c6908b2682ce2881440a91a8cee3c124.ppt
- Количество слайдов: 53

Best Practices for Prevention of Retained Surgical Items Victoria M. Steelman, Ph. D, RN, CNOR, FAAN 1

Victoria Steelman, Ph. D, RN, CNOR, FAAN Dr. Steelman has focused on implementing evidence-based practice (EBP) changes for over 20 years and has extensively published and presented on EBP and perioperative issues, and authored many of the AORN Recommended Practices. She received two AORN Outstanding Achievement awards for this work. In 2008, she received the AORN Award for Excellence in recognition of her contributions to perioperative nursing. In 2007, she was inducted into the American Academy of Nursing in recognition of the national and global impact of her work. She is currently the President-Elect of AORN. 3

Disclosure Information Speaker: Victoria M. Steelman, RN, Ph. D, CNOR, FAAN Planning Committee: Ellice Mellinger MS, BSN, RN, CNOR Discloses no conflict AORN’s policy is that the subject matter experts for this product must disclose any financial relationship in a company providing grant funds and/or a company whose product(s) may be discussed or used during the educational activity. Financial disclosure will include the name of the company and/or product and the type of financial relationship, and includes relationships that are in place at the time of the activity or were in place in the 12 months preceding the activity. Disclosures for this activity are indicated according to the following numeric categories: 1. Consultant/Speaker’s Bureau: Consultant to RF Surgical Systems, Inc. 2. Employee 3. Stockholder 4. Product Designer 5. Grant/Research Support : Principal Investigator , University of Iowa, RF Surgical Grant 6. Other relationship (specify) : RF Surgical - Honoraria 7. Has no financial interest: Accreditation Statement AORN is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center's Commission on Accreditation. AORN is provider-approved by the California Board of Registered Nursing, Provider Number CEP 13019. AORN IS PLEASED TO PROVIDE THIS WEBINAR ON THIS IMPORTANT TOPIC. HOWEVER, THE VIEWS EXPRESSED IN THIS WEBINAR ARE THOSE OF THE PRESENTERS AND DO NOT NECESSARILY REPRESENT THE VIEWS OF, AND SHOULD NOT BE ATTRIBUTED TO AORN. 4

