009a83b679d6664da4387fdd6e64fdc2.ppt
- Количество слайдов: 24

Bevacizumab (Bev) in combination with XELOX or FOLFOX-4: updated efficacy results from XELOX-1 / NO 16966, a randomized phase III trial in first-line metastatic colorectal cancer Saltz L 1, Clarke S 2, Diaz-Rubio E 3, Scheithauer W 4, Figer A 5 Wong R 6, Koski S 7, Lichinitser M 8, Yang T 9, Cassidy J 10 1 Memorial Sloan Kettering Cancer Center, New York, USA, 2 University of Sydney and Sydney Cancer Centre, Sydney, Australia, 3 Hospital Clínico San Carlos, Madrid, Spain, 4 Vienna University Medical School, Vienna, Austria, 5 Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, 6 Cancer Care Manitoba, St Boniface General Hospital, Winnipeg, MB, Canada, 7 Cross Cancer Institute, Edmonton, AB, Canada, 8 Russian Cancer Research Center, Moscow, Russian Federation, 9 Chang-Gung Memorial Hospital, Taipei, Taiwan, 10 Glasgow University, Glasgow, Scotland

Introduction NO 16966 (XELOX-1) started as a multinational, 2 -arm, open-label, randomized phase III comparison of XELOX (oxaliplatin 130 mg/m 2 i. v. day 1 + capecitabine 1000 mg/m 2 orally bid days 1− 14, every 3 weeks) vs. FOLFOX-4 (oxaliplatin 85 mg/m 2 i. v. day 1 + 5 -FU 400 mg/m 2 i. v. day 1 + folinic acid 200 mg/m 2 i. v. day 1)1 (Figure 1). After pivotal phase III data for bevacizumab became available, 2 the protocol was amended to a partially blinded randomized, 2 x 2 factorial design with two co-primary objectives. Previously reported results showed that in terms of progression- free survival (PFS), bevacizumab is superior to placebo when combined with oxaliplatin-based chemotherapy (XELOX / FOLFOX-4). 3 Here we present updated overall survival data with an additional 1 year of follow-up.

