ce3e394f7a88a782ea09536d0fdda513.ppt
- Количество слайдов: 30
Bettelheim / Brown / Campbell / Farrell / Torres Introduction to General, Organic, and Biochemistry 11 e Chapter 10 Organic Chemistry William H. Brown Beloit College www. cengage. com/chemistry/bettelheim 1

• • • Topics to Cover on the First Day of Lecture for Chem 1506 My web site – google russell moser • Has everything you need to know for both lecture and lab • My office hours • Tutoring schedule for WB 5039 Using web site to find information – click [Lecture Classes], then [Chem 1506…] • You will find • Syllabus has two parts • Course description - 650 total points - No makeup tests – attendance – tutoring • Fall Syllabus - We will try to stick to this schedule, particularly tests
![• Using web site to find information – click [Lecture Classes], then [Chem • Using web site to find information – click [Lecture Classes], then [Chem](https://present5.com/presentation/ce3e394f7a88a782ea09536d0fdda513/image-3.jpg)
• Using web site to find information – click [Lecture Classes], then [Chem 1506…] • You will find • Student Information • Information about classes averages and your current grade after a test. • YSU Blackboard • Signin with YSU username and password. Search course catalog for “moser”. Select [enroll] & [submit]. Select [Home Page]. Select appropriate power point lecture slides. • Directions to use OWLv 2 • New students to create an account (use YSU e-mail address) • Or enter your e-mail address and password and get to your assignments
• • On Home page you will find • My Class and Tutoring Schedule • Tutoring Schedule in WB 5039 Not up-to-date yet
