526dfaf980998c28a40fa14052d04120.ppt
- Количество слайдов: 29

BETH ISRAEL DEACONNESS DATA WAREHOUSE PROJECT February 27, 2009
BACKGROUND BIDPO/BIDMC would like to engage MAe. HC to provide a quality data warehouse service to: • Enable automated extraction and aggregation of selected clinical data from member physicians’ e. CW and Web. OMR EHR systems • Develop selected clinical quality measures for BIDPO internal benchmarking, reporting to health plans and case management • Create a demonstration of emerging HITEP II quality data set standards MAe. HC quality data center (QDC) currently aggregates clinical data from participants in MAe. HC pilot projects • Automated, longitudinal, and patient-centric • 20 core measures Current project will require • • Slide title Measure definition and specification according to BIDPO requirements Creation of HITSP-compliant CCD interfaces from e. CW and Web. OMR to MAe. HC QDC Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
AGENDA MAe. HC QDC current status BIDMC/BIDPO goals Accomplishing our goals Roles and Responsibilities Timeline Next Steps Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
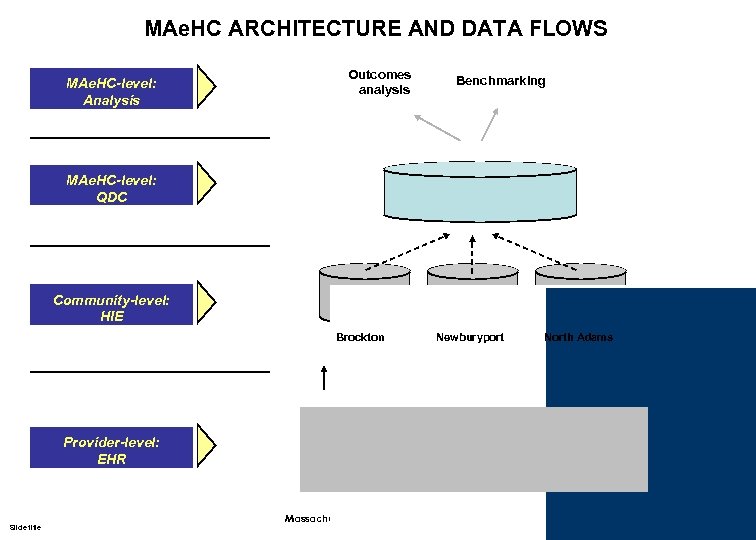
MAe. HC ARCHITECTURE AND DATA FLOWS MAe. HC-level: Analysis Outcomes analysis Benchmarking MAe. HC-level: QDC Community-level: HIE Brockton Newburyport North Adams Provider-level: EHR Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
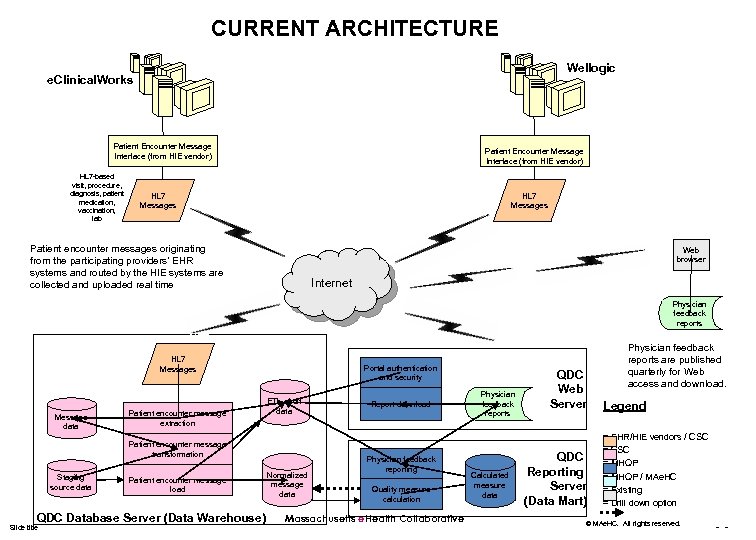
CURRENT ARCHITECTURE Wellogic e. Clinical. Works Patient Encounter Message Interface (from HIE vendor) HL 7 -based visit, procedure, diagnosis, patient medication, vaccination, lab Patient Encounter Message Interface (from HIE vendor) HL 7 Messages Patient encounter messages originating from the participating providers’ EHR systems and routed by the HIE systems are collected and uploaded real time Web browser Internet Physician