XELOX-1 / NO 16966 study design The study was double-blind with regard to bevacizumab and placebo administration, but not for capecitabine and 5 -FU, since these are administered orally and intravenously, respectively (Figure 1). Recruitment occurred in two phases as the protocol was amended to include a placebo-controlled comparison with bevacizumab.
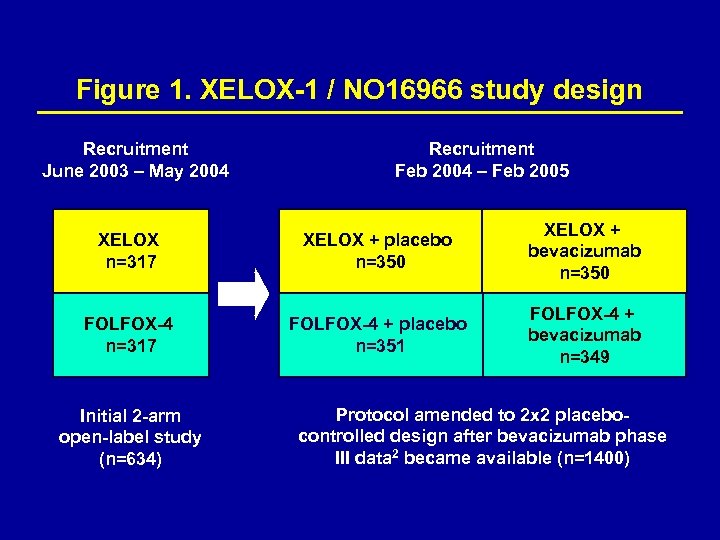
Figure 1. XELOX-1 / NO 16966 study design Recruitment June 2003 – May 2004 Recruitment Feb 2004 – Feb 2005 XELOX n=317 XELOX + placebo n=350 XELOX + bevacizumab n=350 FOLFOX-4 n=317 FOLFOX-4 + placebo n=351 FOLFOX-4 + bevacizumab n=349 Initial 2 -arm open-label study (n=634) Protocol amended to 2 x 2 placebocontrolled design after bevacizumab phase III data 2 became available (n=1400)
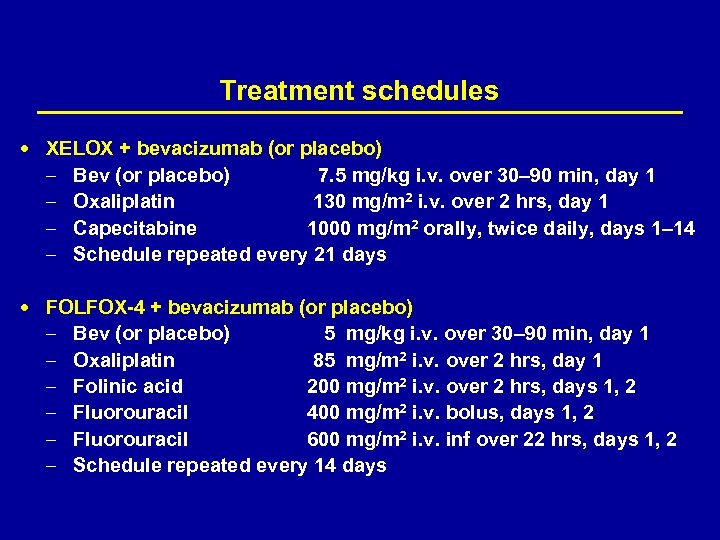
Treatment schedules XELOX + bevacizumab (or placebo) – – Bev (or placebo) 7. 5 mg/kg i. v. over 30– 90 min, day 1 Oxaliplatin 130 mg/m 2 i. v. over 2 hrs, day 1 Capecitabine 1000 mg/m 2 orally, twice daily, days 1– 14 Schedule repeated every 21 days FOLFOX-4 + bevacizumab (or placebo) – – – Bev (or placebo) 5 mg/kg i. v. over 30– 90 min, day 1 Oxaliplatin 85 mg/m 2 i. v. over 2 hrs, day 1 Folinic acid 200 mg/m 2 i. v. over 2 hrs, days 1, 2 Fluorouracil 400 mg/m 2 i. v. bolus, days 1, 2 Fluorouracil 600 mg/m 2 i. v. inf over 22 hrs, days 1, 2 Schedule repeated every 14 days
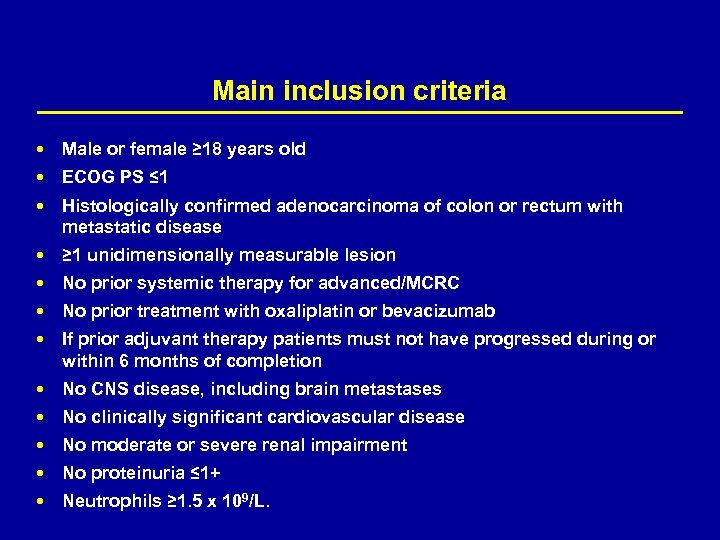
Main inclusion criteria Male or female ≥ 18 years old ECOG PS ≤ 1 Histologically confirmed adenocarcinoma of colon or rectum with metastatic disease ≥ 1 unidimensionally measurable lesion No prior systemic therapy for advanced/MCRC No prior treatment with oxaliplatin or bevacizumab If prior adjuvant therapy patients must not have progressed during or within 6 months of completion No CNS disease, including brain metastases No clinically significant cardiovascular disease No moderate or severe renal impairment No proteinuria ≤ 1+ Neutrophils ≥ 1. 5 x 109/L.
