9ae9c3b44375ad315c504c845ba72959.ppt
- Количество слайдов: 49

Best Practices for OINDP Pharmaceutical Development Programs Leachables and Extractables II. OINDP Container Closure Systems PQRI Leachables & Extractables Working Group PQRI Training Course September 20 -21, 2006 Washington, DC

Container Closure System Components ► ► Primary Packaging Components, which are or may be in direct contact with the dosage form. These include: containers (e. g. , ampules, vials, bottles), container liners, closures (e. g. , screw caps, stoppers, metering valves), closure liners, stopper overseals, container inner seals, administration ports, overwraps, etc. Secondary Packaging Components, which are not or will not be in direct contact with the dosage form. These include container labels, administration accessories, shipping containers, etc. Note that even though secondary packaging components are not in direct contact with the drug product, they may still contribute leachables under certain conditions.
Critical Components “Critical components” of an OINDP container closure system are defined as those that contact either the patient or the formulation, components that affect the mechanics of the overall performance of the device, or any necessary secondary protective packaging. ”
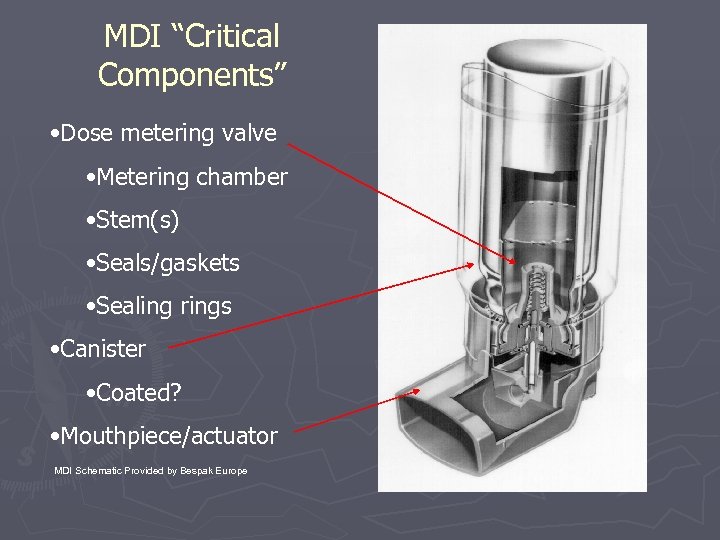
MDI “Critical Components” • Dose metering valve • Metering chamber • Stem(s) • Seals/gaskets • Sealing rings • Canister • Coated? • Mouthpiece/actuator MDI Schematic Provided by Bespak Europe

OINDP Container Closure System Components
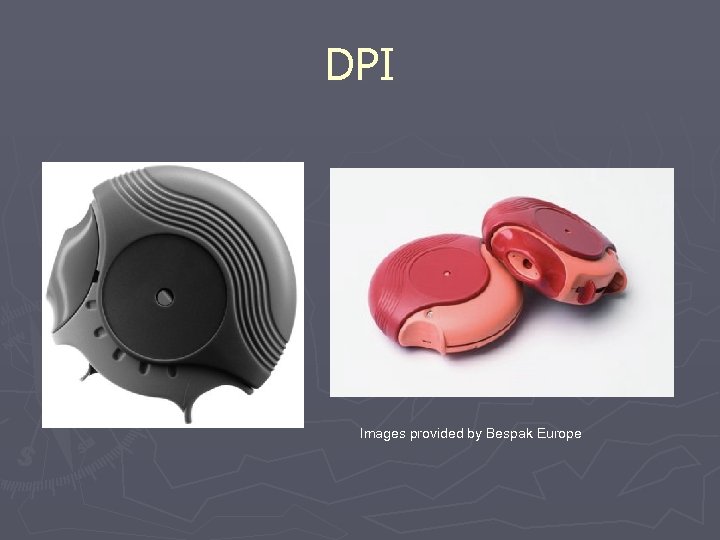
DPI Images provided by Bespak Europe
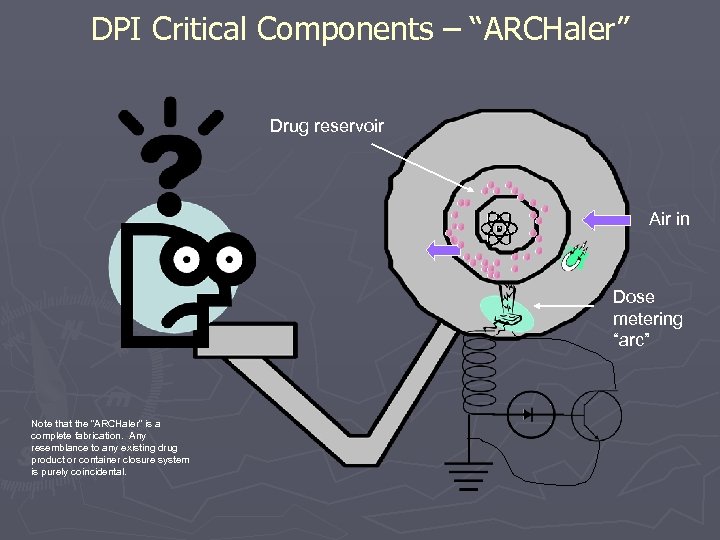
DPI Critical Components – “ARCHaler” Drug reservoir Air in Dose metering “arc” Note that the “ARCHaler” is a complete fabrication. Any resemblance to any existing drug product or container closure system is purely coincidental.

