e4c7aaf3814e56c7090e40987cc19ed5.ppt
- Количество слайдов: 55

Best Practices for OINDP Pharmaceutical Development Programs Leachables and Extractables IV. Analysis of Leachables and Extractables PQRI Leachables & Extractables Working Group September 2006 PQRI Training Course September 20 -21, 2006 Washington, DC PQRI Training Course 1
Key Points to Consider ► Identification of leachables and extractables is a problem in Trace Organic Analysis. ► “Trace Organic Analysis” can be defined as the qualitative and quantitative analysis of a complex mixture of trace level organic compounds contained in a complex matrix. ► Other similar problems include: § § § Analysis of pollutants in environmental matrices Organic geochemical analysis Metabolite profiling in biological matrices September 2006 PQRI Training Course 2
Key Points to Consider (continued) ► Trace Organic Analysis problems require (in general): § Some knowledge of the chemical nature of the analyte mixture and matrix (Supplier Information). § Removal/extraction of the complex mixture of organic compounds from the matrix. § Separation of the complex mixture of organic compounds into individual chemical entities. § Compound specific detection of the individual chemical entities within the complex mixture. September 2006 PQRI Training Course 3
Compound Specific Detectors (from the ridiculous to the sublime) ► Single-crystal X-ray Spectrometer ► FTIR (Fourier Transform Infrared Spectrophotometer) ► NMR (Nuclear Magnetic Resonance Spectrometer) ► Mass Spectrometer § GC/MS § LC/MS September 2006 PQRI Training Course 4

September 2006 PQRI Training Course 5
What is a Mass Spectrometer? ? ? ? ► Sample inlet system ► Ion Source ► Mass analyzer ► Detector September 2006 PQRI Training Course 6