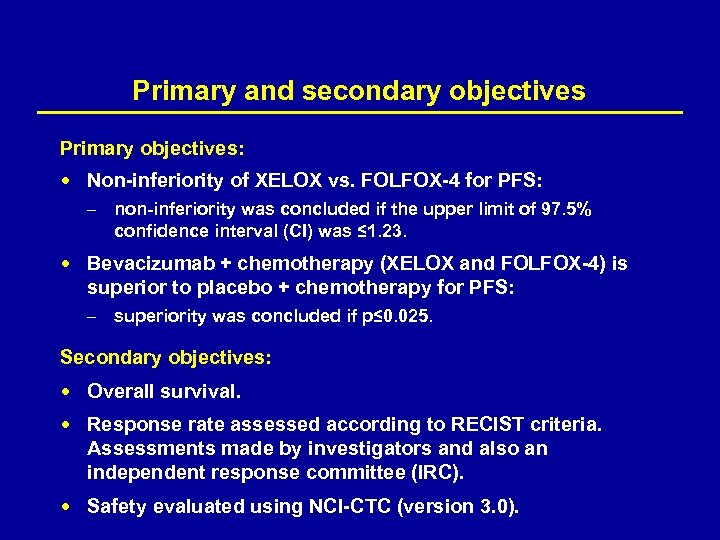
Primary and secondary objectives Primary objectives: Non-inferiority of XELOX vs. FOLFOX-4 for PFS: – non-inferiority was concluded if the upper limit of 97. 5% confidence interval (CI) was ≤ 1. 23. Bevacizumab + chemotherapy (XELOX and FOLFOX-4) is superior to placebo + chemotherapy for PFS: – superiority was concluded if p≤ 0. 025. Secondary objectives: : Overall survival. Response rate assessed according to RECIST criteria. Assessments made by investigators and also an independent response committee (IRC). Safety evaluated using NCI-CTC (version 3. 0).

Study populations ITT (intent-to-treat) = all randomized patients. EPP (eligible patient population) = ITT minus major protocol violators and patients not receiving at least 1 dose of study drug. Used for the XELOX non-inferiority analyses due to health authority requirements. Safety population = all patients receiving at least one dose of the respective study drug.
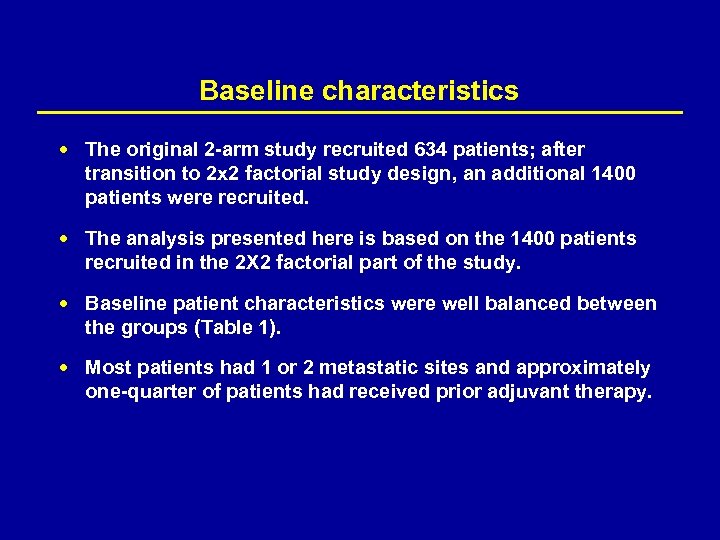
Baseline characteristics The original 2 -arm study recruited 634 patients; after transition to 2 x 2 factorial study design, an additional 1400 patients were recruited. The analysis presented here is based on the 1400 patients recruited in the 2 X 2 factorial part of the study. Baseline patient characteristics were well balanced between the groups (Table 1). Most patients had 1 or 2 metastatic sites and approximately one-quarter of patients had received prior adjuvant therapy.