16 -5
Organic Chemistry Organic chemistry: The study of the compounds of carbon. • Organic compounds are made up of carbon and only a few other elements. • Chief among these are hydrogen, oxygen, and nitrogen. • Also present in some organic compounds are sulfur, phosphorus, and a halogen (fluorine, chlorine, bromine, or iodine). 6
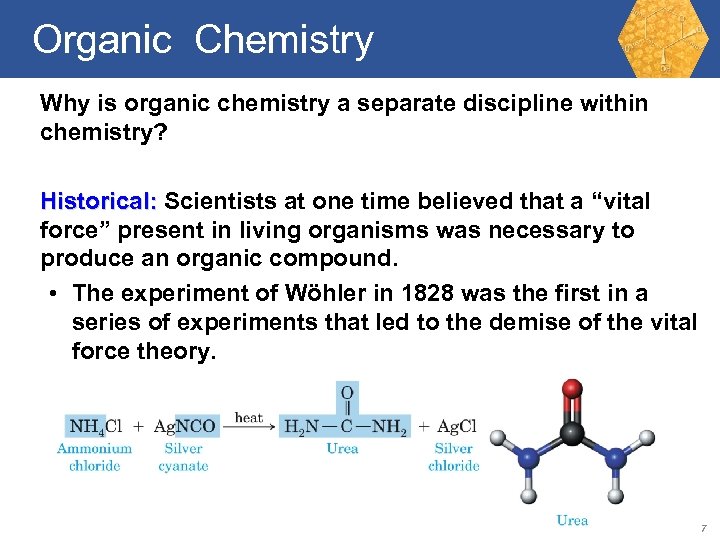
Organic Chemistry Why is organic chemistry a separate discipline within chemistry? Historical: Scientists at one time believed that a “vital force” present in living organisms was necessary to produce an organic compound. • The experiment of Wöhler in 1828 was the first in a series of experiments that led to the demise of the vital force theory. 7
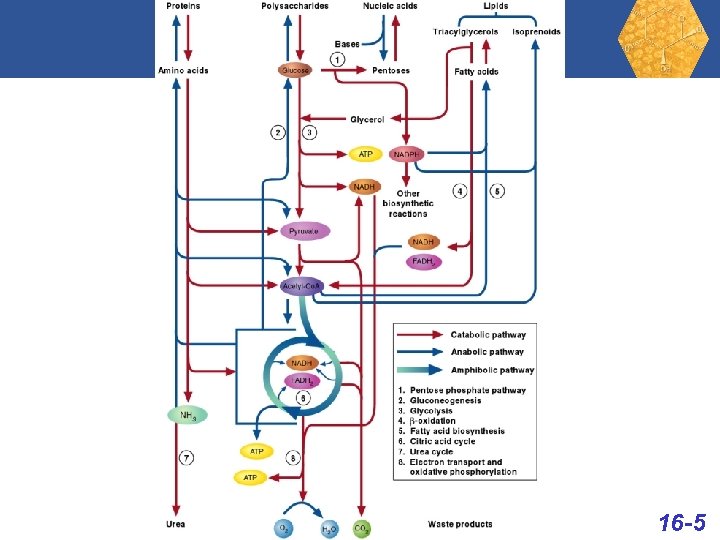
Organic Chemistry The sheer number of organic compounds • Chemists have discovered or made over 10 million organic compounds and an estimated 100, 000 new ones are discovered or made each year. • By comparison, chemists have discovered or made an estimated 1. 7 million inorganic compounds. • Thus, approximately 85% of all known compounds are organic. The link to biochemistry • Carbohydrates, lipids, proteins, enzymes, nucleic acids, hormones, vitamins, and almost all other chemicals in living systems are organic compounds. 8
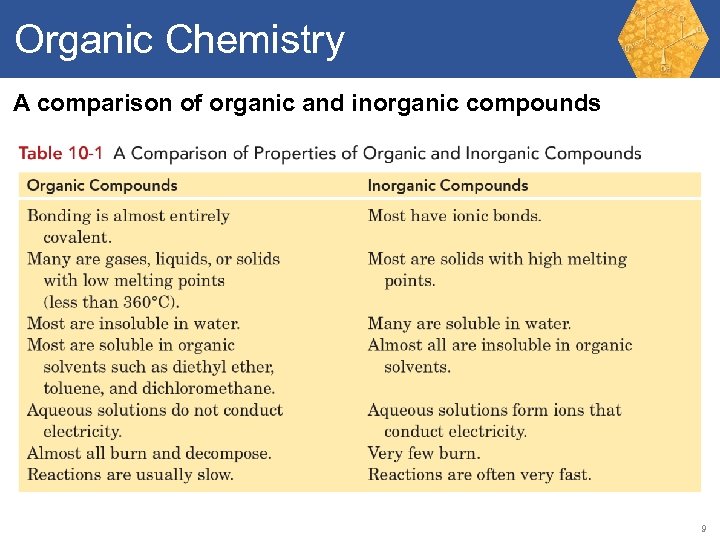
Organic Chemistry A comparison of organic and inorganic compounds 9
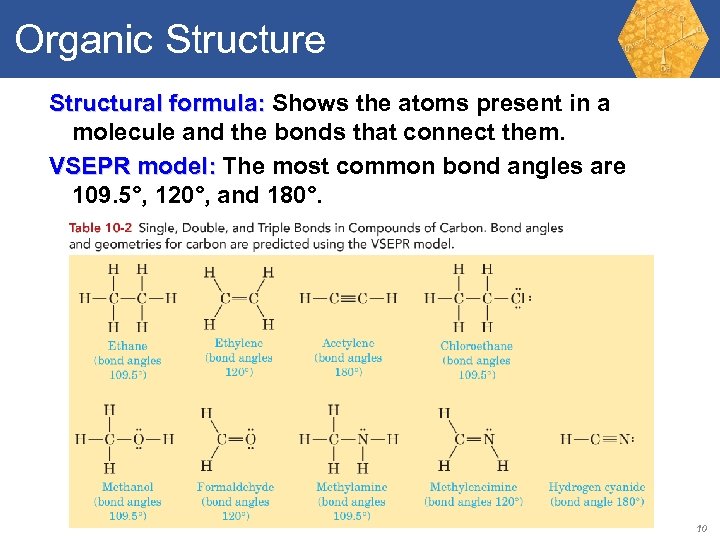
Organic Structure Structural formula: Shows the atoms present in a molecule and the bonds that connect them. VSEPR model: The most common bond angles are 109. 5°, 120°, and 180°. 10