Objectives 1. Describe the incidence of retained surgical items and outcomes to patients 2. Discuss recommendations of the Association of peri. Operative Registered Nurses (AORN) 3. List steps of a proactive risk analysis for evaluating the processes used to prevent retained surgical sponges. 4. Describe the use of a multidisciplinary process to evaluate adjunct technology for prevention of retained surgical sponges 5
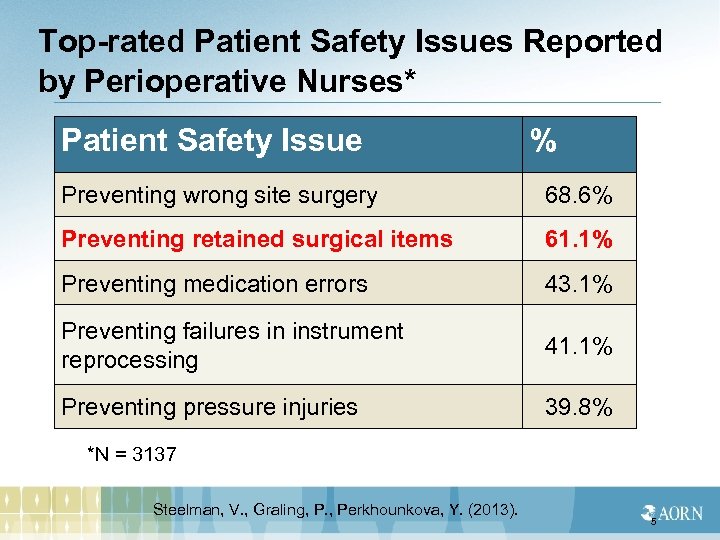
Top-rated Patient Safety Issues Reported by Perioperative Nurses* Patient Safety Issue % Preventing wrong site surgery 68. 6% Preventing retained surgical items 61. 1% Preventing medication errors 43. 1% Preventing failures in instrument reprocessing 41. 1% Preventing pressure injuries 39. 8% *N = 3137 Steelman, V. , Graling, P. , Perkhounkova, Y. (2013). 5
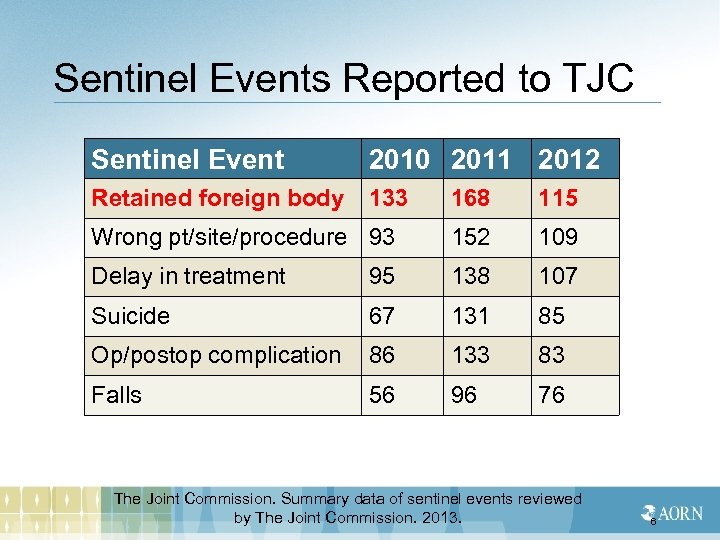
Sentinel Events Reported to TJC Sentinel Event 2010 2011 2012 Retained foreign body 133 168 115 Wrong pt/site/procedure 93 152 109 Delay in treatment 95 138 107 Suicide 67 131 85 Op/postop complication 86 133 83 Falls 56 96 76 The Joint Commission. Summary data of sentinel events reviewed by The Joint Commission. 2013. 6
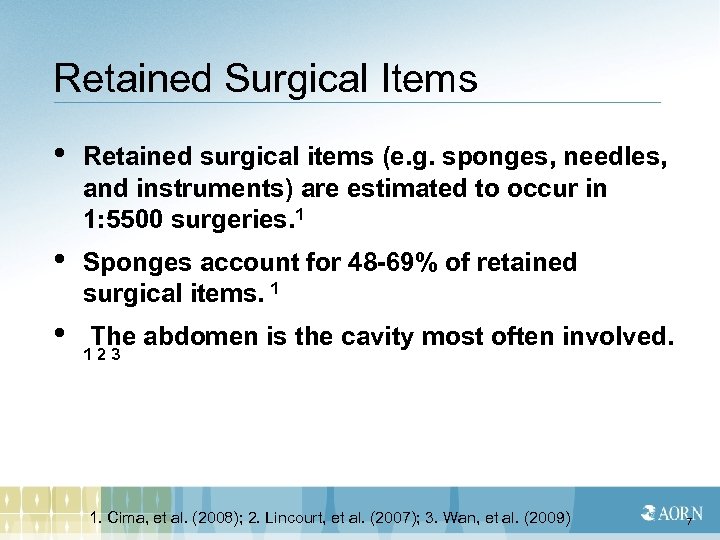
Retained Surgical Items • Retained surgical items (e. g. sponges, needles, and instruments) are estimated to occur in 1: 5500 surgeries. 1 • Sponges account for 48 -69% of retained surgical items. 1 • The abdomen is the cavity most often involved. 1 2 3 1. Cima, et al. (2008); 2. Lincourt, et al. (2007); 3. Wan, et al. (2009) 7