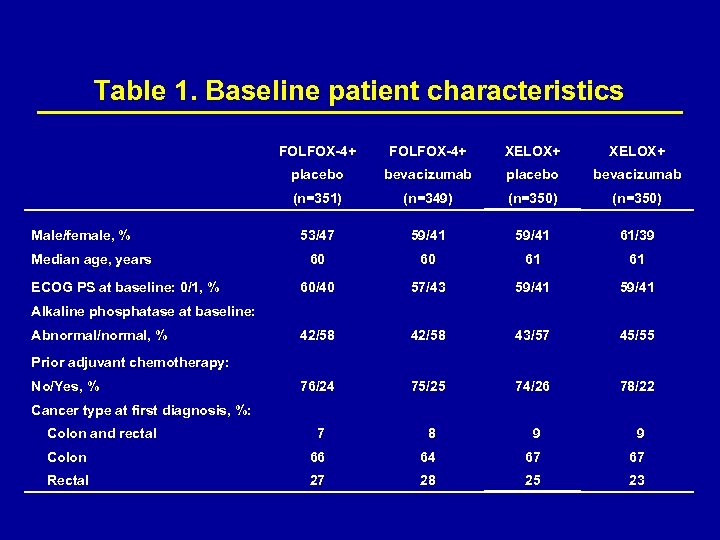
Table 1. Baseline patient characteristics FOLFOX-4+ XELOX+ placebo bevacizumab (n=351) (n=349) (n=350) 53/47 59/41 61/39 60 60 61 61 60/40 57/43 59/41 42/58 43/57 45/55 76/24 75/25 74/26 78/22 7 8 9 9 Colon 66 64 67 67 Rectal 27 28 25 23 Male/female, % Median age, years ECOG PS at baseline: 0/1, % Alkaline phosphatase at baseline: Abnormal/normal, % Prior adjuvant chemotherapy: No/Yes, % Cancer type at first diagnosis, %: Colon and rectal

Efficacy The co-primary objective was met: the addition of bevacizumab to oxaliplatin-based chemotherapy significantly improved PFS, HR=0. 83 [97. 5% CI 0. 72– 0. 95, p=0. 0023 (Figure 2)]. 3, 4 The overall survival analysis presented here includes 12 additional months of follow-up. Pre-specified on-treatment definition (events occuring within 28 days of last dose only) showed significant improvement in PFS in bevacizumab-treated patients (Figure 3).