September 2006 PQRI Training Course 7
Sample Inlet Systems ► Direct Insertion Probes (EI/CI/FAB) ► Batch Inlet Systems (AGIS/heated septum inlets/”targets”) ► Gas Chromatographs ► Liquid Chromatographs (with or without separation in an HPLC column) September 2006 PQRI Training Course 8
Ionization Processes ► ► ► ► ► EI CI FI FAB LSIMS TSP ESI APCI MALDI - September 2006 electron ionization chemical ionization field ionization fast atom bombardment liquid secondary ion thermospray electrospray atmospheric pressure CI matrix assisted laser desorption PQRI Training Course 9
Mass Analyzers ► Magnetic sector (high resolution) ► Quadrupole filter (“benchtop”/Triple Quad. ) ► Time-of-flight (scan rate/mass range/accurate mass measurements) ► Quadrupole ion trap (MSn) ► Ion cyclotron resonance (FTMS/ultrahigh resolution/mass range/accurate mass measurements) September 2006 PQRI Training Course 10

GC/MS – Circa Late 1980 s Circa 2006 September 2006 PQRI Training Course 11
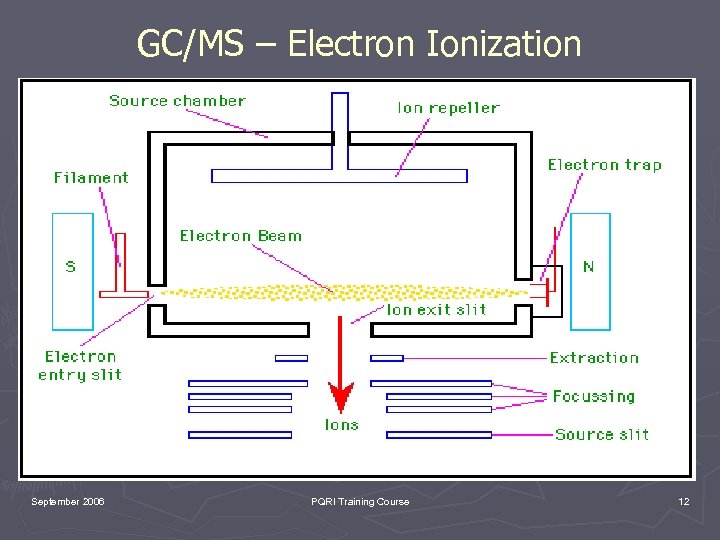
GC/MS – Electron Ionization September 2006 PQRI Training Course 12
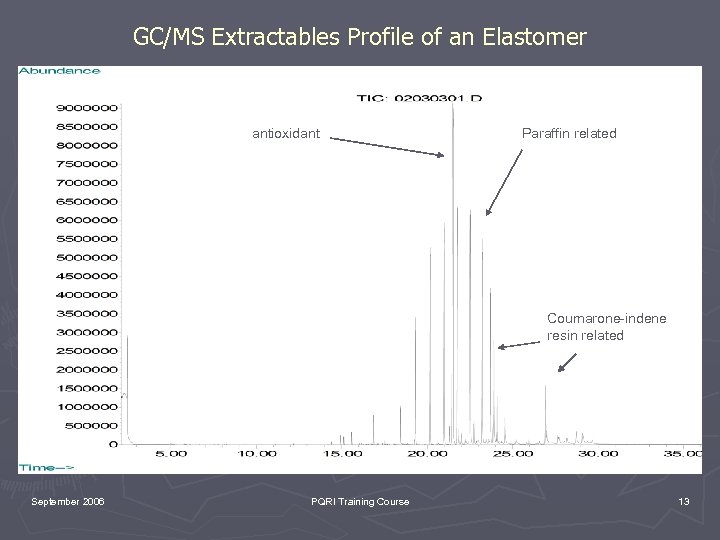
GC/MS Extractables Profile of an Elastomer antioxidant Paraffin related Coumarone-indene resin related September 2006 PQRI Training Course 13
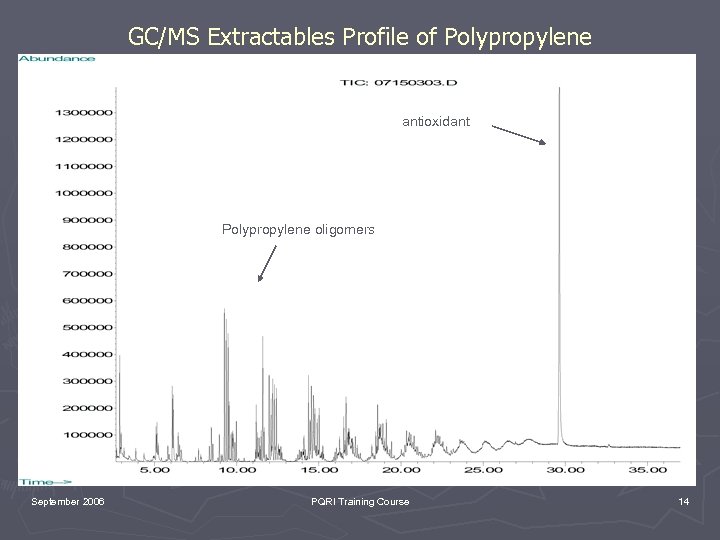
GC/MS Extractables Profile of Polypropylene antioxidant Polypropylene oligomers September 2006 PQRI Training Course 14
Interpreting a Mass Spectrum ► Confirm molecular weight!!!!!! § Adduct ions/multiply charged ions (LC/MS) § Alternate ionization techniques (EI/CI and APCI/ESI) § Note features of the molecular ion (obvious heteroatoms/nitrogen rule) ► Rationalize significant fragmentation processes: § Structures of fragment ions § Mechanisms for fragment ion formation September 2006 PQRI Training Course 15