feedback reports HL 7 Messages Message data Patient encounter message extraction Portal authentication and security ETL audit data Patient encounter message transformation Staging source data Patient encounter message load QDC Database Server (Data Warehouse) Slide title Normalized message data Report download Physician feedback reporting Quality measure calculation Massachusetts e. Health Collaborative Physician feedback reports Calculated measure data QDC Web Server QDC Reporting Server (Data Mart) Physician feedback reports are published quarterly for Web access and download. Legend = EHR/HIE vendors / CSC = MHQP / MAe. HC = Existing = Drill down option © MAe. HC. All rights reserved. - -
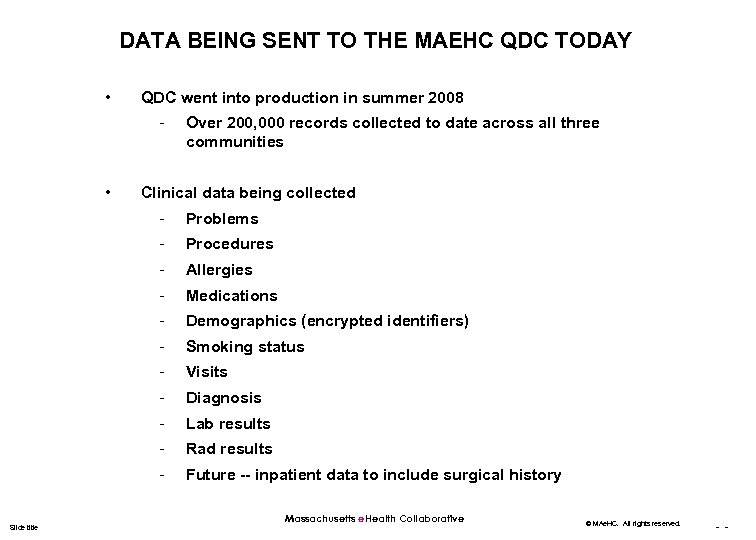
DATA BEING SENT TO THE MAEHC QDC TODAY • QDC went into production in summer 2008 - • Over 200, 000 records collected to date across all three communities Clinical data being collected - Procedures - Allergies - Medications - Demographics (encrypted identifiers) - Smoking status - Visits - Diagnosis - Lab results - Rad results Slide title Problems Future -- inpatient data to include surgical history Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
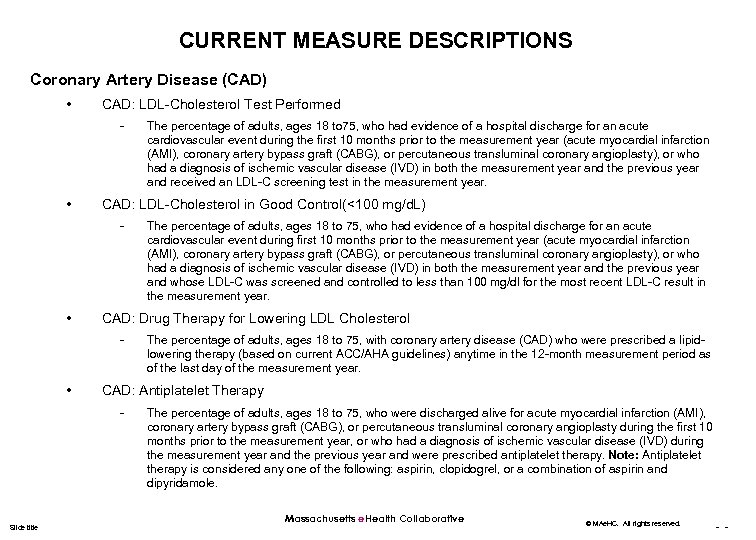
CURRENT MEASURE DESCRIPTIONS Coronary Artery Disease (CAD) • CAD: LDL-Cholesterol Test Performed - • CAD: LDL-Cholesterol in Good Control(<100 mg/d. L) - • The percentage of adults, ages 18 to 75, with coronary artery disease (CAD) who were prescribed a lipidlowering therapy (based on current ACC/AHA guidelines) anytime in the 12 -month measurement period as of the last day of the measurement year. CAD: Antiplatelet Therapy - Slide title The percentage of adults, ages 18 to 75, who had evidence of a hospital discharge for an acute cardiovascular event during first 10 months prior to the measurement year (acute myocardial infarction (AMI), coronary