What are some potential sources for leachables and extractables? ► ► ► Chemical additives present in individual elastomeric/polymeric container closure system components, including contaminants in such additives (e. g. PAHs and N-nitrosamines). Monomers and higher molecular weight oligomers derived from incomplete polymerization reactions. Migrants from secondary packaging components, such as inks and label adhesives. Surface residues, such as heavy oils and degreasing agents on the surfaces of metal canisters and containers. Chemical additives on the surfaces of container closure system component fabrication machinery, such as mould release agents, antistatic and antislip agents, etc. Chemical entities from the storage environment (i. e. , “very” secondary packaging components), such as volatiles from cardboard shipping containers or plastic storage bags.
Examples of Chemical Additives
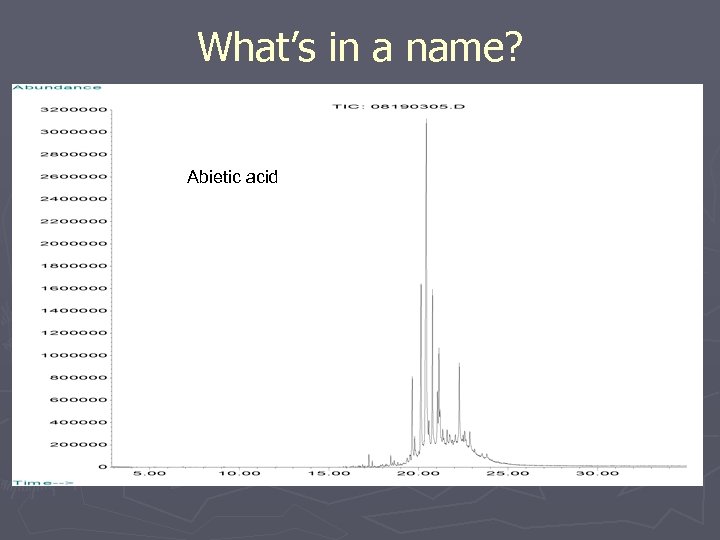
What’s in a name? Abietic acid
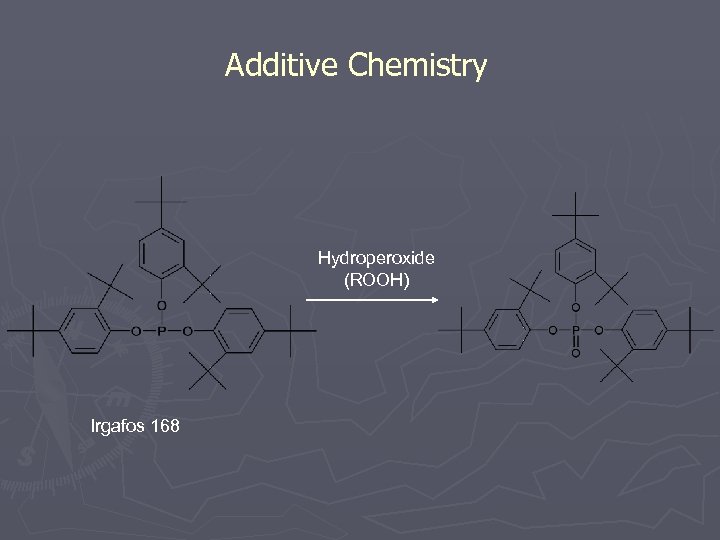
Additive Chemistry Hydroperoxide (ROOH) Irgafos 168
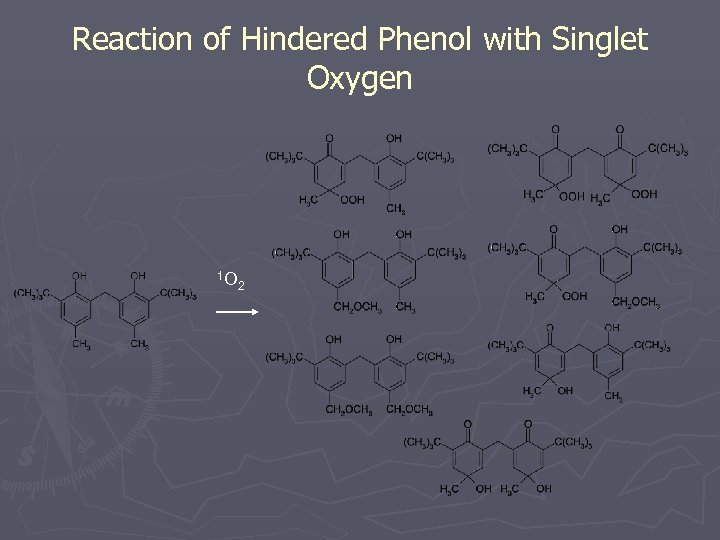
Reaction of Hindered Phenol with Singlet Oxygen 1 O 2