Organic Structure Among neutral (uncharged) organic compounds: • Carbon normally forms four covalent bonds and has no unshared pairs of electrons. • Hydrogen forms one covalent bond and no unshared pairs of electrons. • Nitrogen normally forms three covalent bonds and has one unshared pair of electrons. • Oxygen normally forms two covalent bonds and has two unshared pairs of electrons. • Halogen normally forms one covalent bond and has three unshared pairs of electrons. 11

Functional Groups Functional group: An atom or group of atoms within a molecule that shows a characteristic set of predictable physical and chemical properties. Functional groups are important because • They undergo the same types of chemical reactions no matter in what organic molecule they are found. • To a large measure, they determine the chemical and physical properties of a molecule. • They are the units by which we divide organic compounds into families. • They provide the basis on which we derive names for organic compounds. 12
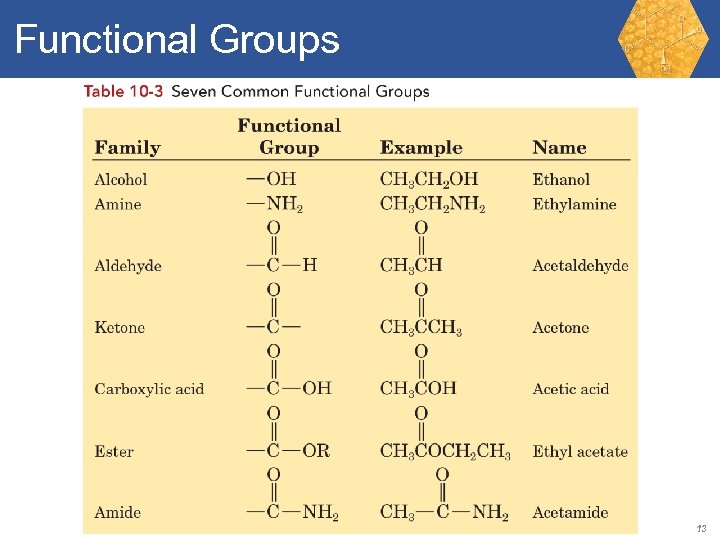
Functional Groups 13
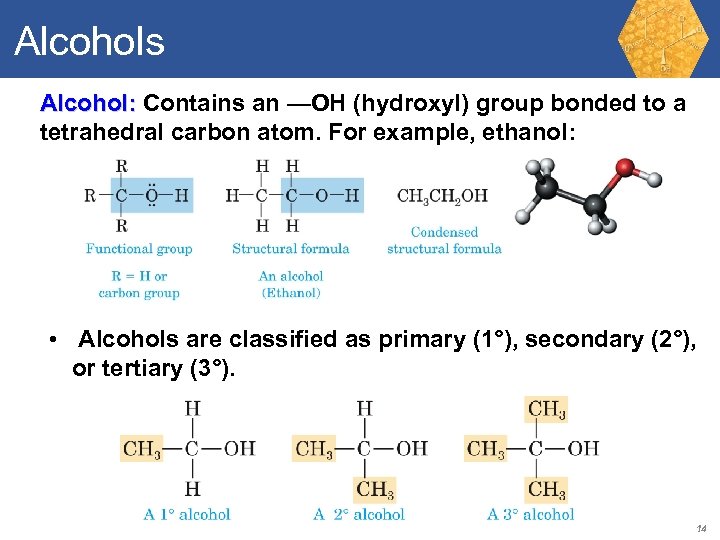
Alcohols Alcohol: Contains an —OH (hydroxyl) group bonded to a tetrahedral carbon atom. For example, ethanol: • Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°). 14

• Important Definition • R group: An abbreviation for any group of atoms that may include carbons, hydrogens, halogens, oxygens and nitrogens that are attached to a functional group or represent a side chain to the rest of a molecule.