Outcomes of Retained Surgical Items • • • Reoperation 69% Readmission/prolonged stay 43% Sepsis/infection 43% Fistula/bowel obstruction 15% Visceral perforation 7% Death 2% Gawande AA, et. al. (2003) 8
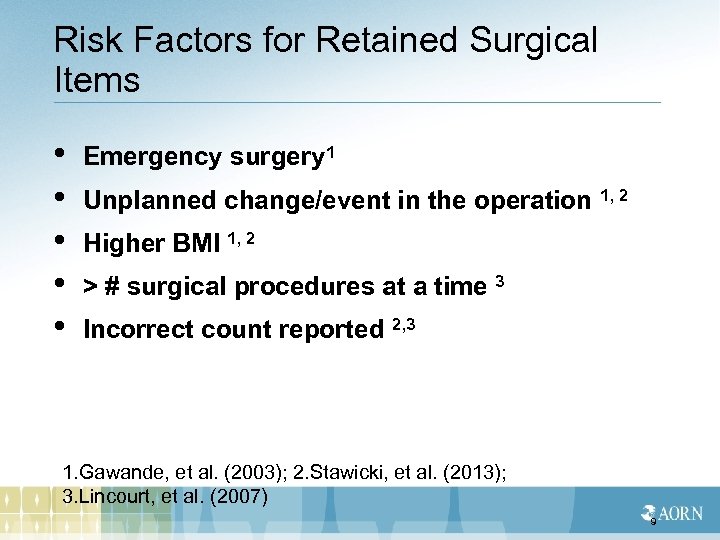
Risk Factors for Retained Surgical Items • • • Emergency surgery 1 Unplanned change/event in the operation 1, 2 Higher BMI 1, 2 > # surgical procedures at a time 3 Incorrect count reported 2, 3 1. Gawande, et al. (2003); 2. Stawicki, et al. (2013); 3. Lincourt, et al. (2007) 9
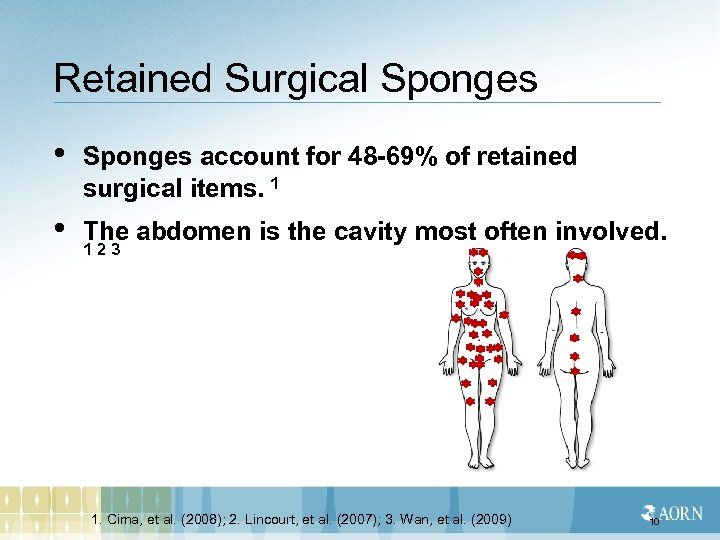
Retained Surgical Sponges • Sponges account for 48 -69% of retained surgical items. 1 • The abdomen is the cavity most often involved. 1 2 3 1. Cima, et al. (2008); 2. Lincourt, et al. (2007); 3. Wan, et al. (2009) 10
Tissue Reactions to Retained Surgical Items • Metal - Inert, identified in a manner similar to a surgical implant • Gauze - Fibrous response • adhesions, encapsulation and granuloma - Exudative Inflammatory response • Abscess, chronic internal/external fistula Zantvoord, et al. (2008) 11

Sponges Migrate • • • Intestine Bladder Airway/lung Thorax Stomach Retroperitoneum When sponges migrate into these non-sterile tissues, infection, sepsis, and death can occur. Zantvoord, et al. (2008) 12