Information Required ► ► ► The elastomeric/polymeric or other material constituting the principal structure of the component (e. g. , High Density Polyethylene, Ethylene-Propylene-Diene rubber, stainless steel, etc. ) The polymerization/cross-linking/curing process, or processes, for the component base polymer, including any chemical additives employed. The compounding/fabrication process, or processes, including any additives designed to assist in compounding/fabrication. All individual chemical additives/ingredients in the component, including the composition and chemistry of each individual additive. Any cleaning/washing processes for finished components, including knowledge of cleaning, washing, or other agents. The storage/shipping environment for both components and drug product, if the potential for environmental leaching exists.

Raw Materials – Supply Chain

Raw Materials - Supply Chain

Component Fabrication Images provided by Bespak Europe Moulding machines
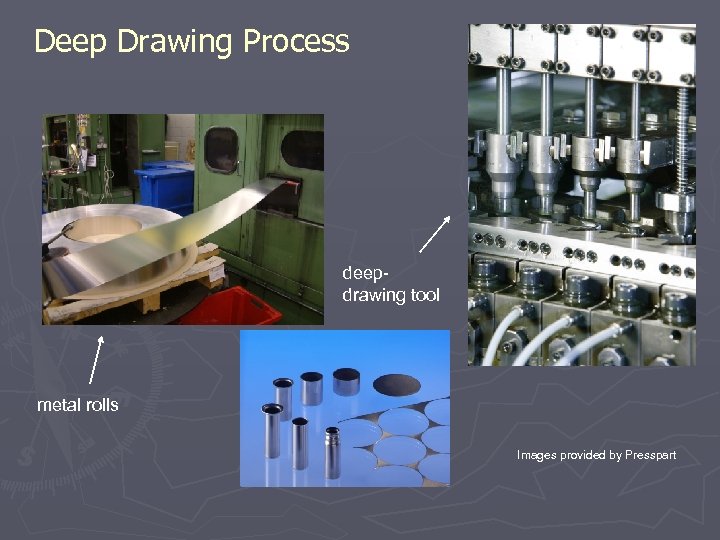
Deep Drawing Process deepdrawing tool metal rolls Images provided by Presspart
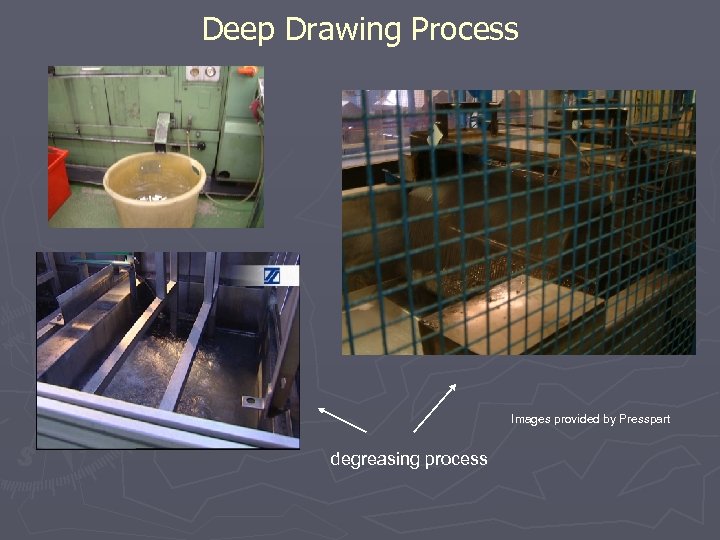
Deep Drawing Process finished canisters Images provided by Presspart degreasing process
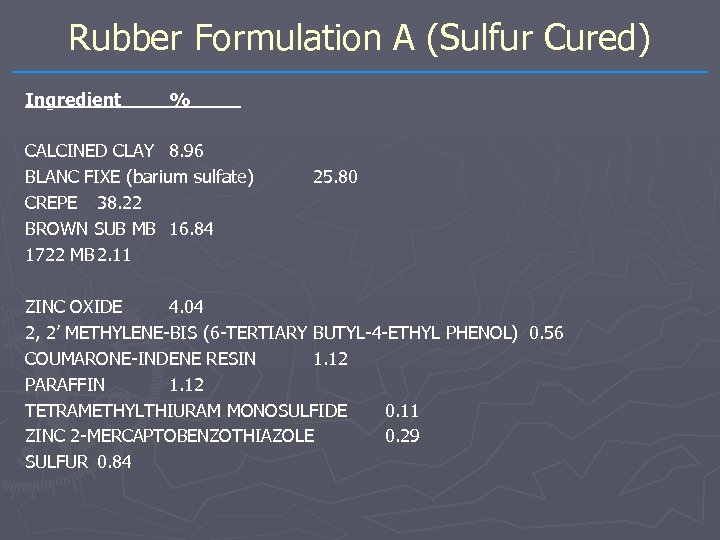