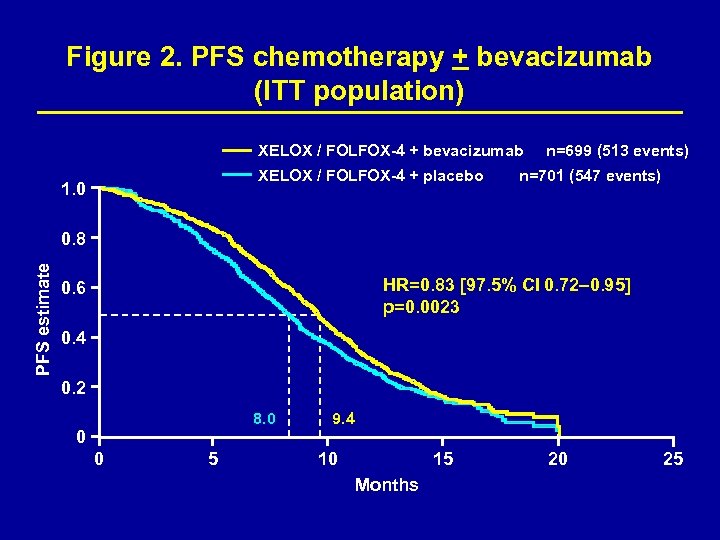
Figure 2. PFS chemotherapy + bevacizumab (ITT population) XELOX / FOLFOX-4 + bevacizumab XELOX / FOLFOX-4 + placebo 1. 0 n=699 (513 events) n=701 (547 events) PFS estimate 0. 8 HR=0. 83 [97. 5% CI 0. 72– 0. 95] p=0. 0023 0. 6 0. 4 0. 2 8. 0 9. 4 0 0 5 10 15 Months 20 25
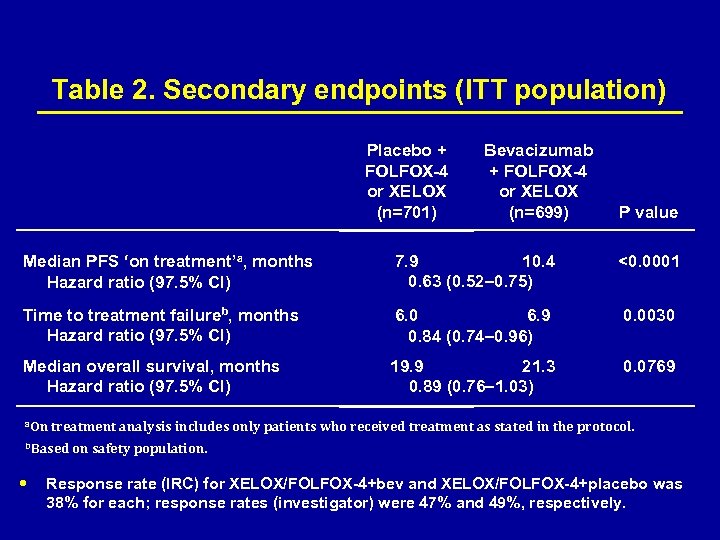
Table 2. Secondary endpoints (ITT population) Placebo + FOLFOX-4 or XELOX (n=701) Bevacizumab + FOLFOX-4 or XELOX (n=699) P value Median PFS ‘on treatment’a, months Hazard ratio (97. 5% CI) 7. 9 10. 4 0. 63 (0. 52– 0. 75) <0. 0001 Time to treatment failureb, months Hazard ratio (97. 5% CI) 6. 0 6. 9 0. 84 (0. 74– 0. 96) 0. 0030 Median overall survival, months Hazard ratio (97. 5% CI) 19. 9 21. 3 0. 89 (0. 76– 1. 03) 0. 0769 a. On treatment analysis includes only patients who received treatment as stated in the protocol. b. Based on safety population. Response rate (IRC) for XELOX/FOLFOX-4+bev and XELOX/FOLFOX-4+placebo was 38% for each; response rates (investigator) were 47% and 49%, respectively.
