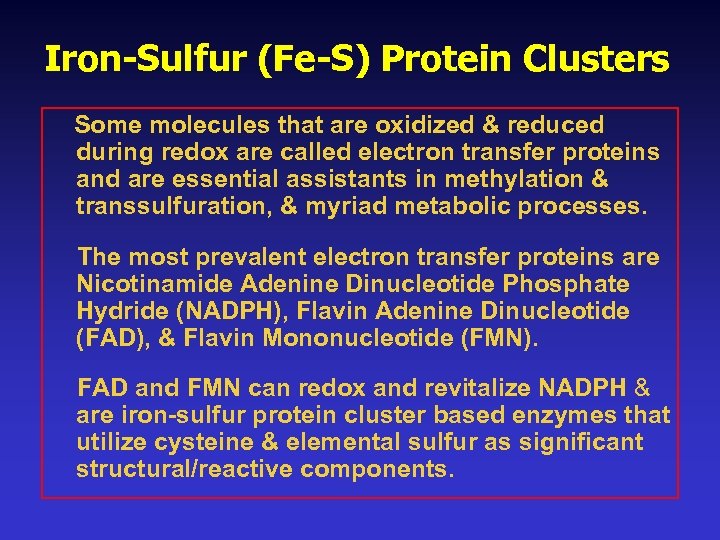
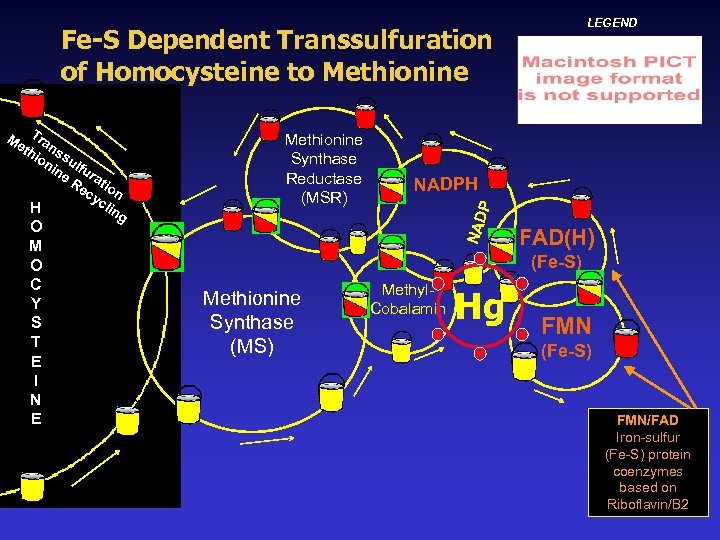
Iron-Sulfur (Fe-S) Protein Clusters Some molecules that are oxidized & reduced during redox are called electron transfer proteins and are essential assistants in methylation & transsulfuration, & myriad metabolic processes. The most prevalent electron transfer proteins are Nicotinamide Adenine Dinucleotide Phosphate Hydride (NADPH), Flavin Adenine Dinucleotide (FAD), & Flavin Mononucleotide (FMN). FAD and FMN can redox and revitalize NADPH & are iron-sulfur protein cluster based enzymes that utilize cysteine & elemental sulfur as significant structural/reactive components.
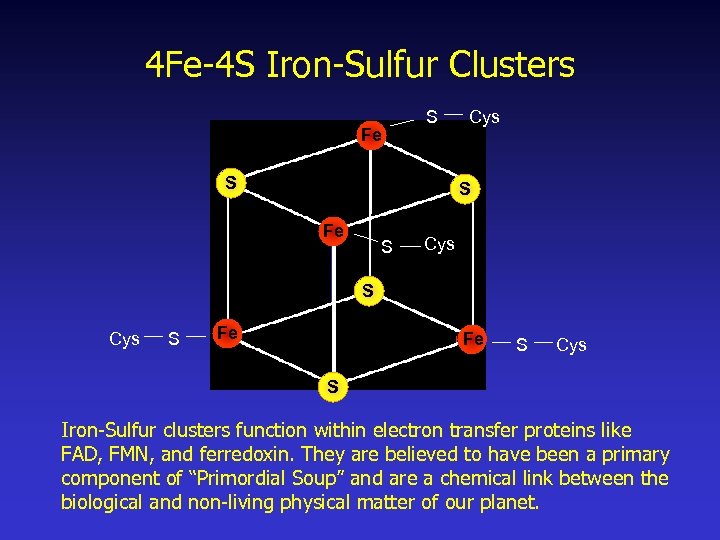
4 Fe-4 S Iron-Sulfur Clusters Fe S S Cys S Fe Fe S Cys S Iron-Sulfur clusters function within electron transfer proteins like FAD, FMN, and ferredoxin. They are believed to have been a primary component of “Primordial Soup” and are a chemical link between the biological and non-living physical matter of our planet.
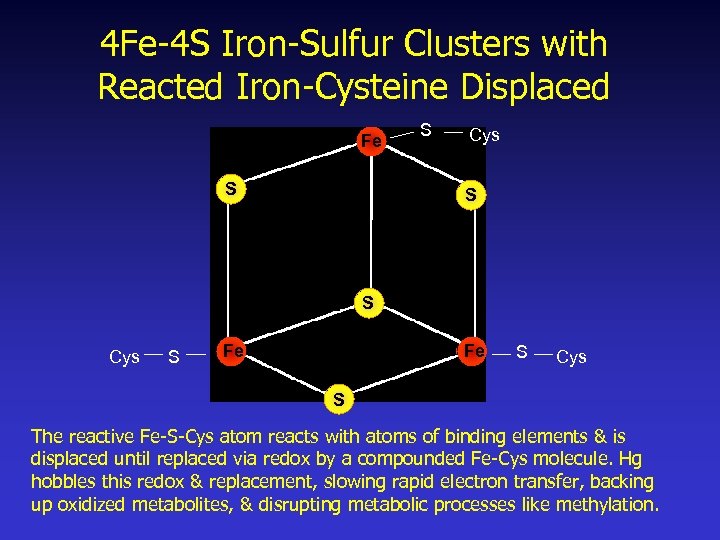
4 Fe-4 S Iron-Sulfur Clusters with Reacted Iron-Cysteine Displaced Fe S S Cys S Fe Fe S Cys S The reactive Fe-S-Cys atom reacts with atoms of binding elements & is displaced until replaced via redox by a compounded Fe-Cys molecule. Hg hobbles this redox & replacement, slowing rapid electron transfer, backing up oxidized metabolites, & disrupting metabolic processes like methylation.
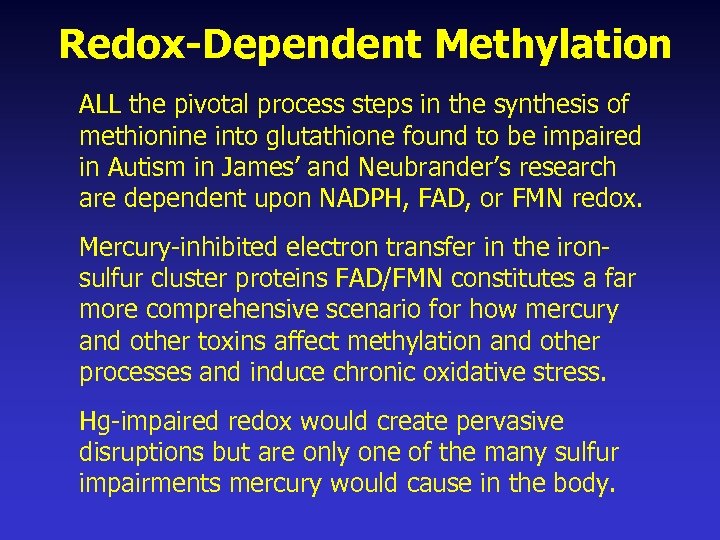
Redox-Dependent Methylation ALL the pivotal process steps in the synthesis of methionine into glutathione found to be impaired in Autism in James’ and Neubrander’s research are dependent upon NADPH, FAD, or FMN redox. Mercury-inhibited electron transfer in the ironsulfur cluster proteins FAD/FMN constitutes a far more comprehensive scenario for how mercury and other toxins affect methylation and other processes and induce chronic oxidative stress. Hg-impaired redox would create pervasive disruptions but are only one of the many sulfur impairments mercury would cause in the body.
Interactive Circular Bucket Brigades Empty Full One dumps into another Dump Fill Dump Fill Dump
A Simplified Reduction & Oxidation or Redox Model Primary Chemical Process Second Redox Process First Recycling/ Redox Process Third Redox Fourth Redox Fifth Redox
LEGEND Fe-S Dependent Transsulfuration of Homocysteine to Methionine O M O C Y S T E I N E NADPH P Methionine Synthase Reductase (MSR) NAD Me Tra th nss io ni ulfu ne r Re atio cy n cli H ng FAD(H) (Fe-S) Methionine Synthase (MS) Methyl. Cobalamin Hg FMN (Fe-S) FMN/FAD Iron-sulfur (Fe-S) protein coenzymes based on Riboflavin/B 2
Reduction of 5, 10 -Methylene Tetrahydrofolate by MTHFR A N D & sis te e la nth Fo Sy D A N D 5, 10 Methelene Tetrahydro. Folate Reductase (MTHFR) H NADH NA 5 M E T H Y L T E T R A H Y R D O F O L A T E LEGEND Hg FAD(H) Kreb’s Cycle (Fe-S) FMN/FAD Iron-sulfur (Fe-S) protein coenzymes based on riboflavin
Primary & Secondary Pathway of MS Redox The primary pathway of redoxing or recycling Methionine Synthase is via Methionine Synthase Reductase, with secondary redox accomplished by Methylcobalamin. Both pathways are dependent upon NADPH and/or FMN/FAD iron-sulfur dependent redox.