artery bypass graft (CABG), or percutaneous transluminal coronary angioplasty), or who had a diagnosis of ischemic vascular disease (IVD) in both the measurement year and the previous year and whose LDL-C was screened and controlled to less than 100 mg/dl for the most recent LDL-C result in the measurement year. CAD: Drug Therapy for Lowering LDL Cholesterol - • The percentage of adults, ages 18 to 75, who had evidence of a hospital discharge for an acute cardiovascular event during the first 10 months prior to the measurement year (acute myocardial infarction (AMI), coronary artery bypass graft (CABG), or percutaneous transluminal coronary angioplasty), or who had a diagnosis of ischemic vascular disease (IVD) in both the measurement year and the previous year and received an LDL-C screening test in the measurement year. The percentage of adults, ages 18 to 75, who were discharged alive for acute myocardial infarction (AMI), coronary artery bypass graft (CABG), or percutaneous transluminal coronary angioplasty during the first 10 months prior to the measurement year, or who had a diagnosis of ischemic vascular disease (IVD) during the measurement year and the previous year and were prescribed antiplatelet therapy. Note: Antiplatelet therapy is considered any one of the following: aspirin, clopidogrel, or a combination of aspirin and dipyridamole. Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
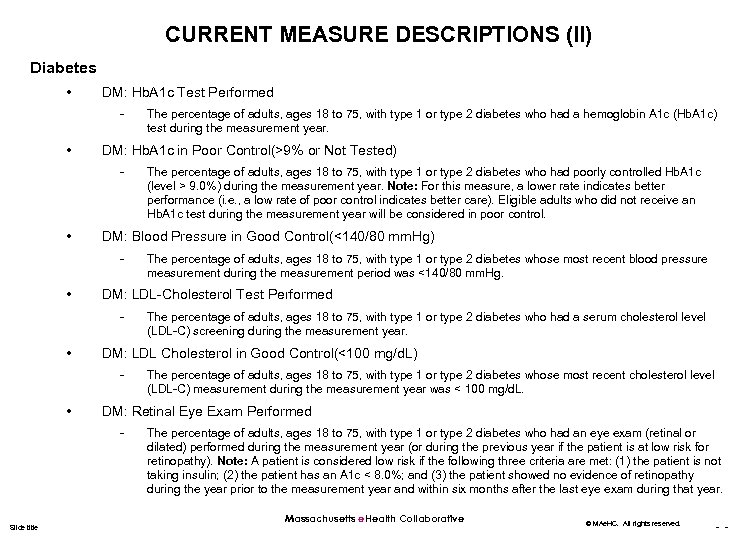
CURRENT MEASURE DESCRIPTIONS (II) Diabetes • DM: Hb. A 1 c Test Performed - • DM: Hb. A 1 c in Poor Control(>9% or Not Tested) - • The percentage of adults, ages 18 to 75, with type 1 or type 2 diabetes whose most recent cholesterol level (LDL-C) measurement during the measurement year was < 100 mg/d. L. DM: Retinal Eye Exam Performed - Slide title The percentage of adults, ages 18 to 75, with type 1 or type 2 diabetes who had a serum