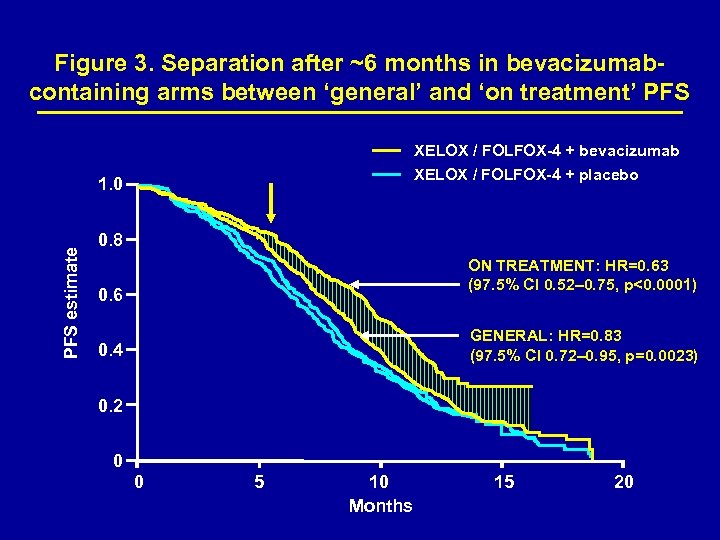
Figure 3. Separation after ~6 months in bevacizumabcontaining arms between ‘general’ and ‘on treatment’ PFS XELOX / FOLFOX-4 + bevacizumab XELOX / FOLFOX-4 + placebo PFS estimate 1. 0 0. 8 ON TREATMENT: HR=0. 63 (97. 5% CI 0. 52– 0. 75, p<0. 0001) 0. 6 GENERAL: HR=0. 83 (97. 5% CI 0. 72– 0. 95, p=0. 0023) 0. 4 0. 2 0 0 5 10 Months 15 20
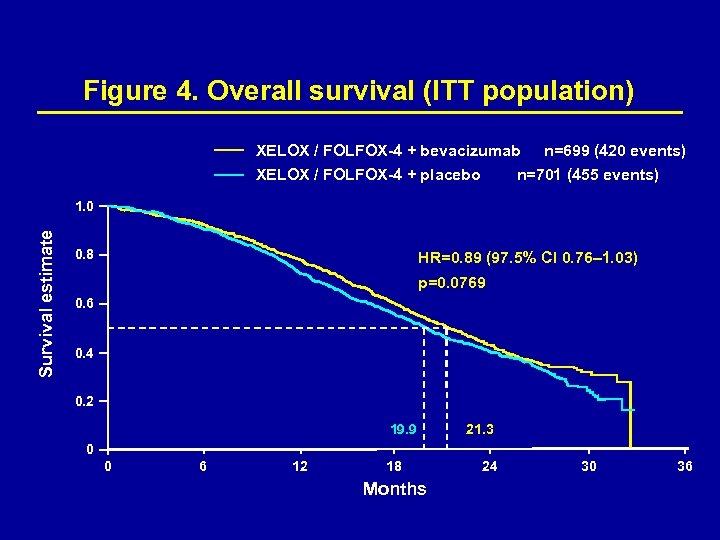
Figure 4. Overall survival (ITT population) XELOX / FOLFOX-4 + bevacizumab XELOX / FOLFOX-4 + placebo n=699 (420 events) n=701 (455 events) Survival estimate 1. 0 0. 8 HR=0. 89 (97. 5% CI 0. 76– 1. 03) p=0. 0769 0. 6 0. 4 0. 2 19. 9 21. 3 0 0 6 12 18 Months 24 30 36

Treatment exposure Duration of treatment was similar in bevacizumab- and placebo-containing arms. Median treatment duration: – Bevacizumab + XELOX 6. 1 months (range 0– 15. 3) – Placebo + XELOX 5. 4 months (range 0– 15. 7) – Bevacizumab + FOLFOX-4 6. 3 months (0– 14. 9) – Placebo + FOLFOX-4 6. 0 months (range 0– 15. 6). Only 29% of bevacizumab recipients and 44– 50% of placebo recipients were treated until disease progression. a a. Includes non-progressive patients stopping treatment at week 48 (end of primary treatment phase)