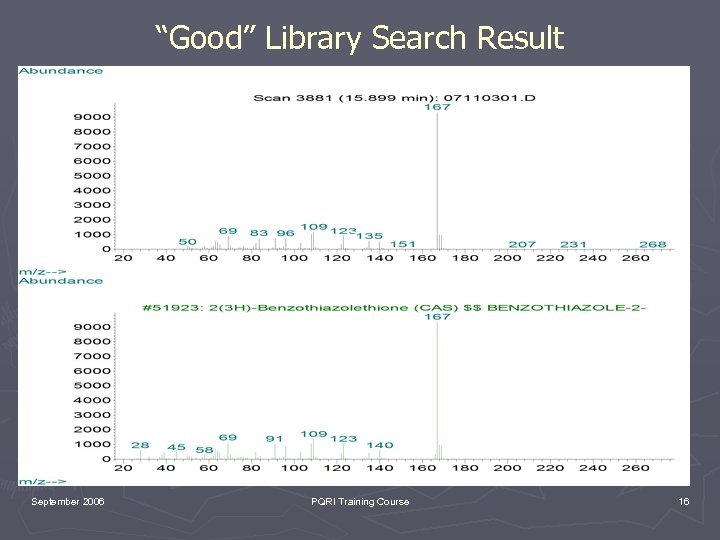
“Good” Library Search Result September 2006 PQRI Training Course 16
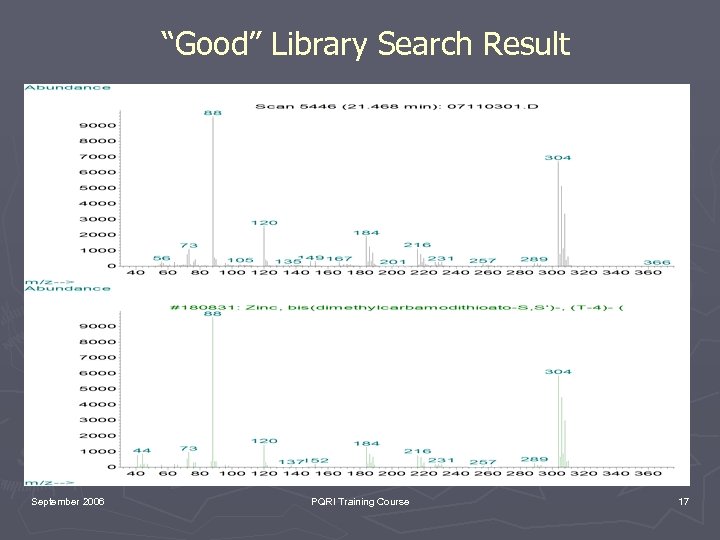
“Good” Library Search Result September 2006 PQRI Training Course 17
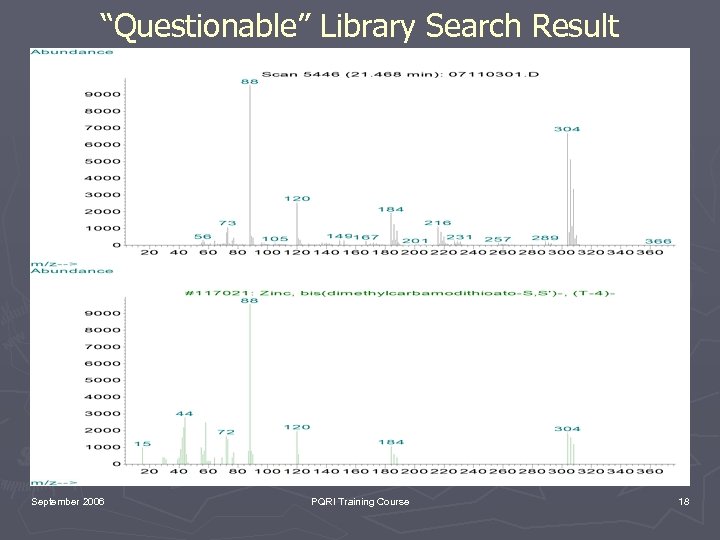
“Questionable” Library Search Result September 2006 PQRI Training Course 18
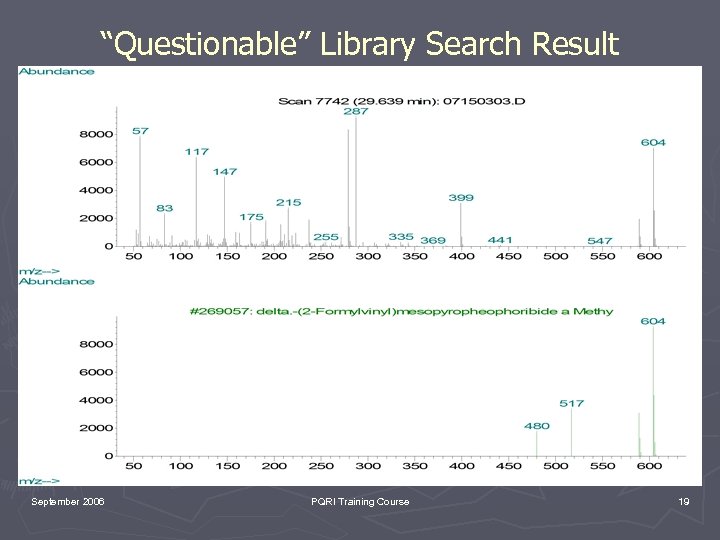
“Questionable” Library Search Result September 2006 PQRI Training Course 19
![Example of Chemical Ionization (ammonia reagent gas) CI+ (ammonia) [M+NH 4]+ EI . M+ Example of Chemical Ionization (ammonia reagent gas) CI+ (ammonia) [M+NH 4]+ EI . M+](https://present5.com/presentation/e4c7aaf3814e56c7090e40987cc19ed5/image-20.jpg)
Example of Chemical Ionization (ammonia reagent gas) CI+ (ammonia) [M+NH 4]+ EI . M+ (? ? ? ) September 2006 PQRI Training Course 20
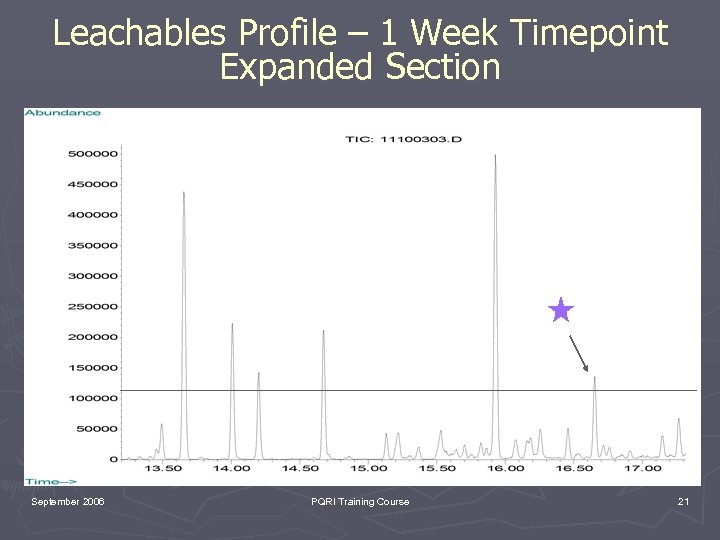
Leachables Profile – 1 Week Timepoint Expanded Section September 2006 PQRI Training Course 21
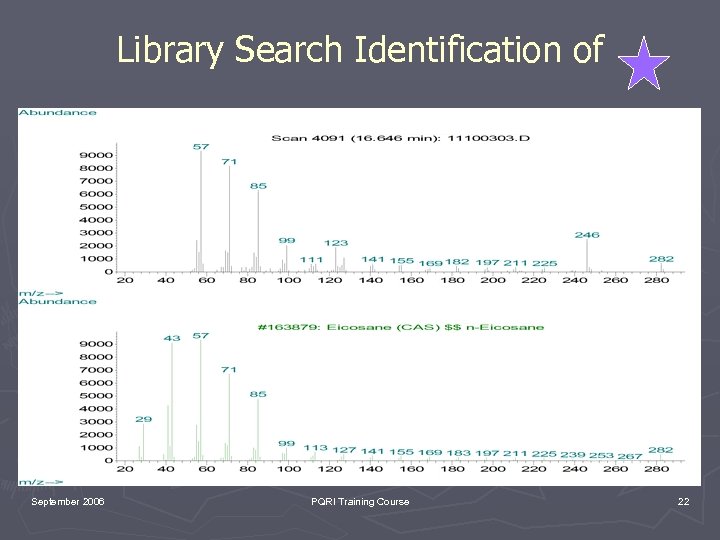
Library Search Identification of September 2006 PQRI Training Course 22
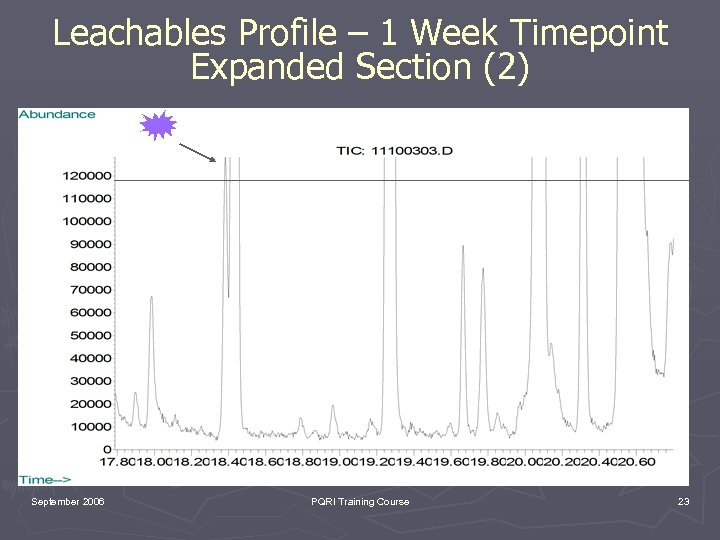
Leachables Profile – 1 Week Timepoint Expanded Section (2) September 2006 PQRI Training Course 23
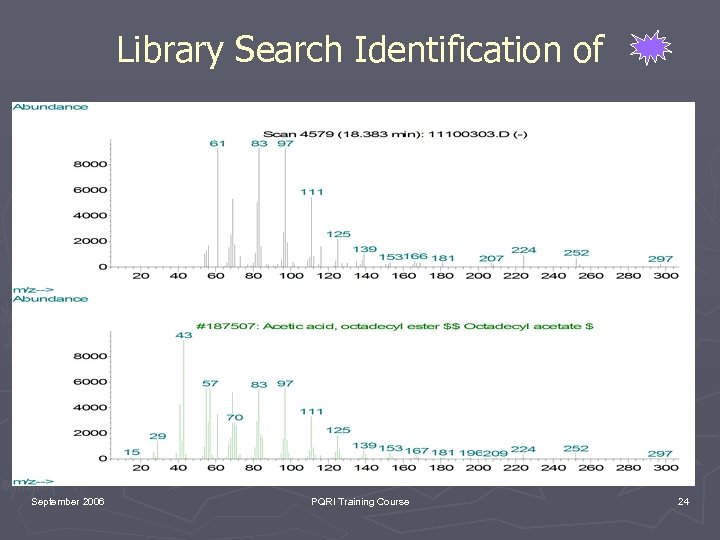
Library Search Identification of September 2006 PQRI Training Course 24

Key Points to Consider ► EI is under “kinetic control”. Therefore, EI spectra are reproducible and spectral libraries can be employed (remember limitations of libraries!!!!!!!). ► CI is under “thermodynamic control”. It is useful for molecular weight confirmation but no libraries. ► GC/MS provides both a chromatographic retention time and mass spectrum (spectra) which can be compared with an authentic reference material (Good luck finding it). September 2006 PQRI Training Course 25