cholesterol level (LDL-C) screening during the measurement year. DM: LDL Cholesterol in Good Control(<100 mg/d. L) - • The percentage of adults, ages 18 to 75, with type 1 or type 2 diabetes whose most recent blood pressure measurement during the measurement period was <140/80 mm. Hg. DM: LDL-Cholesterol Test Performed - • The percentage of adults, ages 18 to 75, with type 1 or type 2 diabetes who had poorly controlled Hb. A 1 c (level > 9. 0%) during the measurement year. Note: For this measure, a lower rate indicates better performance (i. e. , a low rate of poor control indicates better care). Eligible adults who did not receive an Hb. A 1 c test during the measurement year will be considered in poor control. DM: Blood Pressure in Good Control(<140/80 mm. Hg) - • The percentage of adults, ages 18 to 75, with type 1 or type 2 diabetes who had a hemoglobin A 1 c (Hb. A 1 c) test during the measurement year. The percentage of adults, ages 18 to 75, with type 1 or type 2 diabetes who had an eye exam (retinal or dilated) performed during the measurement year (or during the previous year if the patient is at low risk for retinopathy). Note: A patient is considered low risk if the following three criteria are met: (1) the patient is not taking insulin; (2) the patient has an A 1 c < 8. 0%; and (3) the patient showed no evidence of retinopathy during the year prior to the measurement year and within six months after the last eye exam during that year. Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
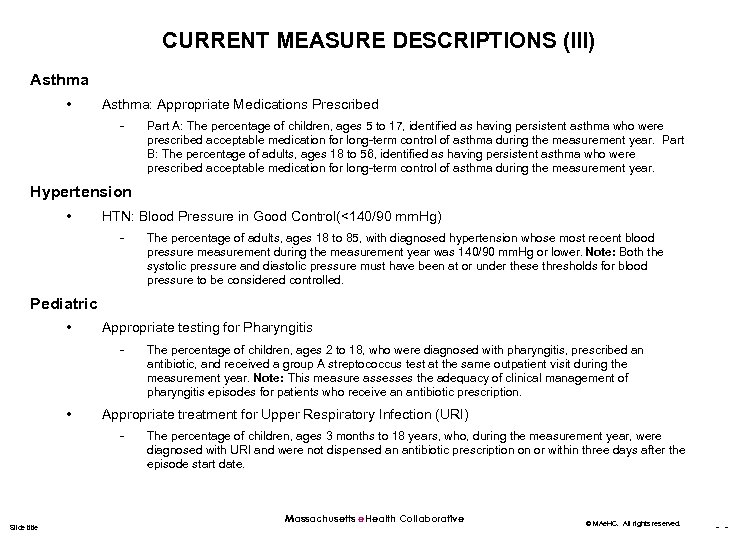
CURRENT MEASURE DESCRIPTIONS (III) Asthma • Asthma: Appropriate Medications Prescribed - Part A: The percentage of children, ages 5 to 17, identified as having persistent asthma who were prescribed acceptable medication for long-term control of asthma during the measurement year. Part B: The percentage of adults, ages 18 to 56, identified