Best Practices Start With • Recommended practices for prevention of retained surgical items • Developed by a multidisciplinary committee AORN (2013) 13

Recommended Practices for Prevention of Retained Surgical Items • Multidisciplinary approach - • • Each team member has a role Work together Accountability: All team members Use a standardized approach Time activities around key events Minimize distractions AORN (2013) 14
Scrub Person • Confirm that instruments and devices are intact when returned from the operative site • Verify integrity and completeness of items when counting • Ensure that the RN circulator can see items when counting • Speak up when a discrepancy exists AORN (2013) 15
Circulating RN • Counts should not be performed during critical portions of the procedure • • Initiate the count • Communicate & document count results Perform the count in concert with the perioperative team AORN (2013) 16

Surgeon & First Assistant • Communicating placement of surgical items in the wound • • Acknowledging awareness of the start of the count • • Performing methodological wound exploration • Notifying scrub person and circulator when items are returned to the surgical site after the count Removing soft goods and instruments from sterile field at the start of the count process Accounting for and communicating about surgical items in the surgical field AORN (2013) 17

Anesthesia Provider • Plan milestone actions to avoid undue pressure during counts • • Do not use counted items Verify that throat packs & bite blocks are removed & communicate this to the team AORN (2013) 18

Counting • • All surgical procedures Prior to start of procedure When dispensed onto the sterile field Upon closing a cavity within a cavity - Sponges, soft goods, sharps Upon closing first layer (e. g. fascia) - Sponges, soft goods, sharps Upon final closure Permanent relief of either the scrub person or RN circulator AORN (2013) 19

Needles - All needles should be counted, regardless of size, for all procedures - Needles are counted when the package is opened - Empty suture packages should not be used to reconcile a count - Needles less than 10 mm may not be identified on radiographs AORN (2013) 20
Exceptions to Instrument Counting Based upon facility policy: • Complex procedures involving large numbers of instruments (e. g. AP spinal fusion) • • Trauma • Procedures where the width and depth of the incision is too small to retain an instrument Procedures that require complex instruments with numerous small parts AORN (2013) 21

Sponges • Items should be radiopaque - Towels if used inside the wound • Pocketed sponge bag system should be used • When intentionally packed, document: - Reconciled when confirmed by surgeon - Incorrect if unsure - Communicate upon transfer AORN (2013) 22
Effectiveness of Counts • • Primary measure for prevention of RSI • The limited effectiveness of counts is poorly understood Standard of care for many years 1 Sensitivity 77. 2%2 62% of retained surgical items were detected after the surgical count was reported as correct 3 1. AORN (2013); 2. Egorova, et al. (2008); 3. Cima, et al. (2008) 23

Retained Surgical Items • Should trigger a thorough analysis: - • Processes in place Causes Contributing factors Corrective action Root cause analysis - Reactive Learn from one event 24

Proactive Risk Analyses • Uses collective experiences of personnel - not just from a single event • • Look at processes in place • Prioritize points in the process that require additional control Identify potential failures & causes of these failures 25

Proactive Risk Analyses • Failure Mode and Effect Analysis (FMEA) • Institute for Healthcare Improvement (IHI) • Healthcare Failure Mode and Effect Analysis (HFMEA) National Patient Safety Center, Department of Veterans Affairs (NCPS) http: //www. patientsafety. va. gov/Cog. Aids/HFMEA/index. html#page=page-1 27

Steps of HFMEA 1. 2. 3. 4. 5. Define the topic Assemble the team Graphically describe the process Conduct the analysis Identify actions and outcome measures Definitions based upon the Healthcare Failure Mode and Effect Analysis (HFMEA) from the VA National Center for Patient Safety 28

1. Define the topic Example: • The management of surgical sponges from case preparation in the operating room to surgery completion, in order to prevent inadvertently retained sponges after surgery, 28