Rubber Formulation A (Sulfur Cured) Ingredient % CALCINED CLAY 8. 96 BLANC FIXE (barium sulfate) CREPE 38. 22 BROWN SUB MB 16. 84 1722 MB 2. 11 25. 80 ZINC OXIDE 4. 04 2, 2’ METHYLENE-BIS (6 -TERTIARY BUTYL-4 -ETHYL PHENOL) 0. 56 COUMARONE-INDENE RESIN 1. 12 PARAFFIN 1. 12 TETRAMETHYLTHIURAM MONOSULFIDE 0. 11 ZINC 2 -MERCAPTOBENZOTHIAZOLE 0. 29 SULFUR 0. 84

What do we know? ► Carbon black is a known source of PAHs and has also been shown to be involved in N-nitrosamine formation in rubber (“special cases”). ► Thiurams are known precursors of N-nitrosamines. ► 2 -Mercaptobenzothiazole is a known “special case”. ► Paraffin and Coumarone-indene resin are natural product materials and are likely complex mixtures of related structures. ► Individual additives are likely GC-able.
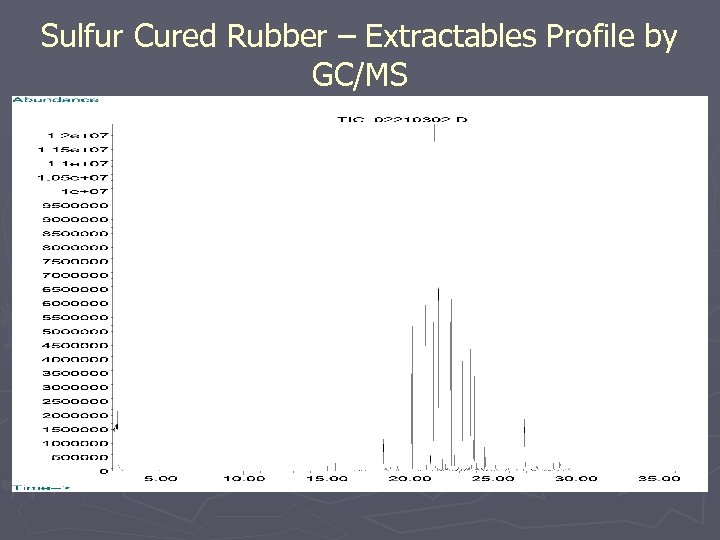
Sulfur Cured Rubber – Extractables Profile by GC/MS
Polypropylene Formulation Ingredient wt % ► Primary Stabilizers Tetrakis (methylene(3, 5 -di-t-butyl 4 -hydroxyhydrocinnamate)) methane Irganox 1010 (Ciba) Anox 20 (Great Lakes) ► 0. 08 wt% Secondary Stabilizers Bis(2, 4 -di-t-butylphenyl)pentaerythritol diphosphite Ultranox 626 (GE) 0. 05 wt%
Polypropylene Formulation Ingredient % ► Corrosion Inhibitors Calcium Stearate 114 -50 (Ferro) ► 0. 03 - 0. 4 wt% Antistatic Vegetable oil derived 90% alpha monoglycerides (soybean) Pationic 901 (Patco) 0. 3 wt% Dimodan HS-KA (Danisco) ► Nucleating Agents 3, 4 -dimethyl dibenzylidene sorbitol Millad 3988 (Milliken) 0. 2 wt%