LC/MS Interfaces/Ionization Processes ► Moving belt/wire (transport device) ► Thermospray (unique ionization process) ► Continuous flow FAB (transport device) ► Particle-beam (transport device) ► Electrospray (unique ionization process) ► APCI (transport device/ionization process) September 2006 PQRI Training Course 26

A “Modern” LC/MS System September 2006 PQRI Training Course 27

“Bench-top” LC/MS Systems September 2006 PQRI Training Course 28
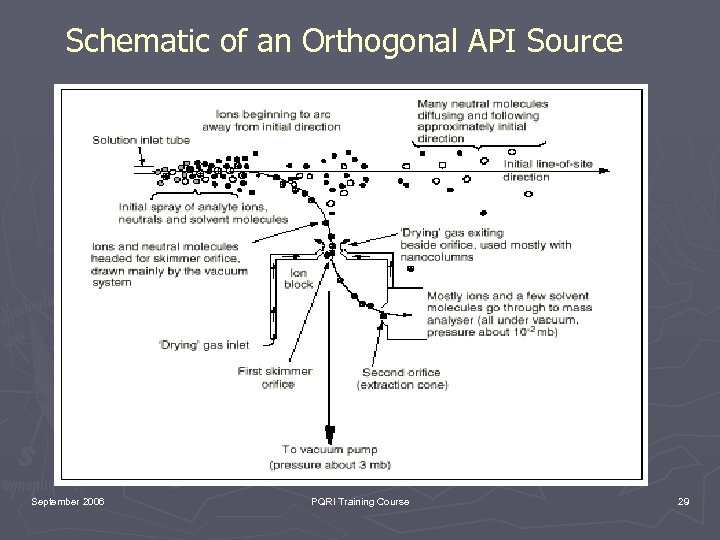
Schematic of an Orthogonal API Source September 2006 PQRI Training Course 29

September 2006 PQRI Training Course 30

Features of Electrospray ► Often reflects solution chemistry (multiply charged ions observed) ► Operates at atmospheric pressure ► Uses a strong electric field ► Flow-rate (optimum performance at u. L/min) ► Soft-ionization process ► Extremely rugged September 2006 PQRI Training Course 31
Features of APCI ► Gas phase ionization process ► Operates at atmospheric pressure ► Flow-rate (optimum performance at m. L/min) ► Uses a corona discharge ► Soft-ionization process ► Extremely rugged September 2006 PQRI Training Course 32
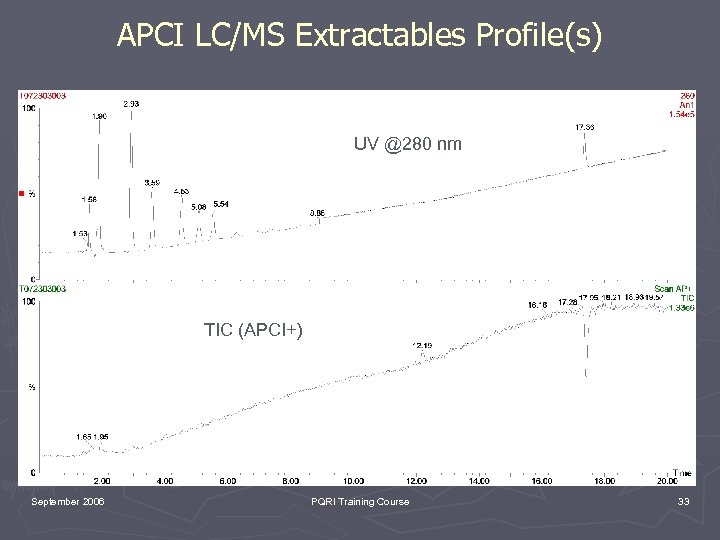
APCI LC/MS Extractables Profile(s) UV @280 nm TIC (APCI+) September 2006 PQRI Training Course 33
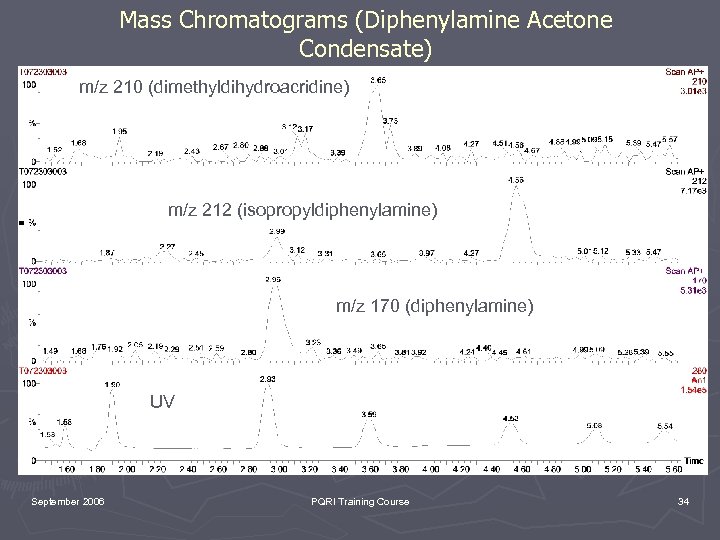
Mass Chromatograms (Diphenylamine Acetone Condensate) m/z 210 (dimethyldihydroacridine) m/z 212 (isopropyldiphenylamine) m/z 170 (diphenylamine) UV September 2006 PQRI Training Course 34