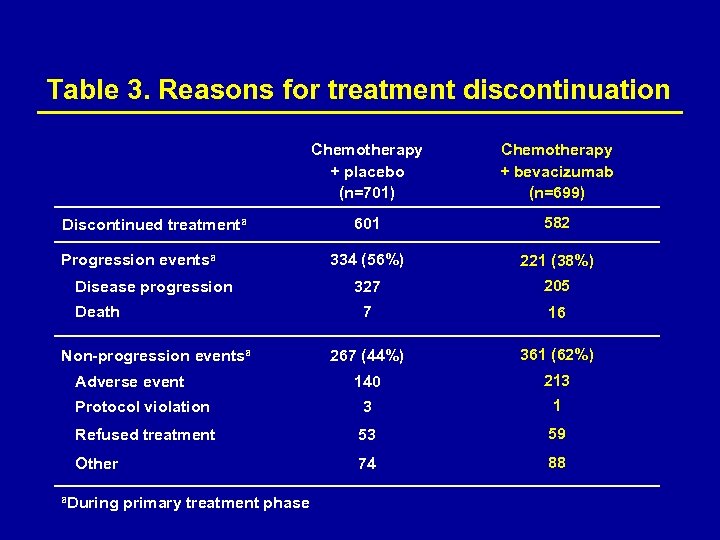
Table 3. Reasons for treatment discontinuation Chemotherapy + placebo (n=701) Chemotherapy + bevacizumab (n=699) 601 582 334 (56%) 221 (38%) 327 205 7 16 267 (44%) 361 (62%) 140 213 Protocol violation 3 1 Refused treatment 53 59 Other 74 88 Discontinued treatmenta Progression eventsa Disease progression Death Non-progression eventsa Adverse event a. During primary treatment phase
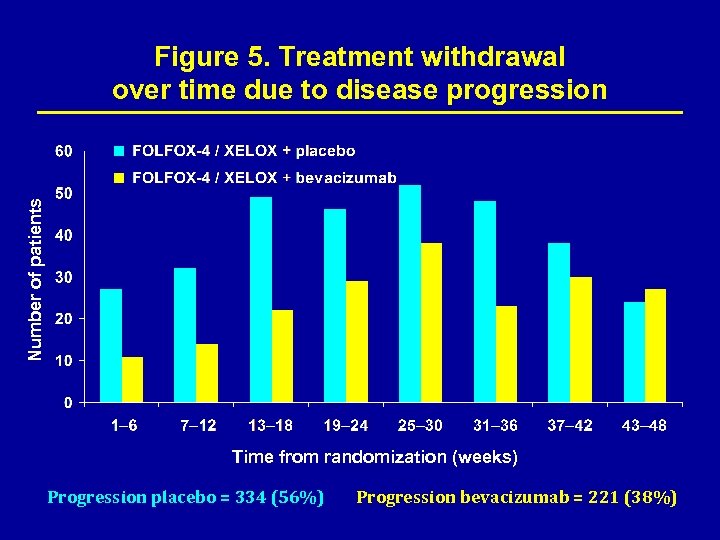
Number of patients Figure 5. Treatment withdrawal over time due to disease progression Time from randomization (weeks) Progression placebo = 334 (56%) Progression bevacizumab = 221 (38%)

Safety A higher proportion of patients discontinued therapy because of AEs in the bevacizumab-containing arms vs. the placebocontaining arms (31% vs. 21%). Most treatment discontinuations were due to chemotherapy- rather than bevacizumab-related events. Most common reasons for treatment discontinuation were neurotoxicity, GI events, general disorders and hematological events. Predefined grade 3/4 AEs of interest to bevacizumab and chemotherapy are presented in Table 3.