Primary & Secondary Pathway of MS Redox Oxidation and redox impairments and not methylation disruptions are more likely the basis for low levels of reduced glutathione, methylcobalamin, & folate. A more complete approach to the problem would involve finding the origin of the oxidative stress/redox impairments and correcting this at its point of genesis if possible.
Disruption of Iron-Sulfur Clusters Chemical toxins known to interfere with Iron-Sulfur proteins by disrupting their normal electron transfer: q Rotenone: mild pesticide developed from legumes but a potent inhibitor q Amytal: a barbiturate that also inhibits folate, Vit. D, and calcium metabolism q Piericidin A: a penicillin-like antibiotic and a potent inhibitor q Heavy Metals: Lead, Mercury, Cadmium are all potent inhibitors

Hg’s Disruption of Iron-Sulfur Clusters In plants, even highly diluted, submicromolar concentrations of Hg can completely inhibit NADPHdependent redox within 15 minutes. Other studies have shown that mercury completely disrupts the “ping pong” to and fro functions of NADPH.
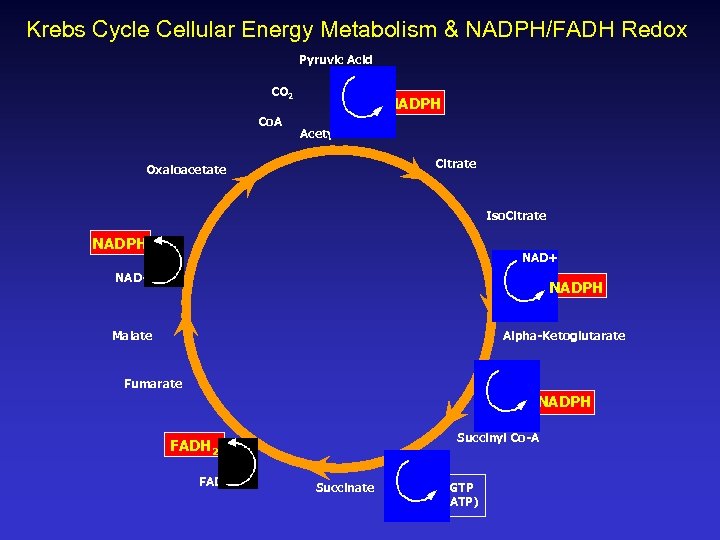
Krebs Cycle Cellular Energy Metabolism & NADPH/FADH Redox Pyruvic Acid CO 2 Co. A NADPH Acetyl-Co. A Citrate Oxaloacetate Iso. Citrate NADPH NAD+ NADPH Malate Alpha-Ketoglutarate NAD+ Fumarate NADPH Succinyl Co-A FADH 2 FAD Succinate GTP (ATP)
Iron-Sulfur Cluster Proteins & The Kreb’s Cycle NADPH & FADH are both reduced via and exert a reactive influence within the Kreb’s Cycle of cellular energy production. It is very likely that specific anomalies of Kreb’s Cycle acids in organic acid tests of Autistic children like pyruvate, citrate, alpha -ketoglutarate, succinate, or malate could be directly attributed to diminished electron transfers from Hg-impacted iron-sulfur proteins that cause biochemical “traffic jams. ”