Alcohols Problem: Draw Lewis structures and condensed structural formulas for the two alcohols with the molecular formula C 3 H 8 O. 16
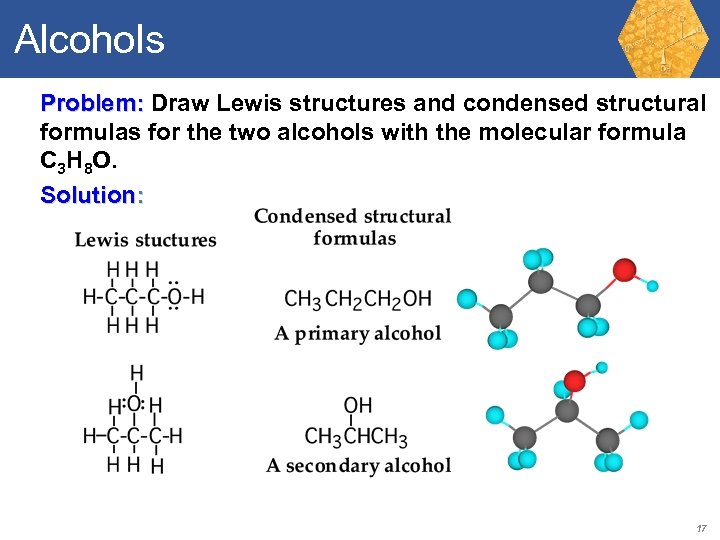
Alcohols Problem: Draw Lewis structures and condensed structural formulas for the two alcohols with the molecular formula C 3 H 8 O. Solution: 17
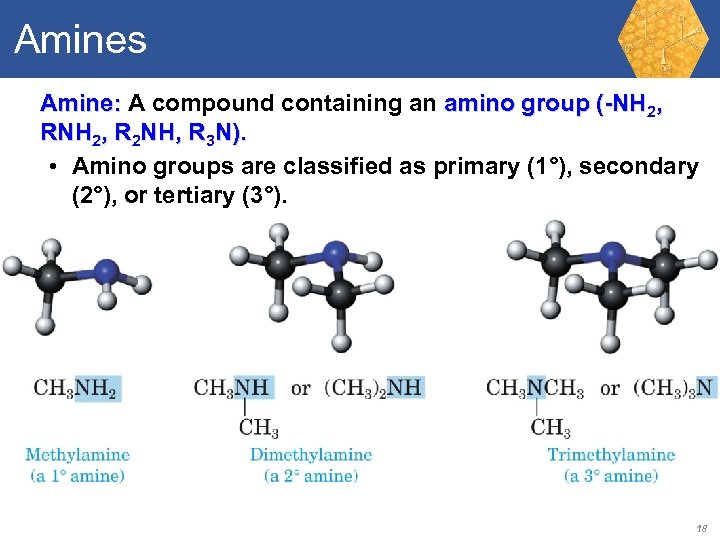
Amines Amine: A compound containing an amino group (-NH 2, R 2 NH, R 3 N). • Amino groups are classified as primary (1°), secondary (2°), or tertiary (3°). 18

Amines Problem: Draw condensed structural formulas for the two primary amines with the molecular formula C 3 H 9 N. 19
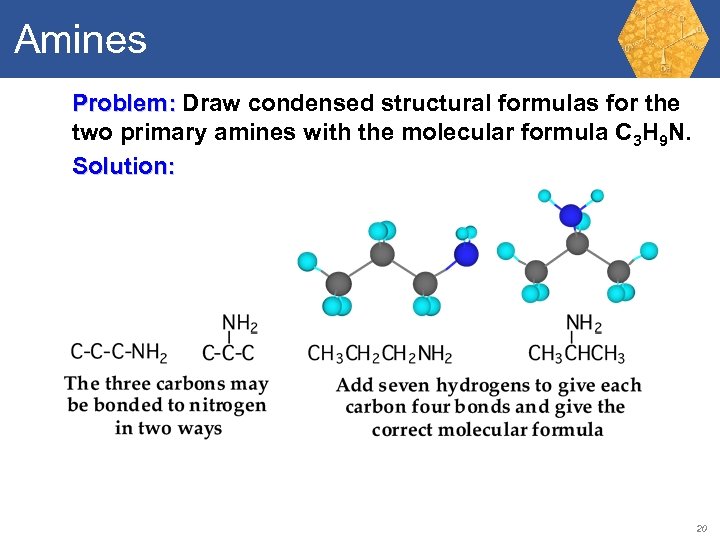
Amines Problem: Draw condensed structural formulas for the two primary amines with the molecular formula C 3 H 9 N. Solution: 20