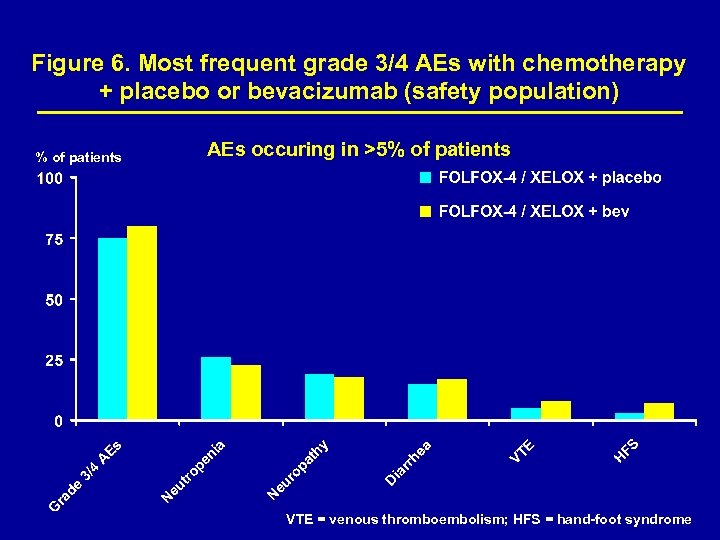
Figure 6. Most frequent grade 3/4 AEs with chemotherapy + placebo or bevacizumab (safety population) % of patients AEs occuring in >5% of patients VTE = venous thromboembolism; HFS = hand-foot syndrome
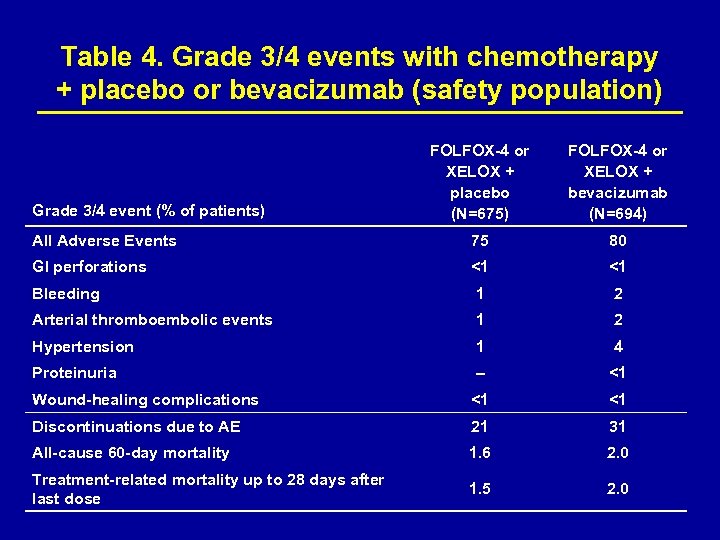
Table 4. Grade 3/4 events with chemotherapy + placebo or bevacizumab (safety population) FOLFOX-4 or XELOX + placebo (N=675) FOLFOX-4 or XELOX + bevacizumab (N=694) All Adverse Events 75 80 GI perforations <1 <1 Bleeding 1 2 Arterial thromboembolic events 1 2 Hypertension 1 4 Proteinuria – <1 Wound-healing complications <1 <1 Discontinuations due to AE 21 31 All-cause 60 -day mortality 1. 6 2. 0 Treatment-related mortality up to 28 days after last dose 1. 5 2. 0 Grade 3/4 event (% of patients)
Conclusions The addition of bevacizumab to front-line oxaliplatin-based chemotherapy significantly improves PFS. The overall safety profile is in line with previous trial experience in colorectal cancer. Analysis of ‘on treatment’ PFS vs. ‘general’ PFS suggests that continuation of bevacizumab until disease progression may be necessary to optimize the effect of bevacizumab on PFS. The observed overall survival difference did not reach statistical significance (p=0. 077).

Acknowledgement Study sponsored by Roche Sincere thanks to: The patients and their families The co-investigators The research nurses and data managers The study management team

References 1. De Gramont A, et al. J Clin Oncol 2000; 18: 2938− 47. 2. Hurwitz H, et al. NEJM 2004; 350: 2335− 42. 3. Saltz L, et al. Proc ASCO GI 2007 (Abstr 238). 4. Cassidy J, et al. Proc ESMO 2006 (Abstr LBA 3). Presented at the ASCO Annual Meeting, 1− 5 June, 2007
009a83b679d6664da4387fdd6e64fdc2.ppt