2. Assemble the Team • • Content experts Methods expert 29
3. Graphically describe the process • • Observation of entire process Not the policy, but the actual practice - • There is always a difference Select one type of surgery as exemplar - Map the process Example: • • • Routine colon resections -3 No relief, 1 circulating RN, 1 ST Day shift 30
Example: Steps of Process Step 1. Room preparation 2. Initial count 3. Adding sponges 4. Removing sponges 5. First closing count 6. Final closing count Steelman & Cullen (2011) 31
4. Conduct the Analysis For each step of the process: a) Identify all failures that could occur in each step b) Identify the causes of these potential failures 32

Examples of Potential Failures • • Added to field, not recorded Miscount- too few sponges counted Miscount- too many sponges counted Part of sponge missing Uncounted towel placed in wound No methodological wound exploration Surgeon closing during count Steelman & Cullen (2011) 33

Examples of Causes • • • Room inadequately cleaned after last case Manufacturing defect Knowledge deficit Not following procedure Distraction Multitasking Emergency event or procedure Time pressure Unable to see- person counting too fast 34
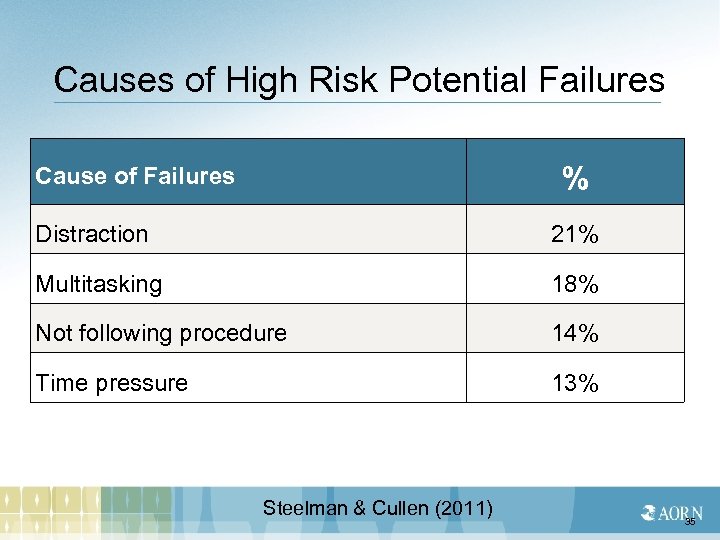
Causes of High Risk Potential Failures % Cause of Failures Distraction 21% Multitasking 18% Not following procedure 14% Time pressure 13% Steelman & Cullen (2011) 35