Iron-Sulfur Clusters and Metabolic Impacts: The hidden role of iron-sulfur protein-dependent redox in methylation transsulfuration pathway impairments cited by Drs. Jill James, James Neubrander, & Richard Deth.
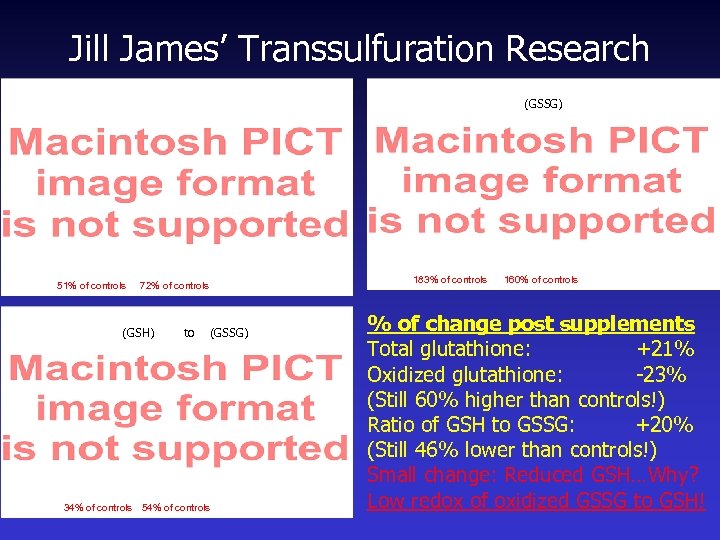
Jill James’ Transsulfuration Research (GSSG) 51% of controls 72% of controls (GSH) to (GSSG) 34% of controls 54% of controls 183% of controls 160% of controls % of change post supplements Total glutathione: +21% Oxidized glutathione: -23% (Still 60% higher than controls!) Ratio of GSH to GSSG: +20% (Still 46% lower than controls!) Small change: Reduced GSH…Why? Low redox of oxidized GSSG to GSH!
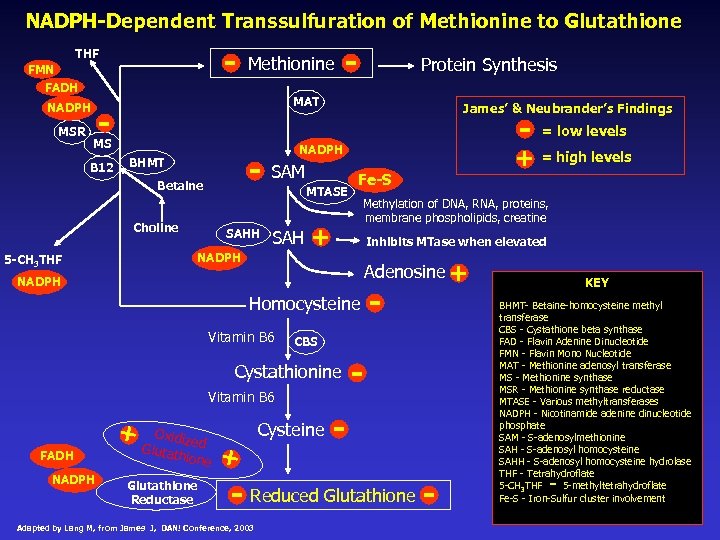
NADPH-Dependent Transsulfuration of Methionine to Glutathione - THF FMN Methionine FADH NADPH MSR Protein Synthesis MAT - MS B 12 - - = low levels NADPH - SAM BHMT Betaine Choline SAHH SAH NADPH 5 -CH 3 THF James’ & Neubrander’s Findings MTASE Fe-S Methylation of DNA, RNA, proteins, membrane phospholipids, creatine + Inhibits MTase when elevated Adenosine + NADPH Homocysteine Vitamin B 6 FADH NADPH Cysteine Oxidiz Glutat ed hione Glutathione Reductase + - - CBS Cystathionine + + = high levels - - Reduced Glutathione Adapted by Lang M, from James J, DAN! Conference, 2003 - KEY BHMT- Betaine-homocysteine methyl transferase CBS - Cystathione beta synthase FAD - Flavin Adenine Dinucleotide FMN - Flavin Mono Nucleotide MAT - Methionine adenosyl transferase MS - Methionine synthase MSR - Methionine synthase reductase MTASE - Various methyltransferases NADPH - Nicotinamide adenine dinucleotide phosphate SAM - S-adenosylmethionine SAH - S-adenosyl homocysteine SAHH - S-adenosyl homocysteine hydrolase THF - Tetrahydroflate 5 -CH 3 THF 5 -methyltetrahydroflate Fe-S - Iron-Sulfur cluster involvement -
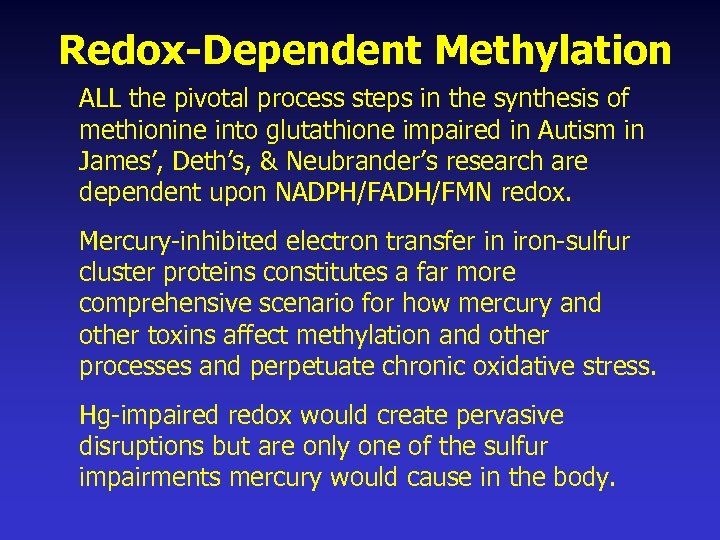
Redox-Dependent Methylation ALL the pivotal process steps in the synthesis of methionine into glutathione impaired in Autism in James’, Deth’s, & Neubrander’s research are dependent upon NADPH/FADH/FMN redox. Mercury-inhibited electron transfer in iron-sulfur cluster proteins constitutes a far more comprehensive scenario for how mercury and other toxins affect methylation and other processes and perpetuate chronic oxidative stress. Hg-impaired redox would create pervasive disruptions but are only one of the sulfur impairments mercury would cause in the body.
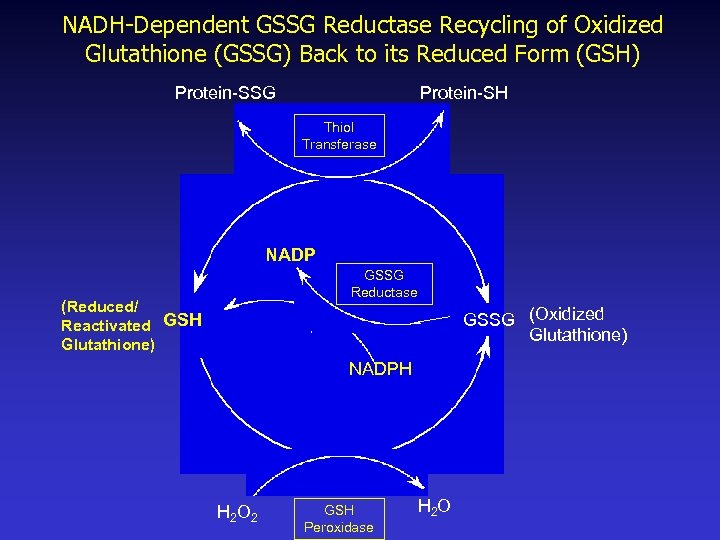
NADH-Dependent GSSG Reductase Recycling of Oxidized Glutathione (GSSG) Back to its Reduced Form (GSH) Protein-SSG Protein-SH Thiol Transferase NADP GSSG Reductase (Reduced/ Reactivated GSH Glutathione) GSSG (Oxidized Glutathione) NADPH H 2 O 2 GSH Peroxidase H 2 O