as having persistent asthma who were prescribed acceptable medication for long-term control of asthma during the measurement year. Hypertension • HTN: Blood Pressure in Good Control(<140/90 mm. Hg) - The percentage of adults, ages 18 to 85, with diagnosed hypertension whose most recent blood pressure measurement during the measurement year was 140/90 mm. Hg or lower. Note: Both the systolic pressure and diastolic pressure must have been at or under these thresholds for blood pressure to be considered controlled. Pediatric • Appropriate testing for Pharyngitis - • Appropriate treatment for Upper Respiratory Infection (URI) - Slide title The percentage of children, ages 2 to 18, who were diagnosed with pharyngitis, prescribed an antibiotic, and received a group A streptococcus test at the same outpatient visit during the measurement year. Note: This measure assesses the adequacy of clinical management of pharyngitis episodes for patients who receive an antibiotic prescription. The percentage of children, ages 3 months to 18 years, who, during the measurement year, were diagnosed with URI and were not dispensed an antibiotic prescription on or within three days after the episode start date. Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
CURRENT MEASURE DESCRIPTIONS (IV) Prevention • PREV: Influenza Vaccination(>=50 yrs) - • PREV: Pneumonia Vaccination(>=65 yrs) - • The percentage of adults, 50 years or older at the beginning of a flu season, who received an influenza vaccination during the one-year measurement period. The percentage of adults, 65 years or older, who have ever received a pneumococcal vaccination. PREV: Colorectal Cancer Screening(50 -80 yrs) - The percentage of adults, ages 50 to 80, who had an - appropriate screening for colorectal cancer. Appropriate screening is considered one or more of the following: · · Colonoscopy during the measurement or