What do we know? ► Polypropylene is known to contain many soluble oligomers. ► Individual additives will likely require analysis by HPLC based methods. ► Individual additives could be both chemically complex and have complex degradation chemistries. ► No reason to suspect the presence of “special cases”
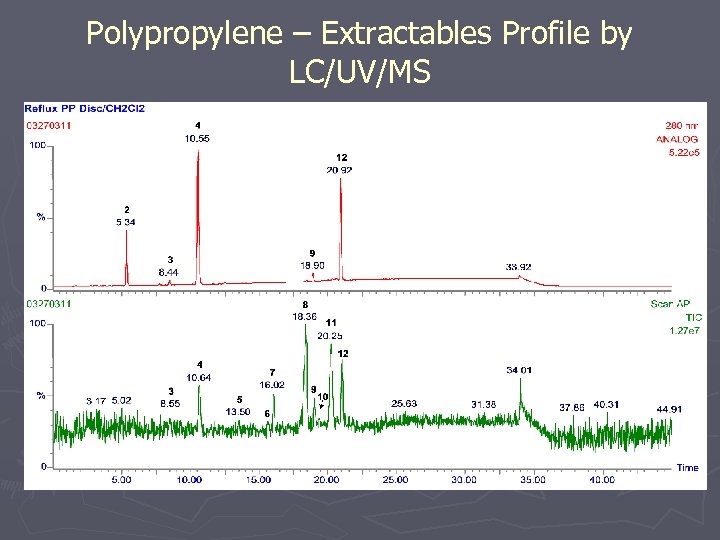
Polypropylene – Extractables Profile by LC/UV/MS
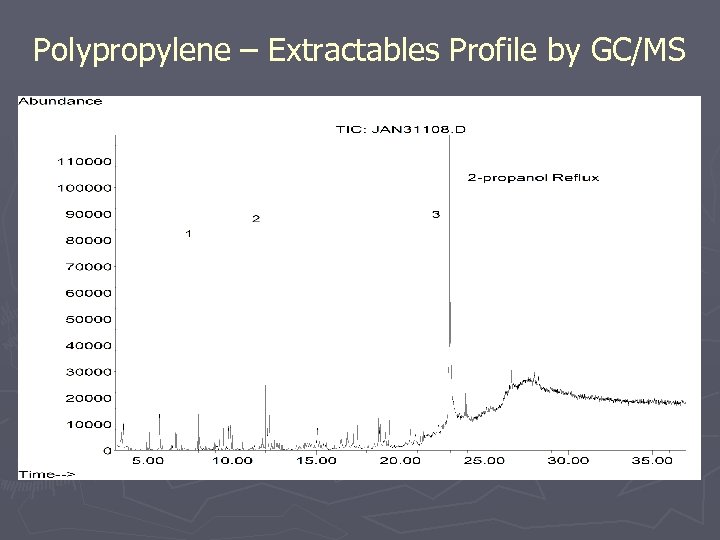
Polypropylene – Extractables Profile by GC/MS

Rubber Formulation B (Peroxide Cured) Ingredient % IMSIL A 25 (silicone dioxide) 24. 01 MISTRON CYPRUBOND (magnesium silicate) 19. 21 BROMOBUTYL 2030 38. 42 VISTALON 404 (ethylene propylene copolymer) 9. 61 WHITE OIL 2 1. 44 420 BLUE MB 0. 12 TITANIUM DIOXIDE 1. 68 PARAFFIN 0. 96 MAGNESIUM OXIDE 0. 60 STEARIC ACID 0. 48 POLYETHYLENE WAX 1. 44 P-800 2. 03