Dr. Neubrander’s Protocol of Methylcobalamin Injections: How do they help metabolically and can they balance disrupted methylation pathways, reduce oxidative stress and maintain a more normal balance of reduced glutathione (GSH) to oxidized glutathione (GSSG)?
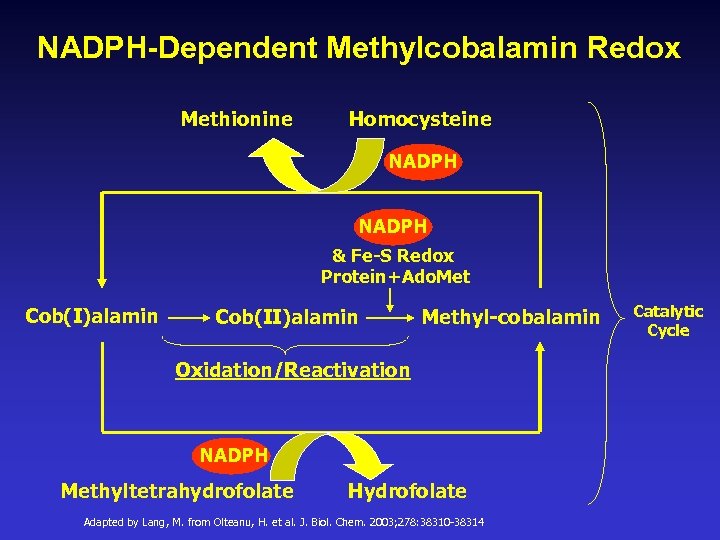
NADPH-Dependent Methylcobalamin Redox Methionine Homocysteine NADPH & Fe-S Redox Protein+Ado. Met Cob(I)alamin Cob(II)alamin Methyl-cobalamin Oxidation/Reactivation NADPH Methyltetrahydrofolate Hydrofolate Adapted by Lang, M. from Olteanu, H. et al. J. Biol. Chem. 2003; 278: 38310 -38314 Catalytic Cycle

Why Do Methylcobalamin B-12 Injections Help Autistic Children with Methylation Impairments? § Chronic redox impairments would leave biochemical processes including, but not limited to, methylation shortchanged and functioning at a diminished rate. § These backups would result in higher levels of oxidized forms of glutathione and cobalamin (B-12), to name just a few.

Why Do Methylcobalamin B-12 Injections Help Autistic Children with Methylation Impairments? § Inhibition of methionine synthase from diminished redox from NADPH/FADH/FMN and methylcobalamin would impact the recycling of homocysteine into methionine, significantly inhibiting glutathione synthesis. §Giving B-12 injections would provide an end of process “jumper cable” solution for the secondary support of methionine synthase that recycles homocysteine back into methionine.
Methylcobalamin & Redox NADPH’s redox of cobalamin to methylcobalamin is yet another example of the role of iron-sulfurdependent redox of methylation chemictry. If NADPH itself is not redoxed effectively, by iron -sulfur dependent redox, then it cannot in turn convert oxidized or less active cobalamin into reduced or active methylcobalamin, thus creating another metabolic logjam. Inhibited NADPH-dependent methylcobalamin redox = inhibited Methionine Synthase, which = incomplete recycling of homcysteine back into methionine which = less available glutathione.