![APCI+ Mass Spectrum of Isopropyldiphenylamine [M+H+Ac. CN]+ [M+H]+ September 2006 PQRI Training Course 35 APCI+ Mass Spectrum of Isopropyldiphenylamine [M+H+Ac. CN]+ [M+H]+ September 2006 PQRI Training Course 35](https://present5.com/presentation/e4c7aaf3814e56c7090e40987cc19ed5/image-35.jpg)
APCI+ Mass Spectrum of Isopropyldiphenylamine [M+H+Ac. CN]+ [M+H]+ September 2006 PQRI Training Course 35
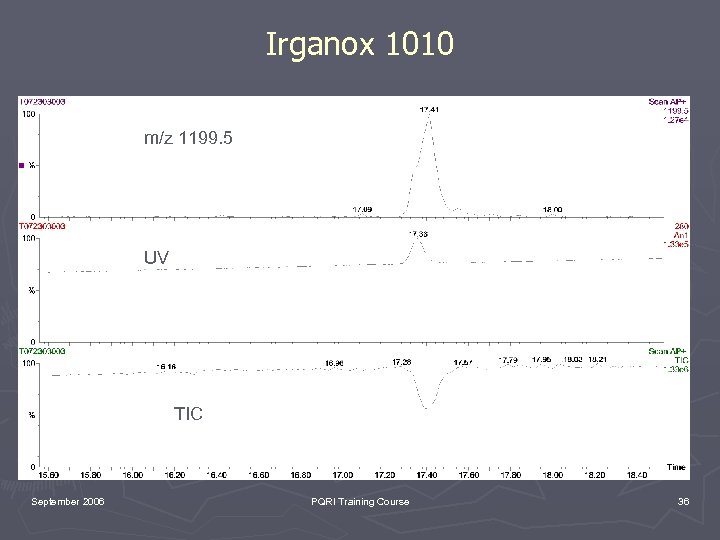
Irganox 1010 m/z 1199. 5 UV TIC September 2006 PQRI Training Course 36
![APCI+ Mass Spectrum Irganox 1010 [M+Na]+ [M+K]+ September 2006 PQRI Training Course 37 APCI+ Mass Spectrum Irganox 1010 [M+Na]+ [M+K]+ September 2006 PQRI Training Course 37](https://present5.com/presentation/e4c7aaf3814e56c7090e40987cc19ed5/image-37.jpg)
APCI+ Mass Spectrum Irganox 1010 [M+Na]+ [M+K]+ September 2006 PQRI Training Course 37
![APCI- Mass Spectrum Irganox 1010 (no induced fragmentation) [M-H]- September 2006 PQRI Training Course APCI- Mass Spectrum Irganox 1010 (no induced fragmentation) [M-H]- September 2006 PQRI Training Course](https://present5.com/presentation/e4c7aaf3814e56c7090e40987cc19ed5/image-38.jpg)
APCI- Mass Spectrum Irganox 1010 (no induced fragmentation) [M-H]- September 2006 PQRI Training Course 38
![APCI- Mass Spectrum Irganox 1010 (induced fragmentation in the ion source) [M-H]- September 2006 APCI- Mass Spectrum Irganox 1010 (induced fragmentation in the ion source) [M-H]- September 2006](https://present5.com/presentation/e4c7aaf3814e56c7090e40987cc19ed5/image-39.jpg)
APCI- Mass Spectrum Irganox 1010 (induced fragmentation in the ion source) [M-H]- September 2006 PQRI Training Course 39
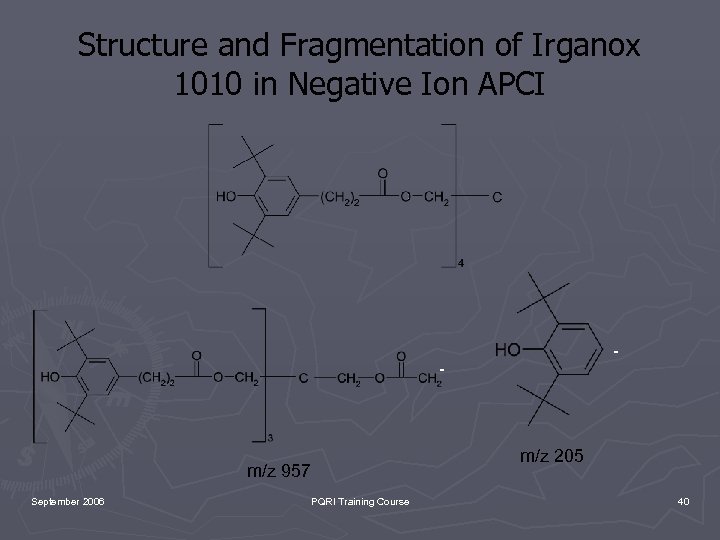
Structure and Fragmentation of Irganox 1010 in Negative Ion APCI - - m/z 957 September 2006 PQRI Training Course m/z 205 40