Calculate a Hazard Score For each failure cause combination in each step: a) Assign a severity score (1 -4) b) Assign a probability score (1 -4) c) Severity X probability = Hazard score (1 -16) 37
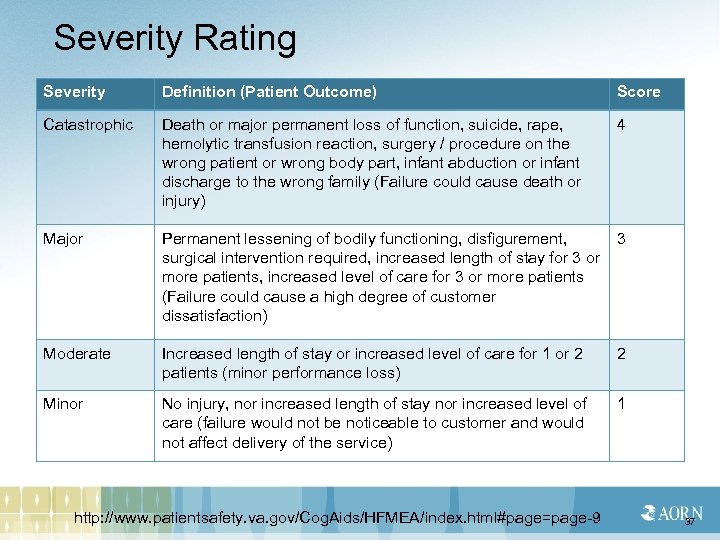
Severity Rating Severity Definition (Patient Outcome) Score Catastrophic Death or major permanent loss of function, suicide, rape, hemolytic transfusion reaction, surgery / procedure on the wrong patient or wrong body part, infant abduction or infant discharge to the wrong family (Failure could cause death or injury) 4 Major Permanent lessening of bodily functioning, disfigurement, surgical intervention required, increased length of stay for 3 or more patients, increased level of care for 3 or more patients (Failure could cause a high degree of customer dissatisfaction) 3 Moderate Increased length of stay or increased level of care for 1 or 2 patients (minor performance loss) 2 Minor No injury, nor increased length of stay nor increased level of care (failure would not be noticeable to customer and would not affect delivery of the service) 1 http: //www. patientsafety. va. gov/Cog. Aids/HFMEA/index. html#page=page-9 37
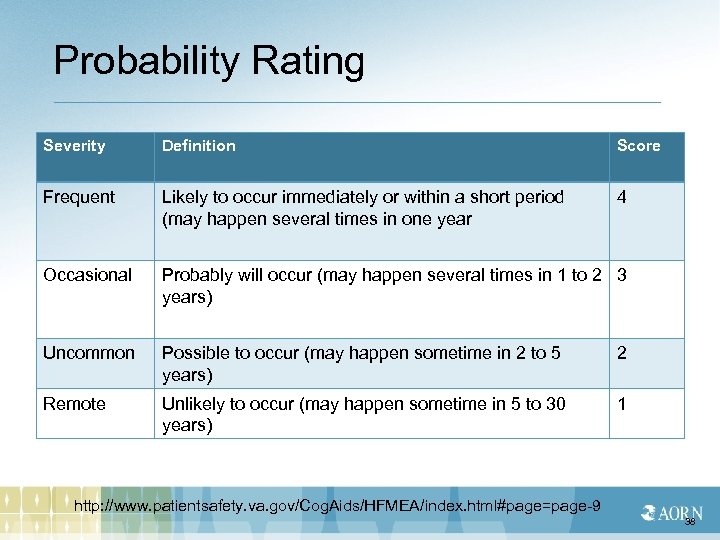
Probability Rating Severity Definition Score Frequent Likely to occur immediately or within a short period (may happen several times in one year 4 Occasional Probably will occur (may happen several times in 1 to 2 3 years) Uncommon Possible to occur (may happen sometime in 2 to 5 years) 2 Remote Unlikely to occur (may happen sometime in 5 to 30 years) 1 http: //www. patientsafety. va. gov/Cog. Aids/HFMEA/index. html#page=page-9 38
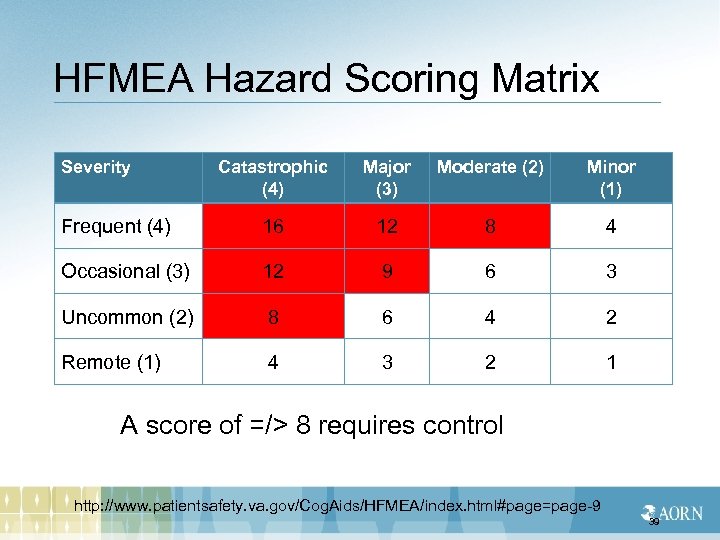
HFMEA Hazard Scoring Matrix Severity Catastrophic (4) Major (3) Moderate (2) Minor (1) Frequent (4) 16 12 8 4 Occasional (3) 12 9 6 3 Uncommon (2) 8 6 4 2 Remote (1) 4 3 2 1 A score of =/> 8 requires control http: //www. patientsafety. va. gov/Cog. Aids/HFMEA/index. html#page=page-9 39
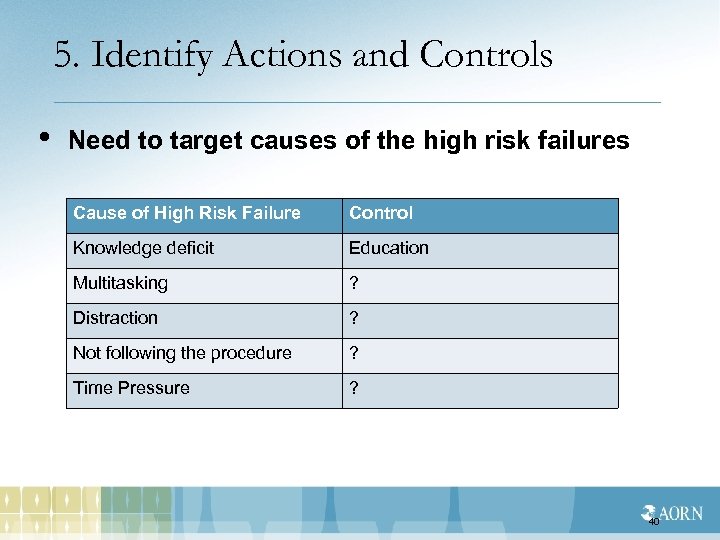
5. Identify Actions and Controls • Need to target causes of the high risk failures Cause of High Risk Failure Control Knowledge deficit Education Multitasking ? Distraction ? Not following the procedure ? Time Pressure ? 40