Research Establishing Redox Control of Transsulfuration Defects in Auxiliary Redox Proteins Lead to Functional Methionine Synthase Deficiency Journal of Biological Chemistry, Aug 1997 Redox control of the transsulfuration & glutathione biosynthesis pathways. Current Opinion in Clinical Nutrition & Metabolic Care, Jan 2002 The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry, Oct 2000 Redox regulation of homocysteine-dependent glutathione synthesis. Redox Report, Aug 2003
Research Establishing Redox Control of Transsulfuration Molecular dissection of human methionine synthase reductase: determination of the flavin redox potentials in full-length enzyme and isolated flavin-binding domains. Biochemistry, Apr 2003 Molecular basis for methionine synthase reductase deficiency in patients belonging to the cbl. E complementation group of disorders in folate/cobalamin metabolism Human Molecular Genetics, Oct 1999
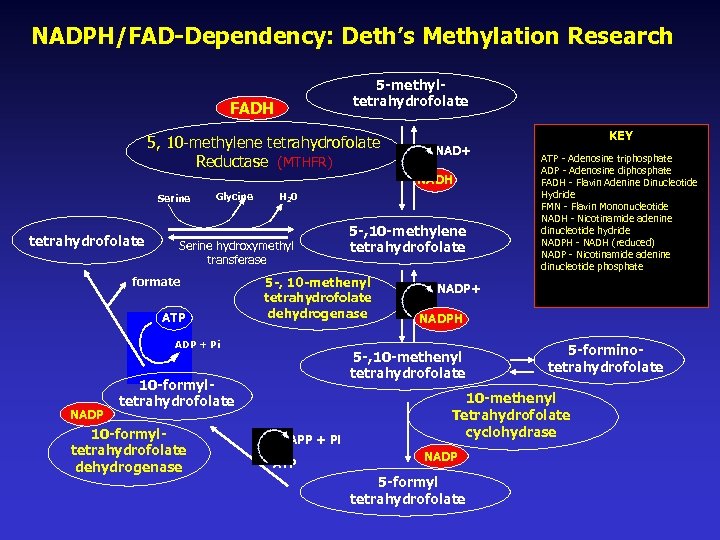
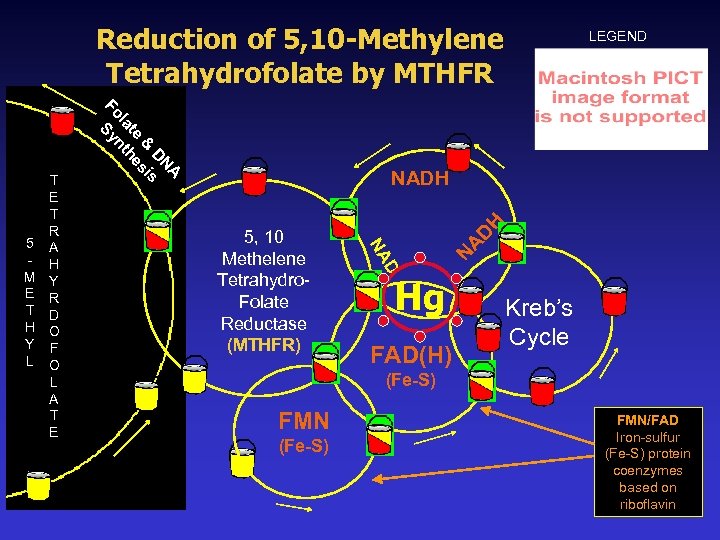
NADPH/FAD-Dependency: Deth’s Methylation Research 5 -methyltetrahydrofolate FADH 5, 10 -methylene tetrahydrofolate Reductase (MTHFR) KEY NAD+ NADH Serine tetrahydrofolate Glycine H 20 Serine hydroxymethyl transferase formate ATP 5 -, 10 -methenyl tetrahydrofolate dehydrogenase ADP + Pi NADP+ NADPH 5 -, 10 -methenyl tetrahydrofolate 10 -formyltetrahydrofolate dehydrogenase 5 -, 10 -methylene tetrahydrofolate APP + Pi ATP - Adenosine triphosphate ADP - Adenosine diphosphate FADH - Flavin Adenine Dinucleotide Hydride FMN - Flavin Mononucleotide NADH - Nicotinamide adenine dinucleotide hydride NADPH - NADH (reduced) NADP - Nicotinamide adenine dinucleotide phosphate 5 -forminotetrahydrofolate 10 -methenyl Tetrahydrofolate cyclohydrase NADP 5 -formyl tetrahydrofolate
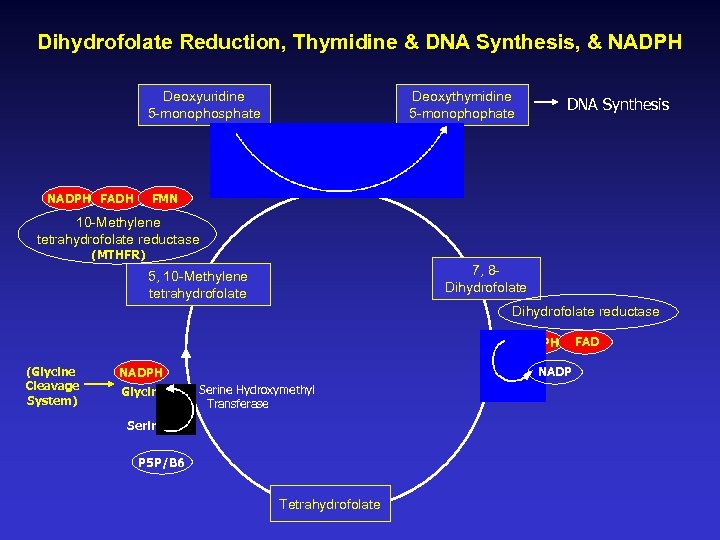
Dihydrofolate Reduction, Thymidine & DNA Synthesis, & NADPH Deoxyuridine 5 -monophosphate NADPH FADH Deoxythymidine 5 -monophophate DNA Synthesis FMN 10 -Methylene tetrahydrofolate reductase (MTHFR) 7, 8 Dihydrofolate 5, 10 -Methylene tetrahydrofolate Dihydrofolate reductase NADPH (Glycine Cleavage System) NADPH Glycine Serine Hydroxymethyl Transferase Serine P 5 P/B 6 Tetrahydrofolate FAD

Research Establishing Redox Involvement in MTHFR Methylenetetrahydrofolate reductase. Steady state and rapid reaction studies on the NADPH-methylenetetrahydrofolate, NADPH -menadione, and methyltetrahydrofolate-menadione oxidoreductase activities of the enzyme. Journal of Biological Chemistry, Sep 1983 Purification & characterization of methylenetetrahydrofolate reductase from human cadaver liver. Journal of Biological Chemistry, Sep 1984 Purification and properties of NADH-dependent 5, 10 methylenetetrahydrofofolate reductase (Met. F) from E. coli. Journal of Bacteriology, Feb 1999 Purification and properties of 5, 10 -methylenetetrahydrofofolate reductase, an iron-sulfur flavoprotein from Clostridium Formicoaceticum. Journal of Biological Chemistry, Sep 1984
Hg-Initiated Oxidative DNA Damage: Do Chronic Redox Impairments in Autism Have a Genesis In Damaged Genetic Code?