![APCI- Mass Spectrum of Stearic Acid [M-H]- September 2006 PQRI Training Course 41 APCI- Mass Spectrum of Stearic Acid [M-H]- September 2006 PQRI Training Course 41](https://present5.com/presentation/e4c7aaf3814e56c7090e40987cc19ed5/image-41.jpg)
APCI- Mass Spectrum of Stearic Acid [M-H]- September 2006 PQRI Training Course 41
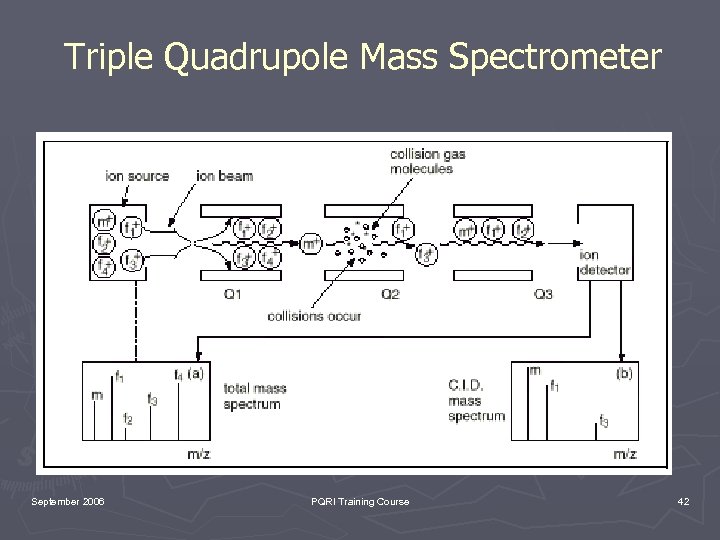
Triple Quadrupole Mass Spectrometer September 2006 PQRI Training Course 42
![MS/MS Spectrum of Irganox 1076 (ESI+; products of m/z 531) [M+H-56]+ [M+H-2 X 56]+ MS/MS Spectrum of Irganox 1076 (ESI+; products of m/z 531) [M+H-56]+ [M+H-2 X 56]+](https://present5.com/presentation/e4c7aaf3814e56c7090e40987cc19ed5/image-43.jpg)
MS/MS Spectrum of Irganox 1076 (ESI+; products of m/z 531) [M+H-56]+ [M+H-2 X 56]+ September 2006 PQRI Training Course 43
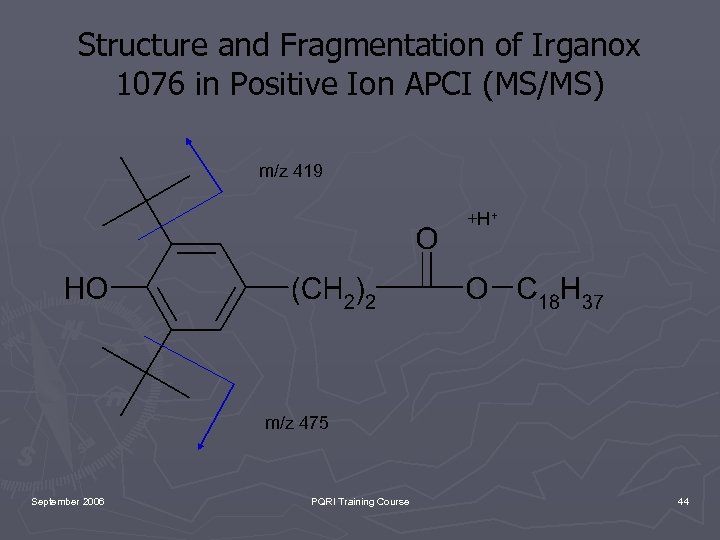
Structure and Fragmentation of Irganox 1076 in Positive Ion APCI (MS/MS) m/z 419 +H+ m/z 475 September 2006 PQRI Training Course 44
![MS/MS Spectrum of Tetramethylthiuram Disulfide (ESI+; products of m/z 241) [M+H]+ September 2006 PQRI MS/MS Spectrum of Tetramethylthiuram Disulfide (ESI+; products of m/z 241) [M+H]+ September 2006 PQRI](https://present5.com/presentation/e4c7aaf3814e56c7090e40987cc19ed5/image-45.jpg)
MS/MS Spectrum of Tetramethylthiuram Disulfide (ESI+; products of m/z 241) [M+H]+ September 2006 PQRI Training Course 45
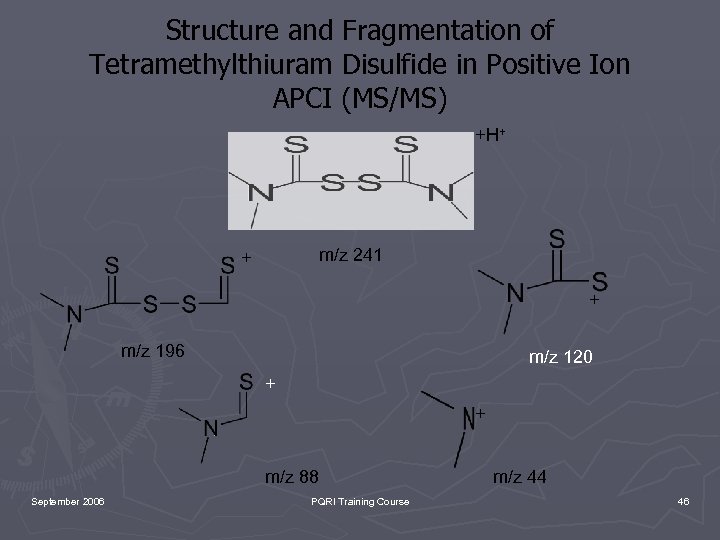
Structure and Fragmentation of Tetramethylthiuram Disulfide in Positive Ion APCI (MS/MS) +H+ m/z 241 + + m/z 196 m/z 120 + + m/z 88 September 2006 PQRI Training Course m/z 44 46

September 2006 PQRI Training Course 47