Control Measures Considered 1. Education would not be effective - Knowledge deficit was not an identified cause 1 2. Enforcement of policy would target 14% of failure points 1 3. Requiring a separate “time out” for closing counts would target 37% of failure points 1 4. Intraoperative radiographs- sensitivity 67% 2 1. Steelman & Cullen (2011): 2. Cima et al. (2008) 41

Recommended Practices for Prevention of Retained Surgical Items Recommendation VII: 1. Perioperative staff members may consider the use of adjunct technologies to supplement manual count procedures. a) A mechanism for evaluating and selecting existing and emerging adjunct technology products should be implemented. AORN (2013) 42

Recommended Practices for Prevention of Retained Surgical Items • Perioperative RNs, physicians, and other health care providers involved in the use of products and medical devices for prevention of RSIs should be part of a multidisciplinary product evaluation and selection committee when the health care organization is evaluating the purchase of adjunct technology • Perioperative personnel should evaluate existing and emerging adjunct technology to determine the application that may be most suitable in their setting. AORN (2013) 43
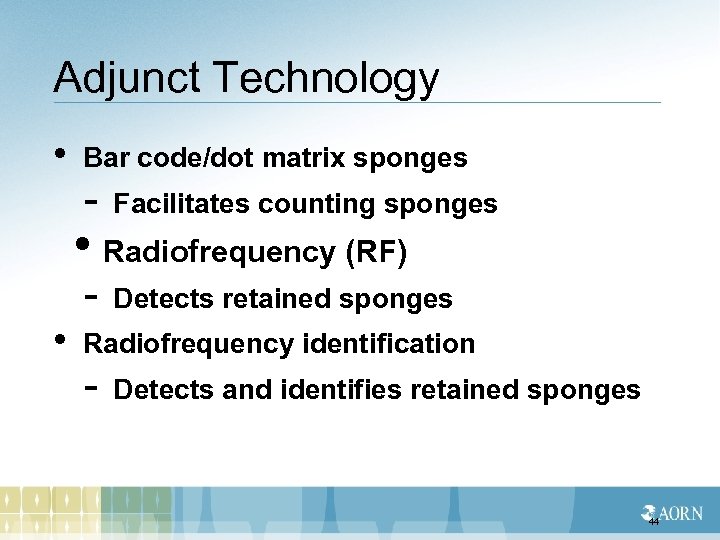
Adjunct Technology • Bar code/dot matrix sponges - Facilitates counting sponges - Detects retained sponges • Radiofrequency (RF) • Radiofrequency identification - Detects and identifies retained sponges 44
Evaluating Adjunct Technology • • Multidisciplinary team • • Evaluate all 3 types of technology Provide an opportunity for those outside of the OR to understand the OR Identify changes in workflow that would be required 45
Steps of a Multidisciplinary Evaluation Two Phases 1. Simulation - Current process Repeat with each of the adjunct technologies Script provided as handout (can be modified) 2. In-use evaluation 47