Iron-sulfur, DNA Mutations, and Methylation Chemistry Genomic research has shown that DNA mutations at the binding sites of Nicotinamide Adenine Dinucleotide Hydride, (NADPH), Flavin Adenine Dinucleotide (FAD, & Flavin Mononucleotide (FMN) are involved with Methionine Synthase Deficiency conditions via diminished redox of Methionine Synthase Reductase.
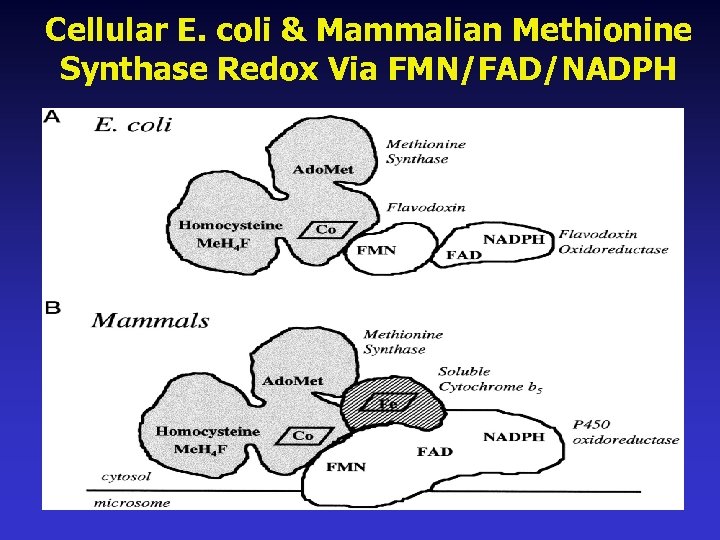
Cellular E. coli & Mammalian Methionine Synthase Redox Via FMN/FAD/NADPH
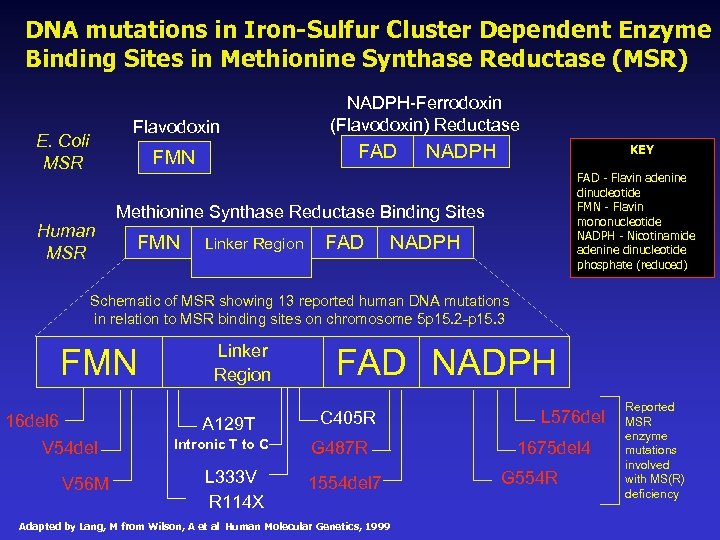
DNA mutations in Iron-Sulfur Cluster Dependent Enzyme Binding Sites in Methionine Synthase Reductase (MSR) Flavodoxin E. Coli MSR FAD FMN Human MSR NADPH-Ferrodoxin (Flavodoxin) Reductase NADPH KEY FAD - Flavin adenine dinucleotide FMN - Flavin mononucleotide NADPH - Nicotinamide adenine dinucleotide phosphate (reduced) Methionine Synthase Reductase Binding Sites FMN Linker Region FAD NADPH Schematic of MSR showing 13 reported human DNA mutations in relation to MSR binding sites on chromosome 5 p 15. 2 -p 15. 3 FMN 16 del 6 Linker Region A 129 T V 54 del V 56 M Intronic T to C L 333 V R 114 X FAD NADPH C 405 R G 487 R 1554 del 7 Adapted by Lang, M from Wilson, A et al Human Molecular Genetics, 1999 L 576 del 1675 del 4 G 554 R Reported MSR enzyme mutations involved with MS(R) deficiency

Research on Hg’s Effects In Oxidative DNA Damage Mercuric chloride damages cellular DNA by a non-apoptotic mechanism. Mutation Research, Oct. 2000 Increased oxidative DNA damage, as assessed by urinary 8 -hydroxy-2'deoxyguanosine concentrations, & serum redox status in persons exposed to mercury. Clinical Chemistry Apr. 2005 Interactions of Hg(II) ions with DNA as revealed by CD measurements. Nucleic Acids Research Mar 1977

Research on Hg’s Effects In Oxidative DNA Damage Oxidative damage to nucleic acids in motor neurons containing mercury. Clinical Chemistry, Apr. 2005 Time course assessment of methylmercury effects on C 6 glioma cells: submicromolar concentr. induce oxidative DNA damage and apoptosis. Journal of Neuroscience Research, Dec 2002
Research: Hg’s DNA Damage & Inhibition of DNA Repair Analysis of metal-induced DNA lesions and DNA-repair replication in mammalian cells. Mutation Research, Mar-Apr 1984 Correlations of DNA strand breaks and their repair with cell survival following acute exposure to mercury and X-rays. Molecular Pharmacology, Jul 1983
Research on Hg’s Inhibition of DNA Repair Use of mammalian DNA repairdeficient mutants to assess the effects of toxic metal compounds on DNA. Biochemical Pharmacology May 1984 Differences in the effects of Hg (II) on DNA repair induced in Chinese Hamster ovary cells by ultraviolet or X-rays. Molecular Pharmacology, Feb, 1986.