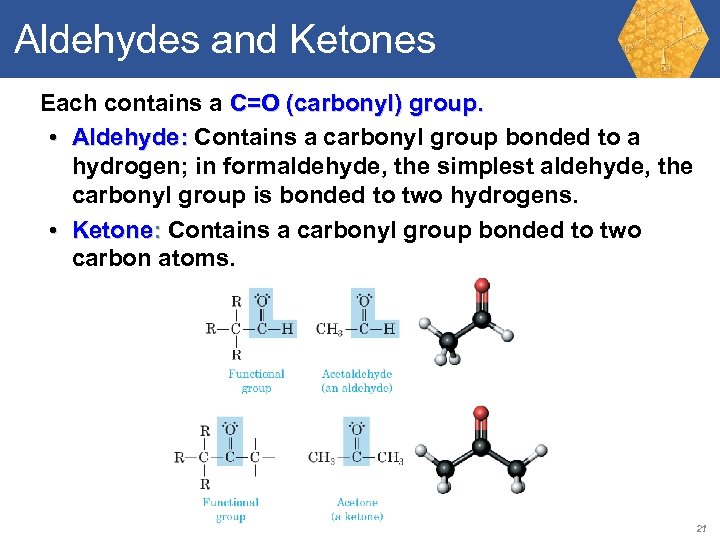
Aldehydes and Ketones Each contains a C=O (carbonyl) group. • Aldehyde: Contains a carbonyl group bonded to a hydrogen; in formaldehyde, the simplest aldehyde, the carbonyl group is bonded to two hydrogens. • Ketone: Contains a carbonyl group bonded to two carbon atoms. 21
Aldehydes and Ketones Problem: Draw condensed structural formulas for the two aldehydes with the molecular formula C 4 H 8 O. 22
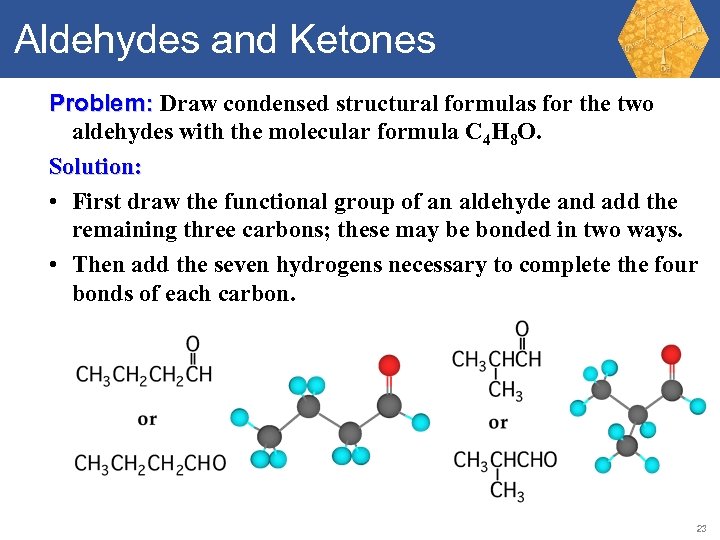
Aldehydes and Ketones Problem: Draw condensed structural formulas for the two aldehydes with the molecular formula C 4 H 8 O. Solution: • First draw the functional group of an aldehyde and add the remaining three carbons; these may be bonded in two ways. • Then add the seven hydrogens necessary to complete the four bonds of each carbon. 23