Rubber Formulation B (Peroxide Cured) Ingredient % CONTENTS OF 420 BLUE MB SBR-3 PIGMENT BLUE 15 ANOX 9 CONTENTS OF P-800 2, 5 DI METHYL-2, 5 -DI (T-BUTYL PEROXIDE) HEXANE PRECIPITATED SILICA 32. 00 68. 00

What do we know? ► Registry Number: 78 -63 -7 ► Formula: C 16 H 34 O 4 ► CA Index Name: Peroxide, (1, 1, 4, 4 -tetramethyl-1, 4 -butanediyl)bis[(1, 1 -dimethyl) (9 CI) ► Other Names: Peroxide, (1, 1, 4, 4 -tetramethylene)bis[tert-butyl (6 CI, 8 CI); (1, 1, 4, 4 Tetramethyltetramethylene)bis(tert-butyl peroxide); 101 XL; 2, 5 -Bis(tert-butyldioxy)-2, 5 -dimethylhexane; 2, 5 -Bis(tertbutylperoxy)-2, 5 -dimethylhexane; 2, 5 -Di(t-butylperoxy)-2, 5 -dimethylhexane; 2, 5 -Di(tert-butylperoxy)-2, 5 dimethylhexane; 2, 5 -Di-tert-butyl-2, 5 -dimethylhexyl peroxide; 2, 5 -Dimethyl-2, 5 -bis(tert-butyldioxy)hexane; 2, 5 Dimethyl-2, 5 -bis(tert-butylperoxy)hexane; 2, 5 -Dimethyl-2, 5 -di(tertbutylperoxy)hexane; 2, 5 -Dimethyl-di(tert-butyl)peroxyhexane; 2, 5 -Dimethylhexane-2, 5 -di-tert-butylperoxide; 2, 5 Methyl-2, 5 -di(tert-butylperoxy)hexane; 25 B 40; AD 40 C; APO 40 S; C 15 (peroxide); C 8 (vulcanizer); C 8 A; CR 05; CT 8 (crosslinking agent); HC 4 (peroxide); Interox DHBP 45 IC/G; Kayahexa AD 40; Kayahexa AD 40 C; L 101; LX 101; Link-Cup DBPH; Luperco 101 X 45; Luperco 101 XL; Luperox 101 XL 45; Lupersol 101 XL; Lupersol L 101; NSC 38203; Perhexa 2. 5 B 40; Perhexa 25 B 40; RC 4 (peroxide); RC 450 P; RC 8; RPZ 101; Sanperox APO; TC 8 (catalyst); Trigonox 101 -40 D; Trigonox 101 -40 MD-GR; Trigonox 101 -50; Trigonox 101 E 10; Trigonox 101 E 5; Trigonox XQ 8; Varox 50; Varox DBPH 50; Varox Liquid; Yinox 101 ► 2261 references in chemistry database (7 related to analytical studies)
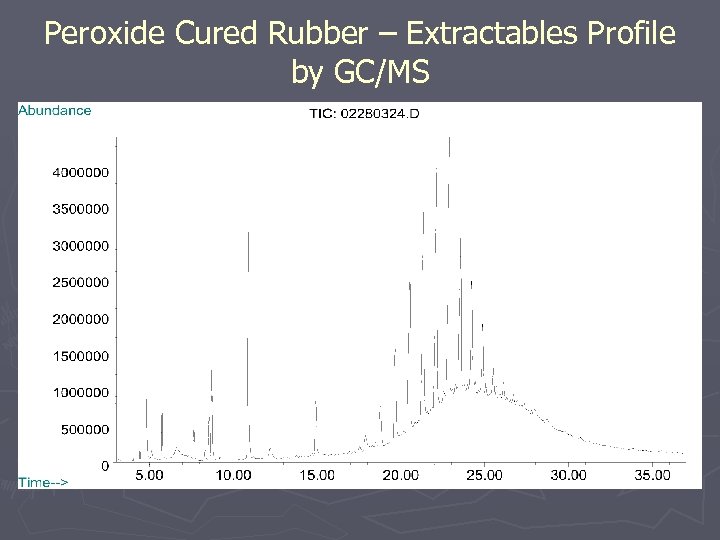
Peroxide Cured Rubber – Extractables Profile by GC/MS

Summary of PQRI Recommendations The pharmaceutical development team should obtain all available information on the composition and manufacturing/fabrication processes for each component type to the extent possible, and determine which components are “critical, ” before beginning extractables and leachables studies on a given OINDP and its associated container/closure system components. ► Component formulation should inform component selection. ► Risk Assessment should be performed during the selection of components and materials. ► Extractables testing, including Controlled Extraction Studies and the development and validation of Routine extractables testing methods, should be accomplished for all critical OINDP components. ►

Best Practices for OINDP Pharmaceutical Development Programs Leachables and Extractables Early Safety Assessment of Potential Leachables PQRI Leachables & Extractables Working Group Douglas J. Ball, MS, DABT PQRI Training Course September 20 -21, 2006 Washington, DC

Early Evaluation Process • Form a team • • • Pharmaceutical Sciences Analytical Chemistry Toxicology Regulatory Review Drug Product Specifications • Determine and agree what are the Critical Components of the DP • Is the actuator trigger a Critical Component?