the nine years prior. PREV: Breast Cancer Screening(42 -69 yrs) - The percentage of women, ages 42 to 69, who received a mammogram during the measurement year or the previous year. PREV: Documentation of Smoking Status - Slide title Double contrast barium enema (DCBE) during the measurement year or the four years prior; or · • Flexible sigmoidoscopy during the measurement year or the four years prior to the measurement year; · • Fecal occult blood test (FOBT) during measurement year; The percentage of adults, 18 years or older at the start of the two-year measurement period, who were asked about their tobacco use one or more times during the two-year measurement period. Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
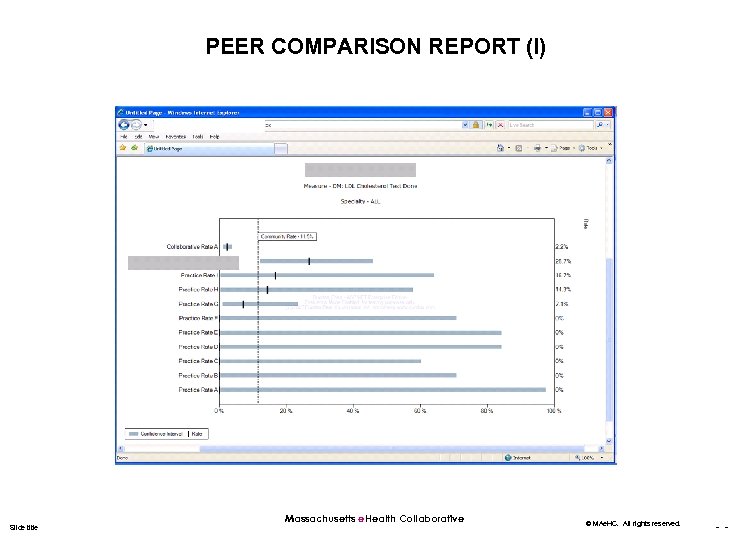
PEER COMPARISON REPORT (I) Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
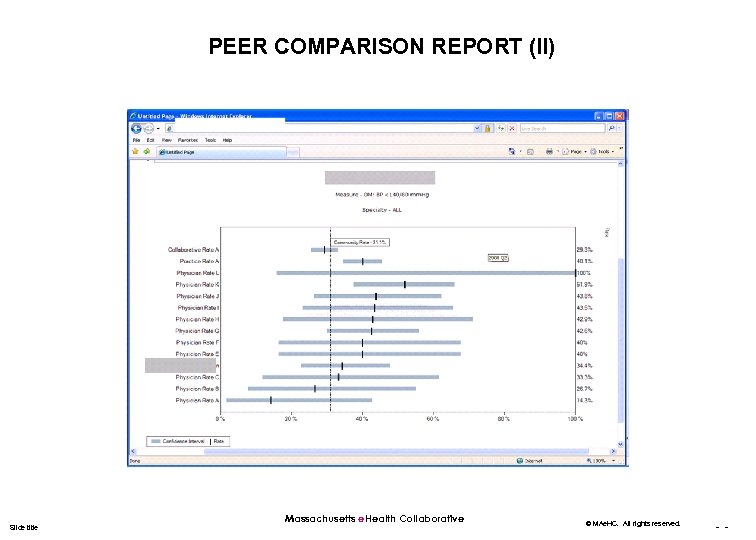
PEER COMPARISON REPORT (II) Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
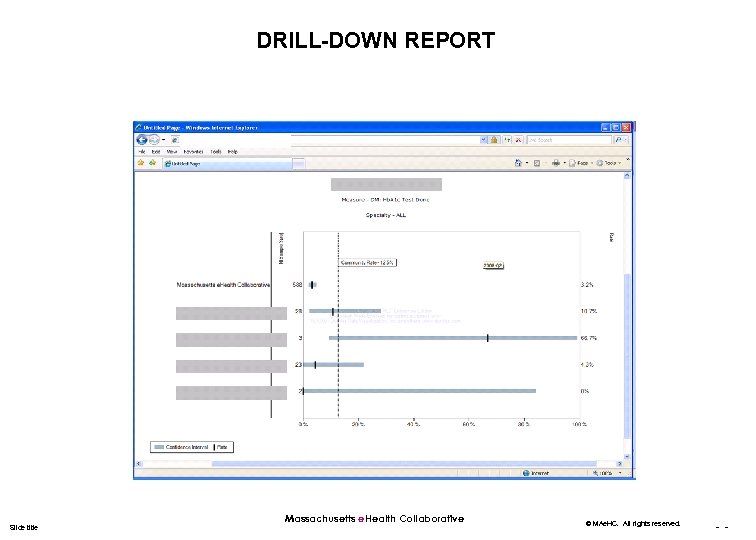
DRILL-DOWN REPORT Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
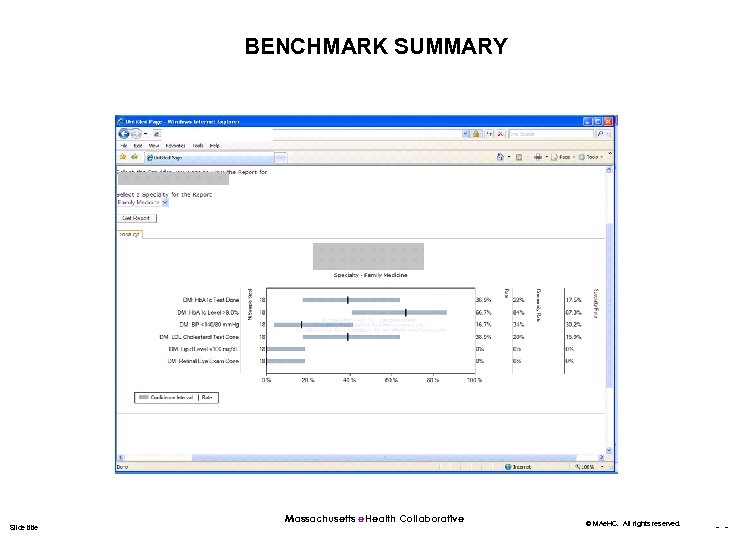
BENCHMARK SUMMARY Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
AGENDA MAe. HC QDC current status BIDMC/BIDPO goals Accomplishing our goals Roles and Responsibilities Timeline Next Steps Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -

BIDPO/BIDMC GOALS Clinical source systems • e. CW through e. HX • Web. OMR Data measures • BID Clinical Standards Group approved measures • HITEP II measures Access and reporting • • Slide title BIDPO-defined enterprise-level and physician-level reports, as required BIDPO enterprise-level access, query, reporting, exporting Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
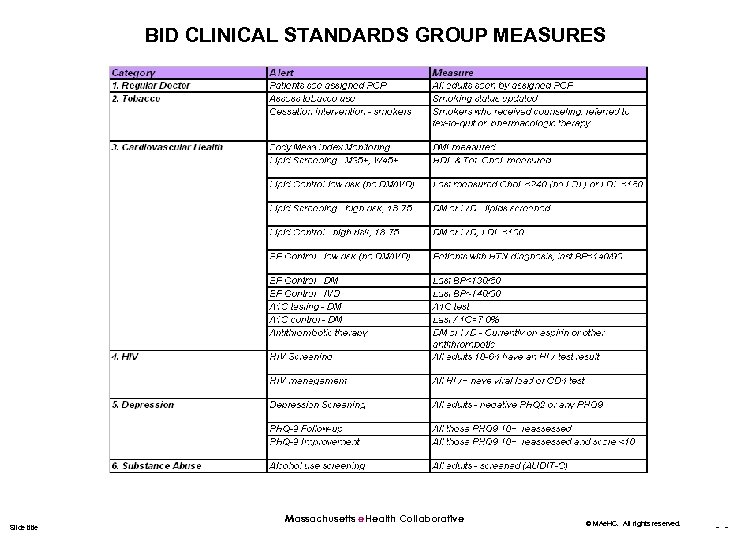
BID CLINICAL STANDARDS GROUP MEASURES Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