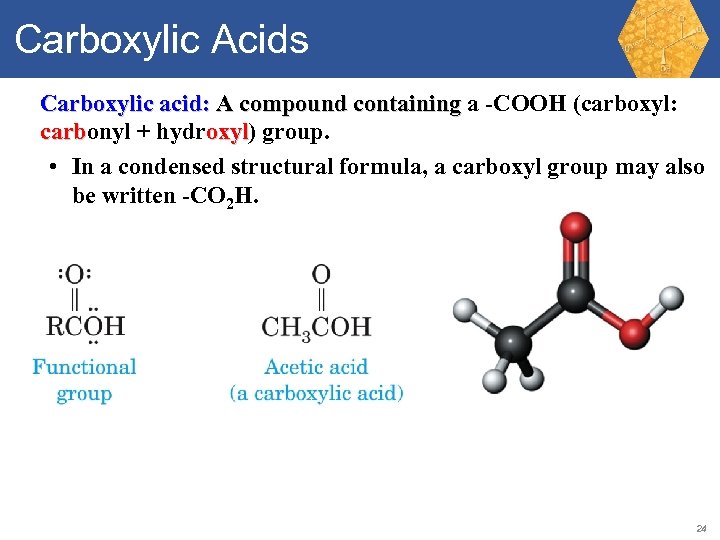
Carboxylic Acids Carboxylic acid: A compound containing a -COOH (carboxyl: carbonyl + hydroxyl) group. carb oxyl • In a condensed structural formula, a carboxyl group may also be written -CO 2 H. 24
Carboxylic Acids Problem: Draw a condensed structural formula for the single carboxylic acid with the molecular formula C 3 H 6 O 2. 25
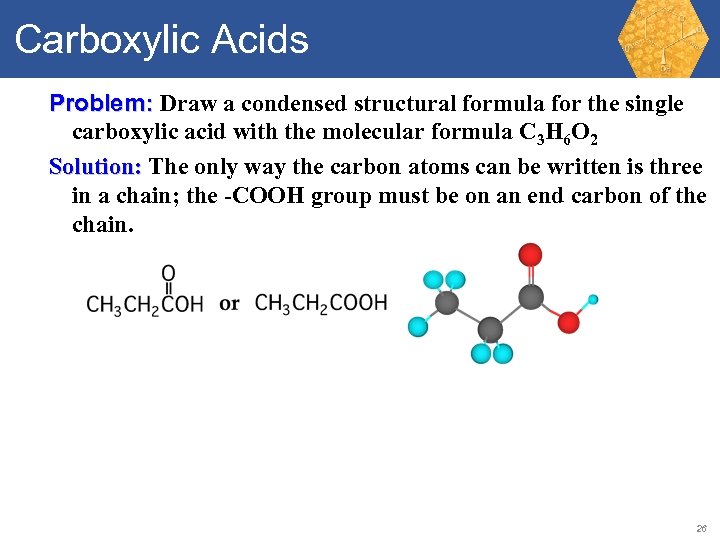
Carboxylic Acids Problem: Draw a condensed structural formula for the single carboxylic acid with the molecular formula C 3 H 6 O 2 Solution: The only way the carbon atoms can be written is three in a chain; the -COOH group must be on an end carbon of the chain. 26