![ESI+ TOF Mass Spectrum of TMTMS [M+Na]+ [2 M+Na]+ [M+H]+ September 2006 PQRI Training ESI+ TOF Mass Spectrum of TMTMS [M+Na]+ [2 M+Na]+ [M+H]+ September 2006 PQRI Training](https://present5.com/presentation/e4c7aaf3814e56c7090e40987cc19ed5/image-48.jpg)
ESI+ TOF Mass Spectrum of TMTMS [M+Na]+ [2 M+Na]+ [M+H]+ September 2006 PQRI Training Course 48
![Expanded ESI+ TOF Mass Spectrum of TMTMS [M+Na]+ September 2006 PQRI Training Course 49 Expanded ESI+ TOF Mass Spectrum of TMTMS [M+Na]+ September 2006 PQRI Training Course 49](https://present5.com/presentation/e4c7aaf3814e56c7090e40987cc19ed5/image-49.jpg)
Expanded ESI+ TOF Mass Spectrum of TMTMS [M+Na]+ September 2006 PQRI Training Course 49
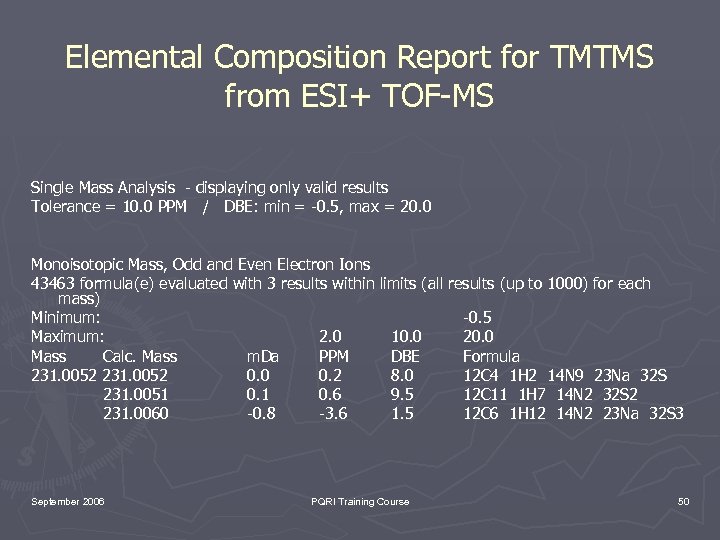
Elemental Composition Report for TMTMS from ESI+ TOF-MS Single Mass Analysis - displaying only valid results Tolerance = 10. 0 PPM / DBE: min = -0. 5, max = 20. 0 Monoisotopic Mass, Odd and Even Electron Ions 43463 formula(e) evaluated with 3 results within limits (all results (up to 1000) for each mass) Minimum: -0. 5 Maximum: 2. 0 10. 0 20. 0 Mass Calc. Mass m. Da PPM DBE Formula 231. 0052 0. 0 0. 2 8. 0 12 C 4 1 H 2 14 N 9 23 Na 32 S 231. 0051 0. 6 9. 5 12 C 11 1 H 7 14 N 2 32 S 2 231. 0060 -0. 8 -3. 6 1. 5 12 C 6 1 H 12 14 N 2 23 Na 32 S 3 September 2006 PQRI Training Course 50
![Expanded ESI+ TOF Mass Spectrum of Irganox 1010 [M+Na]+ [M+NH 4]+ September 2006 PQRI Expanded ESI+ TOF Mass Spectrum of Irganox 1010 [M+Na]+ [M+NH 4]+ September 2006 PQRI](https://present5.com/presentation/e4c7aaf3814e56c7090e40987cc19ed5/image-51.jpg)
Expanded ESI+ TOF Mass Spectrum of Irganox 1010 [M+Na]+ [M+NH 4]+ September 2006 PQRI Training Course 51
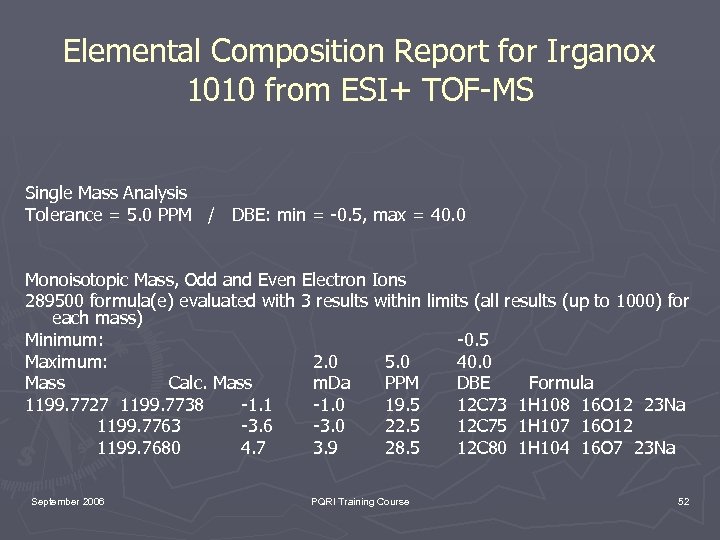