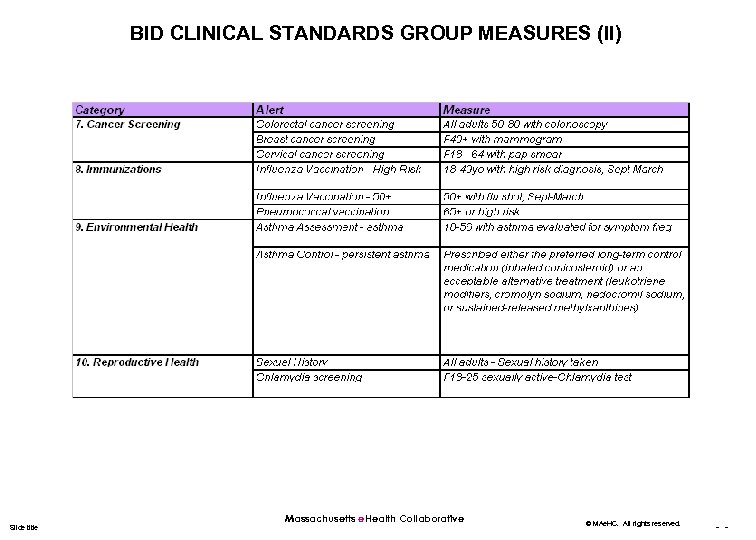
BID CLINICAL STANDARDS GROUP MEASURES (II) Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
HITEP OBJECTIVES HITEP Goals • The intent is to provide a method to encode clinical data obtained during the routine practice of medicine that would then be available to match against the encoded quality measure to determine if the patient or population of patients met any of these specified quality criteria. In so doing, the hope is to provide feedback to clinicians, administrators, policy makers and public health authorities for the purpose of improving the quality of healthcare provided to U. S. patients. HITEP Measures 1. Controlling high BP (logic: could bring in VSs from EHR) 2. Colon cancer screening (logic: complex measure due to multiple modalities and currently requires hybrid review) 3. Overuse measures for adults and children - (logic: requires meds and diagnoses and gets to overuse) a. Avoid antibiotics for child with URI b. Avoid antibiotic use for adult with bronchitis Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
AGENDA MAe. HC QDC current status BIDMC/BIDPO goals Accomplishing our goals Roles and Responsibilities Timeline Next Steps Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -

ACCOMPLISHING OUR GOALS Gap analysis from existing MAe. HC core measures • BIDPO measures • HITEP II measures HITSP/HITEP Specification Review • Measure definition and specification • Transport specification -- upgrade from HL 7 2. x to CCD, in conformance with HITSP specifications Reporting • • Access • Authentication • Slide title Report requirements/specifications Authorization (User roles) Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
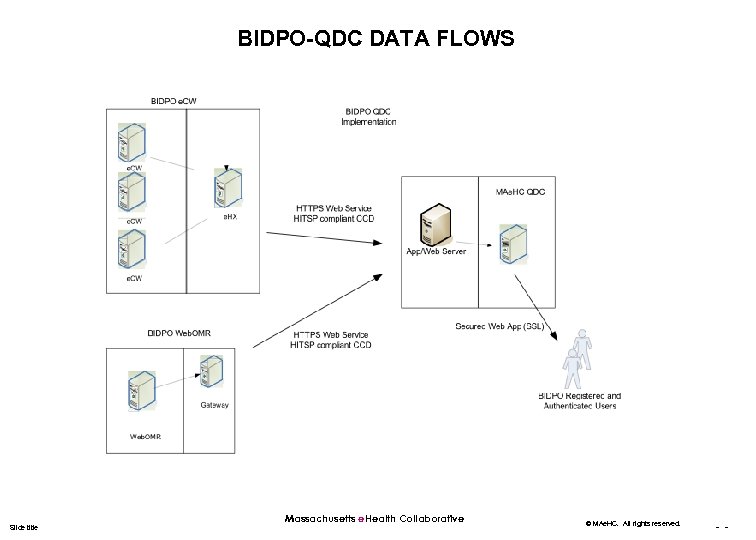
BIDPO-QDC DATA FLOWS Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
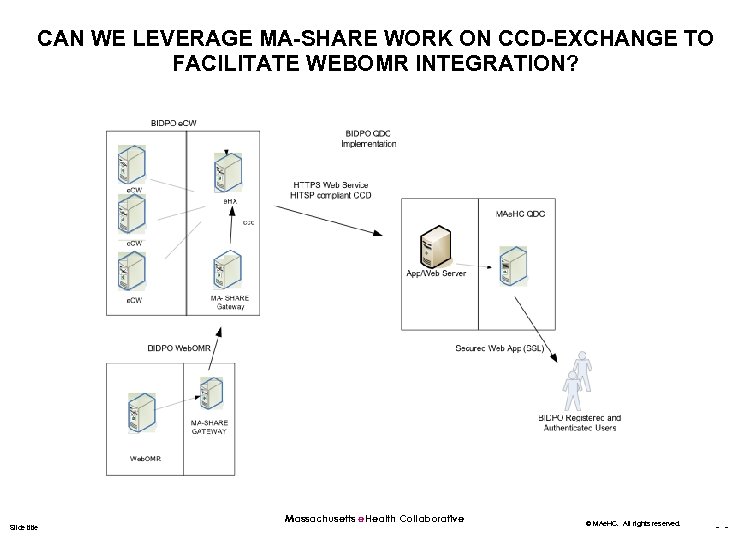