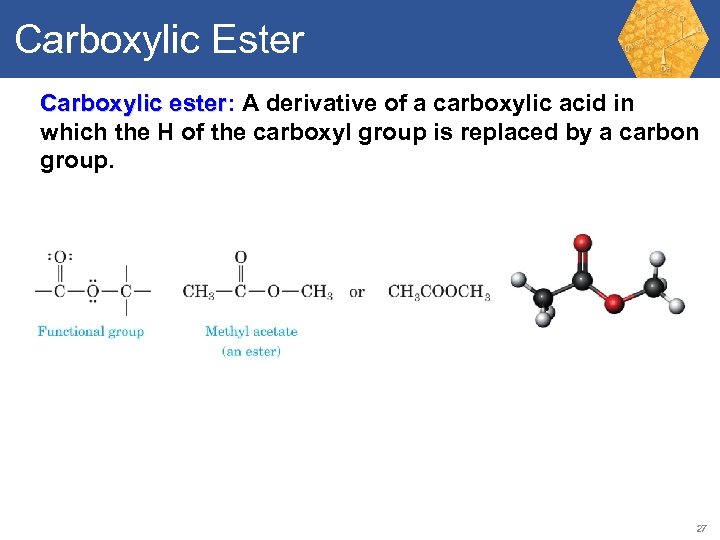
Carboxylic Ester Carboxylic ester: A derivative of a carboxylic acid in ester which the H of the carboxyl group is replaced by a carbon group. 27
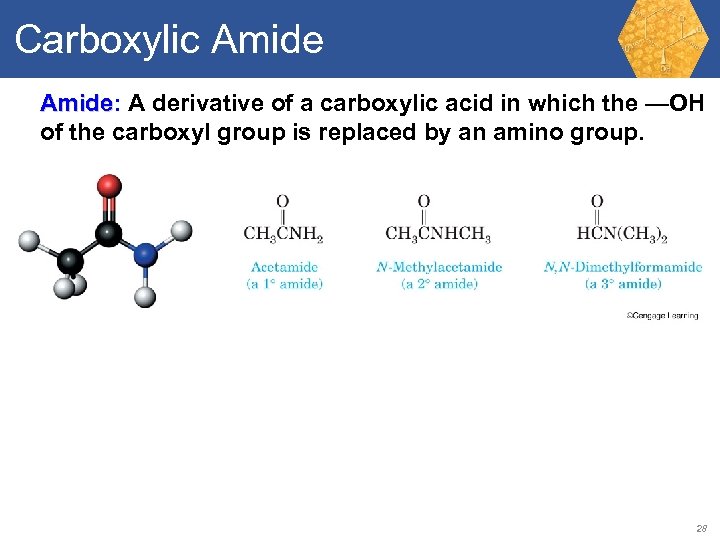
Carboxylic Amide: A derivative of a carboxylic acid in which the —OH Amide of the carboxyl group is replaced by an amino group. 28
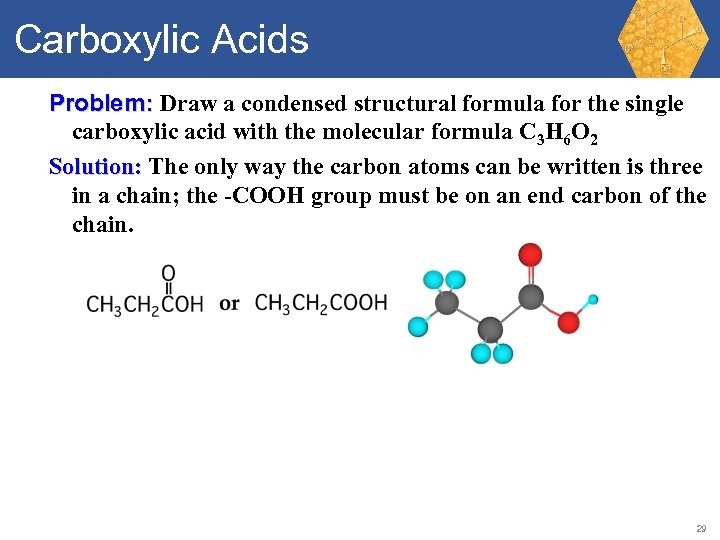
Carboxylic Acids Problem: Draw a condensed structural formula for the single carboxylic acid with the molecular formula C 3 H 6 O 2 Solution: The only way the carbon atoms can be written is three in a chain; the -COOH group must be on an end carbon of the chain. 29
Chapter 10 Organic Chemistry End Chapter 10 30
ce3e394f7a88a782ea09536d0fdda513.ppt