Simulation Participants • • Circulating RN Surgical Technologist (ST) Surgeon Surgical Assistant Anesthesia Provider Quality Manager Safety Officer/Risk Manager 48

Simulation • Current practices (initial, relief, first closing count, final closing count) • • Repeat for each of the technologies All Team Members and observers: • - On a white board or poster board, list: Pros of the technology Cons of the technology Total time required for baseline and each technology. 49

In Use Evaluation • • Input from end-users • Engages all evaluators in change process Evaluate how the technology works with processes during surgery 50

Summary • Preventing retained surgical items is a high priority for action • If you always do what you always did you will always get what you always got. • • Albert Einstein We need to design safer processes 50

References • • • Cima RR, Kollengode A, Garnatz J, Storsveen A, Weisbrod C, Deschamps C. Incidence and characteristics of potential and actual retained foreign object events in surgical patients. J Am Coll Surg. 2008; Jul; 207: 80 -87. Dhillon JS, Park A. Transmural migration of a retained laparotomy sponge. Am Surg. 2002; 68: 603 -05. Egorova NN, Moskowitz A, Gelijns A, et al. Managing the prevention of retained surgical instruments: What is the value of counting? Ann Surg. 2008; 247: 13 -18. Gawande AA, Studdert DM, Orav EJ, Brennan TA, Zinner MJ. Risk factors for retained instruments and sponges after surgery. N Engl J Med. 2003; 348: 229 -235. Kaiser CW, Friedman S, Spurling KP, Slowick T, Kaiser HA. The retained surgical sponge. Ann Surg. 1996; 224: 79 -84. Lincourt AE, Harrell, A, Cristiano, J, Sechrist, C, Kercher, K, Heniford, BT. Retained foreign bodies after surgery. J Surg Res. 2007; 138: 170 -174. 51

References (cont. ) • • • Recommended practices for prevention of retained surgical items. In: Perioperative Standards and Recommended Practices. Denver, CO: AORN, Inc; 2013: 305 -321. Steelman, VM. , Cullen, JJ. Sponges: A Healthcare Failure Mode and Effect Analysis. AORN J. 2011; 94. The Joint Commission. Summary data of sentinel events reviewed by The Joint Commission. 2013. http: //www. jointcommission. org/assets/1/18/2004_4 Q_2012_SE_Stats_Sum mary. pdf VA National Center for Patient Safety. HFMEA. 2013. http: //www. patientsafety. va. gov/Cog. Aids/HFMEA/index. html#page=page-1 Zantvoord Y, van der Weiden RM, van Hooff MH. Transmural migration of retained surgical sponges: A systematic review. Obstet Gynecol Surv. 2008; 63(7): 465 -471. 52

The End
c6908b2682ce2881440a91a8cee3c124.ppt