Supplier Evaluation ► Is the Supplier willing to share information? § What type of data will be shared ► Controlled extraction data ► ISO 10993/USP <87>, <88> ► MSDS ► Other toxicology data reports § May require confidentiality agreements ► Allow ► time to get agreements in place Can the Supplier provide medical grade materials § Has the Supplier filed DMFs for the materials? ► Is Supplier willing to provide DMF § Can non-medical grade material be used ► Can the Supplier provide information on prior use of material for approved DP?

Example Drug Product with Delivery System ► An already approved DP is being revamped § New/Improved delivery system § Global Registration anticipated ► DP evaluation team formed on DP configuration, 5 Critical Components have been Identified ► Based
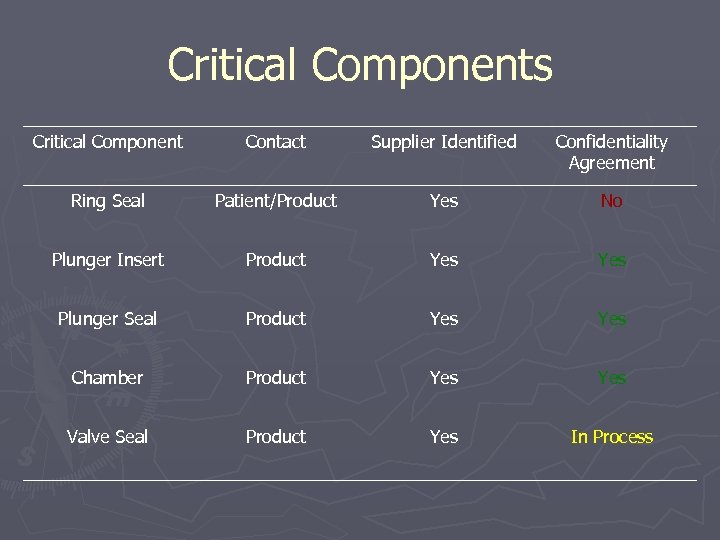
Critical Components Critical Component Contact Supplier Identified Confidentiality Agreement Ring Seal Patient/Product Yes No Plunger Insert Product Yes Plunger Seal Product Yes Chamber Product Yes Valve Seal Product Yes In Process

Ring Seal Supplier has limited experience with pharmaceutical applications ► Is hesitant to provide detailed information on material ► § Trade secret – may compromise exclusivity of material if data shared with DP manufacturer § Will not disclose ►Chemical/Physical composition ►Safety/Risk information

Ring Seal - Options ► Pharmaceutical Sciences ► Analytical Sciences ► Toxicology § Material is “optimal” and compatible with the delivery system § Pharm Sci prefers to stay with this material § Alternatives that were evaluated were not considered “optimal” § Will need to conduct a preliminary extraction study § Will have no data from supplier to compare with § Evaluate extractable profile for potential “red flags” ► PNAs, nitrosamines, MBT, etc. § Potential for extensive in silico risk assessment of extractable profile

Ring Seal – Issues/Risks ► FTE burn by Analytical Chemistry and Toxicology ► Potential exists that an unacceptable extractable is identified § May need to identify alternative material ► Potential of DP delay in development/registration