How to Prevent or Repair Oxidation-Damaged DNA Glutathione & ascorbate are negatively correlated with oxidative DNA damage in human lymphocytes Carcinogenesis, Apr, 1999 Oxidative DNA damage in human lymphocytes: correlations with plasma levels of tocopherol & carotenoids Carcinogenesis, Feb 2000 Lymphocyte Oxidative DNA Damage and Plasma Antioxidants in Alzheimer Disease Arch Neurol, May 1, 2002;
Fe-S Cluster’s Repair of Oxidation-Damaged DNA A Role for iron-sulfur clusters in DNA repair. Curr Opin Chem Biol. Apr 2005 Association of a polynuclear iron-sulfur center with a mutant FNR protein enhances DNA binding. Proceedings, National Academy of Sciences. Mar 1995 Atomic structure of the DNA repair [4 Fe-4 S] enzyme endonuclease III. Science. Oct 1992 A substrate recognition role for the [4 Fe-4 S]2+ cluster of the DNA repair glycosylase Mut. Y. Biochemistry. May 1998

Nutritional Protocols Used Successfully For Autism To Assist in Redox & Hg Detox: What biochemical processes do they support and why do they work?
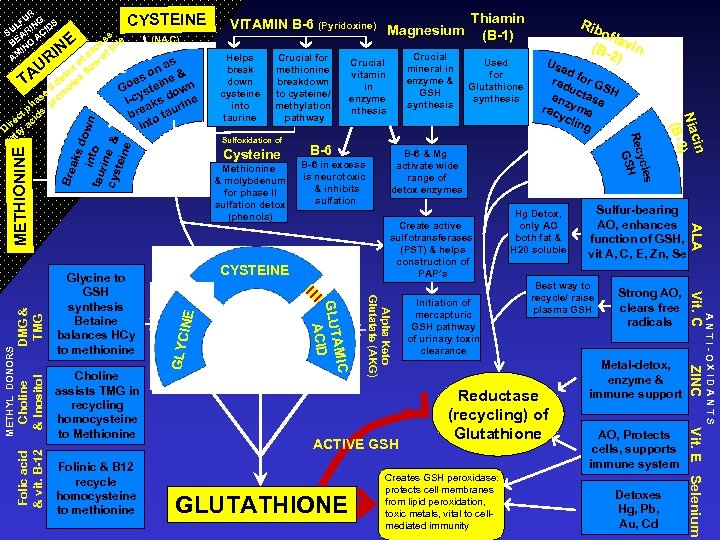
UR LF ING IDS SU AR AC BE NO I AM CYSTEINE Create active sulfotransferases (PST) & helps construction of PAP’s Bre GLYC INE CYSTEINE GLUTATHIONE Best way to recycle/ raise plasma GSH Reductase (recycling) of Glutathione Creates GSH peroxidase: protects cell membranes from lipid peroxidation, toxic metals, vital to cellmediated immunity Sulfur-bearing AO, enhances function of GSH, vit A, C, E, Zn, Se Strong AO, clears free radicals Metal-detox, enzyme & immune support AO, Protects cells, supports immune system Detoxes Hg, Pb, Au, Cd Vit. E Selenium ACTIVE GSH Initiation of mercapturic GSH pathway of urinary toxin clearance Hg Detox, only AO both fat & H 20 soluble ANTI-OXIDANTS DMG & TMG B-6 & Mg activate wide range of detox enzymes ZINC Choline & Inositol B-6 in excess is neurotoxic & inhibits sulfation Vit. C METHYL DONORS Methionine & molybdenum for phase II sulfation detox (phenols) B-6 Us ed red for G S u enz ctase H rec yme ycl ing ALA Folic acid & vit. B-12 Cysteine Used for Glutathione synthesis in Niac ) (B-3 Folinic & B 12 recycle homocysteine to methionine Sulfoxidation of Crucial mineral in enzyme & GSH synthesis Crucial vitamin in enzyme nthesis Alpha Keto Glutatate (AKG) Choline assists TMG in recycling homocysteine to Methionine Crucial for methionine breakdown to cysteine/ methylation pathway AMIC GLUT ACID Glycine to GSH synthesis Betaine balances HCy to methionine Helps break down cysteine into taurine ofla (B- vin 2) s aks dow in n tau to rine cys tein & e RI U METHIONINE TA NE Rib ycle Rec H GS ss e (NAC) ce bil x f e o of as x flow on e & to s es n de e II ot Go stei own y se om l-c aks d rine ha. pr p s bre o tau ct id re ac int Di ty t fa VITAMIN B-6 (Pyridoxine) Magnesium Thiamin (B-1)

All Roads In Effective Nutritional ASD Protocols Lead to Glutathione From the first use of B-6 & Magnesium to Dimethylglycine, Vitamin C, Zinc, and other Antioxidants, N-Acetyl Cysteine, Alpha Lipoic Acid, TTFD/Allithiamine, to today’s use of B-12 and Folate, the primary metabolic benefit has been the enhanced synthesis and redox/recycling of Glutathione.

Supplement Protocol for Enhancing Glutathione Synthesis and Recycling (Redox): B-VITAMINS Avoid tablets and capsules if possible due to poor breakdown and assimilation with sub-optimal gut structure/function. Liquids are better absorbed than other forms and provide 2 -4 x better assimilation. Use optimal daily dosages of all the B-vitamins in the following median levels for a 50 lb. Child, given 3 x/day in divided dosages: Vit B-1 (Thiamin Hydrochloride - 20 -35 mg (GSH synthesis) Vit B-2 (Riboflavin-5 -Phosphate) - 20 -35 mg (Fe-S/flavoprotein building block) Vit B-3 (Niacinamide) - 15 -25 mg (GSH redox) Vit B-3 (Inositol Hexaniacinate) - 20 -30 mg (GSH redox) Vit B-5 (Calcium Pantothenate) - 100 -150 mg (Acetyl Coenzyme A support) or - sulfated B-5: Pantetheine - 50 -75 mg (Enhanced Acetyl Coenzyme A support) Vit B-6 (Pyridoxyl-5 -Phosphate) - 15 -35 mg (Cysteine, GSH, detox enzyme synthesis) or - Pyridoxine Alpha Ketoglutarate - 3 -75 mg (if P-5 -P intolerance) Vit B-12 (Methylcobalamin) sublingual form -. 5 -2 mg (Methylation “jumper cable”) Folinic Acid (5 -methyl. THF), sublingual form -. 5 -2 mg (Methylation “jumper cable”) Biotin - 300 -500 mcg (Sulfur-bearing catalyst) Choline (Citrate) - 60 -100 mg (Lipid cell membrane/myelin & detox support) Inositol (FCC) - 80 -100 mg (Lipid cell membrane support)