Elemental Composition Report for Irganox 1010 from ESI+ TOF-MS Single Mass Analysis Tolerance = 5. 0 PPM / DBE: min = -0. 5, max = 40. 0 Monoisotopic Mass, Odd and Even Electron Ions 289500 formula(e) evaluated with 3 results within limits (all results (up to 1000) for each mass) Minimum: -0. 5 Maximum: 2. 0 5. 0 40. 0 Mass Calc. Mass m. Da PPM DBE Formula 1199. 7727 1199. 7738 -1. 1 -1. 0 19. 5 12 C 73 1 H 108 16 O 12 23 Na 1199. 7763 -3. 6 -3. 0 22. 5 12 C 75 1 H 107 16 O 12 1199. 7680 4. 7 3. 9 28. 5 12 C 80 1 H 104 16 O 7 23 Na September 2006 PQRI Training Course 52

My Philosophy with LC/MS ► For CMC impurity profiling studies use APCI as a primary technique. ► Reproduce the UV chromatogram. ► Use every trick in the book to make APCI work (mobile phase additives, alternate acquisition techniques, etc. ). ► Use electrospray to complement and confirm APCI. September 2006 PQRI Training Course 53
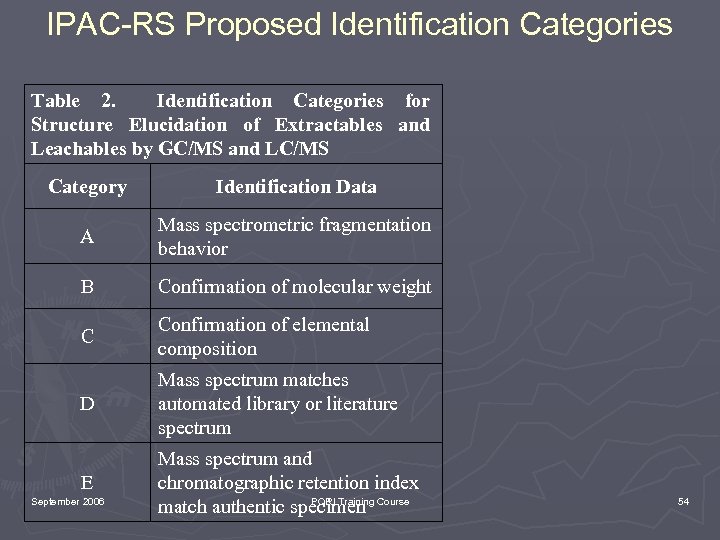
IPAC-RS Proposed Identification Categories Table 2. Identification Categories for Structure Elucidation of Extractables and Leachables by GC/MS and LC/MS Category Identification Data A Mass spectrometric fragmentation behavior B Confirmation of molecular weight C Confirmation of elemental composition D Mass spectrum matches automated library or literature spectrum E Mass spectrum and chromatographic retention index PQRI Training match authentic specimen Course September 2006 54
Identification Categories ►A Confirmed identification means that identification categories A, B (or C), and D (or E) have been fulfilled. ► A Confidentification means that sufficient data to preclude all but the most closely related structures have been obtained. ► A Tentative identification means that data have been obtained that are consistent with a class of molecule only. September 2006 PQRI Training Course 55
e4c7aaf3814e56c7090e40987cc19ed5.ppt