CAN WE LEVERAGE MA-SHARE WORK ON CCD-EXCHANGE TO FACILITATE WEBOMR INTEGRATION? Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
AGENDA MAe. HC QDC current status BIDMC/BIDPO goals Accomplishing our goals Roles and Responsibilities Timeline Next Steps Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
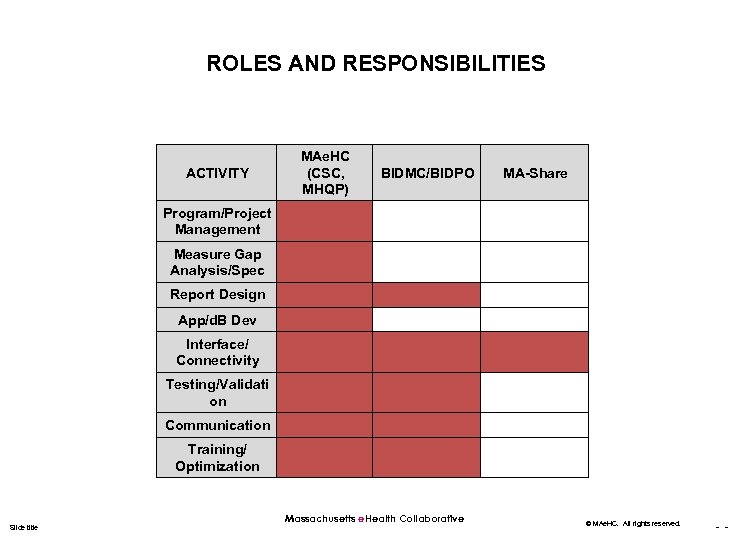
ROLES AND RESPONSIBILITIES ACTIVITY MAe. HC (CSC, MHQP) BIDMC/BIDPO MA-Share Program/Project Management Measure Gap Analysis/Spec Report Design App/d. B Dev Interface/ Connectivity Testing/Validati on Communication Training/ Optimization Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
AGENDA MAe. HC QDC current status BIDMC/BIDPO goals Accomplishing our goals Roles and Responsibilities Timeline Next Steps Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
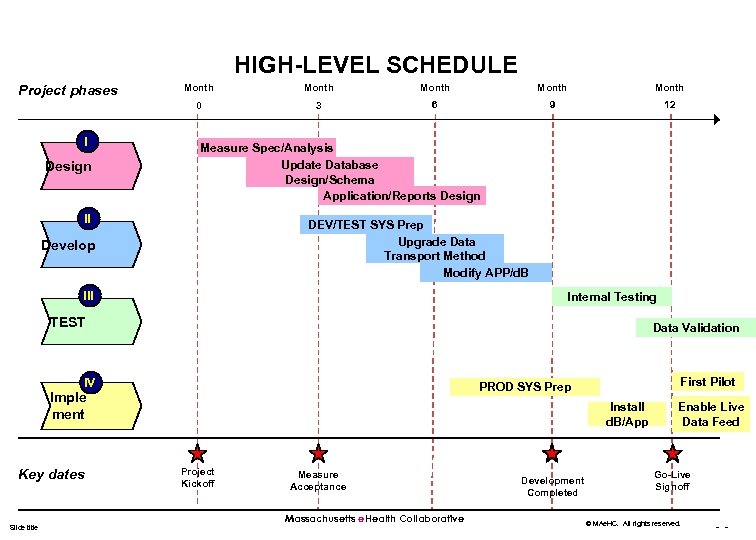
HIGH-LEVEL SCHEDULE I Design Month Month 0 Project phases 3 6 9 12 Measure Spec/Analysis Update Database Design/Schema Application/Reports Design II DEV/TEST SYS Prep Upgrade Data Transport Method Modify APP/d. B Develop III Internal Testing TEST Data Validation IV Imple ment Key dates Slide title First Pilot PROD SYS Prep Install d. B/App Project Kickoff Measure Acceptance Massachusetts e. Health Collaborative Development Completed Enable Live Data Feed Go-Live Signoff © MAe. HC. All rights reserved. - -

AGENDA Current status Goals Accomplishing our goals Roles and Responsibilities Budget Timeline Next Steps Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
NEXT STEPS Confirm measures • TCNY, HITEP, etc. • Detailed gap analysis • Prioritization HITSP Specification/Requirements • Detailed Gap analysis • Roadmap for implementation Determine access and reporting requirements Confirm roles & responsibilities MAe. HC Statement Of Work Legal Framework Slide title Massachusetts e. Health Collaborative © MAe. HC. All rights reserved. - -
526dfaf980998c28a40fa14052d04120.ppt