Supplement Protocol for Enhancing Glutathione Synthesis and Recycling (Redox): ANTIOXIDANTS Use optimal daily dosages of all the antioxidants in the following levels for a 50 lb. child, administered 3 x/day in divided dosages: Pro-vitamin A Mixed carotenoids - 6, 000 IU or QUALITY fish oil* (Redox/gut/immune support) Vit C as Calcium Ascorbate - 750 mg-1 gr. 3 x/day (GSH/Redox support) Lemon Bioflavinoids - 175 -300 mg (Anti-inflammatory & increases Vit. C potential) Vit D-3 (Cholecalciferol - 200 -300 IU (Enhances calcium metabolism & uptake) Vit E (Natural tocopherol)*- 125 -200 IU (GSH redox & cell membrane antioxidant TMG - 100 -750 mg (GSH precursor/methyl donor) L-Glycine - 100 -250 mg (GSH precursor) N-Acetyl Cysteine - 10 -30 mg (GSH precursor & MT synthesis) • Avoid synthetic vitamin A acetate/palmitate and synthetic vitamin E d. L-tocopherols!!! Both are either synthesized from petroleum or contain petroleumderived preservative agents and can also block the assimilation of natural forms of vits A & E-ASK BEFORE YOU BUY!!

Supplement Protocol for Enhancing Glutathione Synthesis and Recycling (Redox): MINERALS Use optimal daily dosages of all the minerals in the following levels for a 50 lb. child, administered 3 x/day in divided dosages: Calcium (Kreb’s Cycle Chelates) - 1200 mg (Prevents cellular uptake of Hg) Chromium (Kreb’s Chelates) - 100 mcg (Blood sugar balance) Magnesium (Kreb’s Chelates)- 375 mg (GSH & MT synthesis & redox) Manganese (Succinate) - 2 -3 mg (GSH & MT synthesis & redox) Molybdenum (Kreb’s Chelates) - 200 mcg (Sulfur process catalyst) Potassium (Alpha-Ketoglutarate) - 100 mg (Nerve cell function & ammonia detox Selenium (Selenomethionine) - 100 mcg (Hg detox & GSH peroxidase cell defense) Zinc (Kreb’s Chelates) - 50 mg (Hg detox, MT synthesis, & GSH redox)
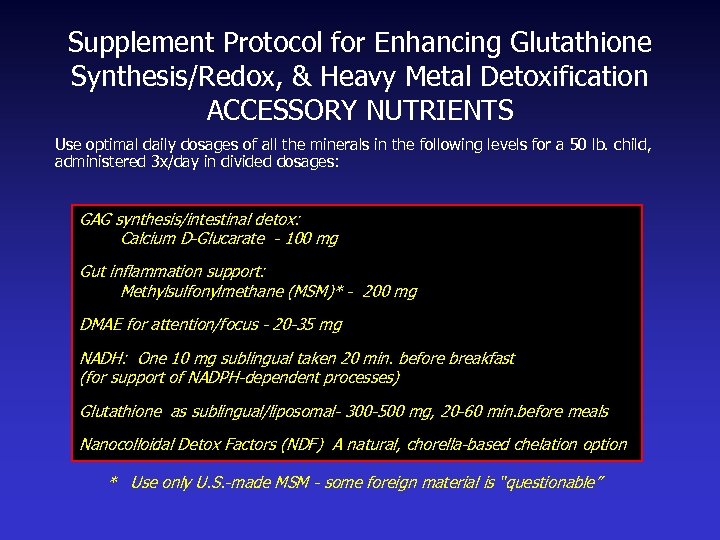
Supplement Protocol for Enhancing Glutathione Synthesis/Redox, & Heavy Metal Detoxification ACCESSORY NUTRIENTS Use optimal daily dosages of all the minerals in the following levels for a 50 lb. child, administered 3 x/day in divided dosages: GAG synthesis/intestinal detox: Calcium D-Glucarate - 100 mg Gut inflammation support: Methylsulfonylmethane (MSM)* - 200 mg DMAE for attention/focus - 20 -35 mg NADH: One 10 mg sublingual taken 20 min. before breakfast (for support of NADPH-dependent processes) Glutathione as sublingual/liposomal- 300 -500 mg, 20 -60 min. before meals Nanocolloidal Detox Factors (NDF) A natural, chorella-based chelation option * Use only U. S. -made MSM - some foreign material is “questionable”

Supplement Protocol for Enhancing Glutathione Synthesis and Recycling (Redox): HERBS Ashwaganda Root (Withania somnifera) support. Supports detoxification via its GSH redox Burdock Root (Arcticum lappa) Promotes liver cleansing, bile flow as well as detoxification via its GSH redox support Chinese Astragalus Root (Astragalus membranaceus) Supports detox. via GSH redox support. Fennel Seed (Agastache foeniculum) Sulfur source. Ginkgo Biloba Leaf (Gingko Biloba) Increases cellular GSH and GSH S-transferase Gotu Kola Leaf (Centella asiatica) Raises levels of GSH. Milk Thistle Seed (Silibum marinum) Sulfur source. Assists GSH redox support. Chinese Sarsaparilla (Smilax Glabra) Used in Chinese medicine to detoxify mercury. Assists in GSH redox and cellular thiol status. Schisandra Fruit (Schisandra chinensis) Liver protectant. Supports detoxification via recycling of glutathione.

Summary 1. Multi-generational exposure to toxins that has affected familial DNA may have set the stage for a susceptibility factor via iron-sulfur-cluster disruptions, increasing the oxidative damage caused by Thimerosal mercury. 2. Mercury is the “rock thrown, ” but sulfur is the “window” that has broken. This scenario is very likely to be the core issue of the pervasive disregulation of metabolism we are seeing in Autism.
Summary 3. Hg-initiated oxidative disruptions in iron-sulfur cluster-dependent redox biochemically precede impairments in glutathione synthesis via the methylation pathway. 4. These sulfur disruptions are the primary reason levels of reduced or active glutathione (GSH) are low & levels of (GSSG) oxidized or spent glutathione are elevated in Autism.

Summary 5. Hg-initiated oxidative DNA damage may facilitate ongoing oxidative stress, crippling antioxidant & global redox processes. This is our biggest challenge in recovering and maintaining the health of our children. 6. Boosting antioxidants, using low dose/varied sulfur & redox support, naturally & gently detoxing metals & supporting DNA repair with iron-sulfur rich Chorella/Spirulina may provide more comprehensive Autism recovery options.

For Our Children…
cac1451bba9685ca6ec639cf5f0de130.ppt