Plunger Insert ► Material is HDPE ► Chemical information supplied § Physical Properties - Yes § Composition - Yes ► Toxicology information supplied § Indirect Food Additive cross reference
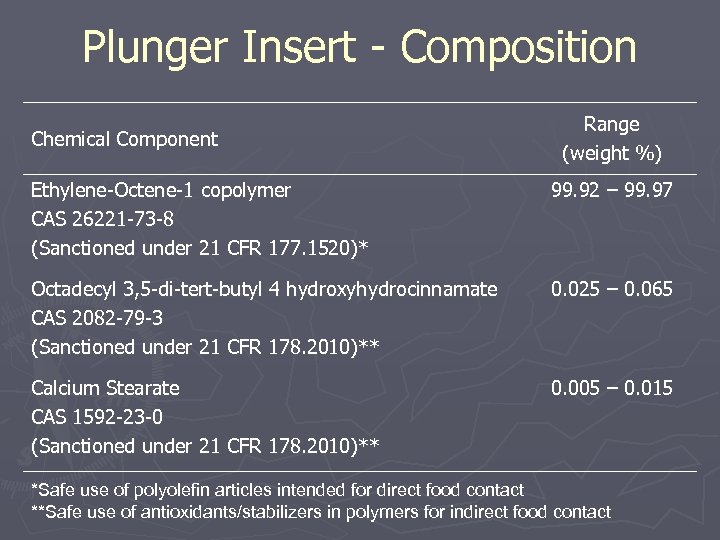
Plunger Insert - Composition Chemical Component Range (weight %) Ethylene-Octene-1 copolymer CAS 26221 -73 -8 (Sanctioned under 21 CFR 177. 1520)* 99. 92 – 99. 97 Octadecyl 3, 5 -di-tert-butyl 4 hydroxyhydrocinnamate CAS 2082 -79 -3 (Sanctioned under 21 CFR 178. 2010)** 0. 025 – 0. 065 Calcium Stearate CAS 1592 -23 -0 (Sanctioned under 21 CFR 178. 2010)** 0. 005 – 0. 015 *Safe use of polyolefin articles intended for direct food contact **Safe use of antioxidants/stabilizers in polymers for indirect food contact

Plunger Seal ► Material is PP ► Chemical information supplied § Physical Properties - No § Composition - Yes ► Toxicology § None information supplied

Plunger Seal - Compositon Chemical Component 1 -propene, polymer with ethene CAS 9010 -79 -1 Content (Weight %) 94. 48 Dimethyl succinate polymer with 4 -hydroxy-2, 2, 6, 6 -tetramethyl-1 piperidineethanol CAS 65447 -77 -0 0. 2 2, 2’-oxamido bis-[ethyl 3 -(3, 5 -di-tert-butyl-4 -hydroxyphenol)]propionate CAS 70331 -94 -1 0. 07 di(stearyl) penta-erythritol diphosphite CAS 3806 -34 -6 0. 1 synthetic hydrotalcite CAS 11097 -59 -9 0. 05 calcium stearate CAS 1592 -23 -0 0. 1 LLDPE CAS 25087 -34 -7 5

Issues ► No Toxicology data supplied § Inquire on availability of toxicology data ISO/USP test results ► MSDS ► Other ► ► Limited Chemical Profile § Additional data requested ► e. g. , antioxidants

Resolution ► Analytical Chemistry § In-house controlled extraction studies will be conducted to obtain comprehensive profile ► Toxicology § Can perform initial assessment on information supplied § May determine bad actors when additional information from controlled extraction studies are evaluated

Initial Risk Assessment (1) ► Obtain chemical structures of each extractant ► Conduct § § § SAR Analysis DEREK - Deductive Estimation of Risk from Existing Knowledge Multi. Case SAR will typically report genotoxicity, mutagenicity, carcinogenicity ► Limited value for reprotoxicology, irritation, sensitization

Risk Assessment (2) Perform Literature Search ► § § § § Agency for Toxic Substances and Disease Registry Chemical Hazards Response Information System Material Safety Datasheet Database New Jersey Hazardous Substance Fact Sheet Micromedex National Toxicology Program Testing Information National Institute of Occupational Safety and Health Registry of Toxic effects of Chemical Substances Occupational Safety and Health Technical Links to Safety and Health Topics Toxnet (National Library of Medicine Specialized Information Services) Registry of Toxic Effects of Chemical Substances Center for Drug Evaluation and Research and the Integrated Risk Information System National Institute of Environmental Health Sciences Environmental Protection Agency Integrated Risk Information System Technical Information Exchange Syste

Risk Assessment (3) ► Evaluate available inforamation § Assume worst case scenario – all extract leaches into DP ► Provide initial risk assessment to team § Identify any/all potential issues § Make recommendations on further use of material based on risk assessment profile

Conclusions ► Data from suppliers may be limited in scope § May not provide a total extract profile § May not provide significant toxicity information ► May require a preliminary extraction study § Obtain more comprehensive profile of the material ► Risk Assessment is only as good as the data that is available
9ae9c3b44375ad315c504c845ba72959.ppt