a602788315a6171a837be2692bb0f561.ppt
- Количество слайдов: 38

Bernie Betlach CLS, MT(ASCP) Laboratory Consultant / Medi-Cal HP Enterprise Services November 2010 1

Paper Claim Submission Clear and Scannable Send Only Necessary Documentation Do Not Use Highlight Markers Do Not Use Pencils Do Not Submit Handwritten Forms 2
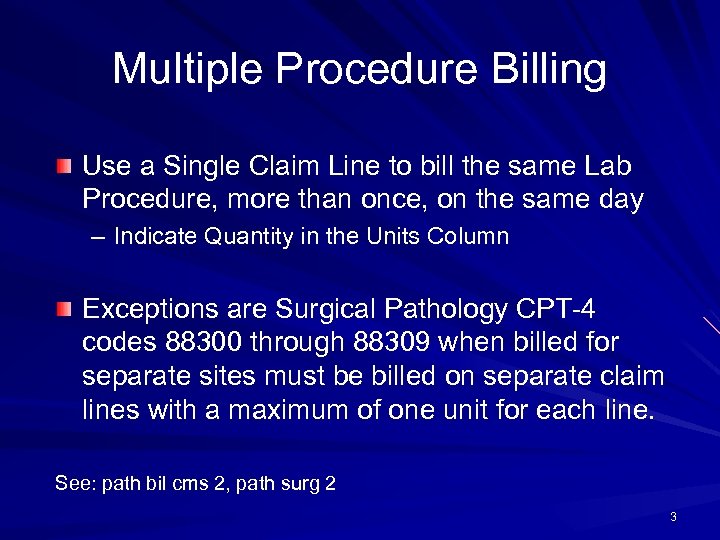
Multiple Procedure Billing Use a Single Claim Line to bill the same Lab Procedure, more than once, on the same day – Indicate Quantity in the Units Column Exceptions are Surgical Pathology CPT-4 codes 88300 through 88309 when billed for separate sites must be billed on separate claim lines with a maximum of one unit for each line. See: path bil cms 2, path surg 2 3

Claim Form Units Field Be sure to state the total number of units On Appeals submit the original claim with the total number of units – Corrected if necessary Medi-Cal will only pay what is billed See: appeal form 1 4

Maximum Reimbursement Laboratory Services are paid at the least amount of the following: – The amount billed – The charge to the general public – Medicare’s maximum allowance – Medi-Cal’s maximum allowance See: Oct. 2008, Bulletin 412 Cal. Code Regs. , tit. 22, § 51529, subd. (a)(2)(B) 5

Maximum Reimbursement (cond’t) The Department is currently asking for Data on what Laboratories are charging other Payers 6

Non Split – Billable Lab Codes Effective in the near future the Medi-Cal Lab Code Split Billing Component will be made consistent with Medicare The ZS Modifier will be eliminated This is being addressed by the “Office of HIPAA Compliance” (OHC) There is no current Timeline Providers will be notified at least 3 Months prior to implementation 7

Laboratory Reservation System Ways of exempting possible Excluded Entities are being studied These Include: – SNFs – Transplant Patients – Cancer Patients 8
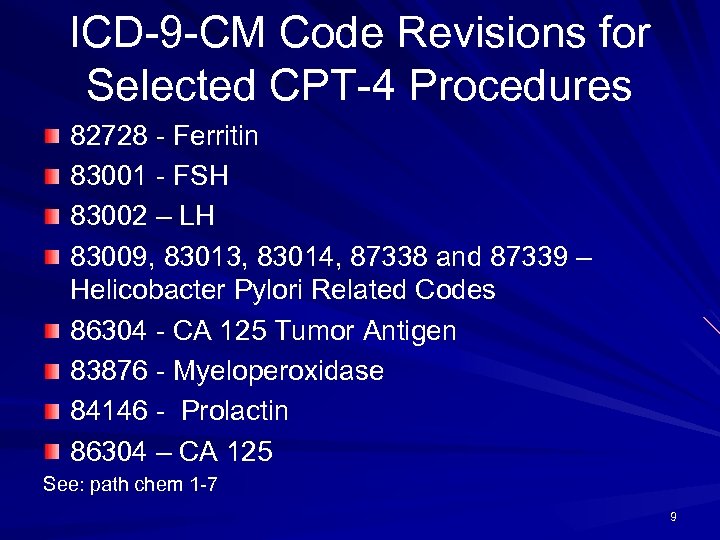
ICD-9 -CM Code Revisions for Selected CPT-4 Procedures 82728 - Ferritin 83001 - FSH 83002 – LH 83009, 83013, 83014, 87338 and 87339 – Helicobacter Pylori Related Codes 86304 - CA 125 Tumor Antigen 83876 - Myeloperoxidase 84146 - Prolactin 86304 – CA 125 See: path chem 1 -7 9

ICD-9 -CM Code Reminder Medi-Cal Claims require Diagnosis Codes to the highest Specificity as indicated in the International Classification of Diseases, 9 th Revision, Clinical Modification, 6 th Edition (ICD-9 -CM) 10

2010 CPT-4 / HCPCS Update Effective September 1, 2010 – The following Pathology and Laboratory codes have been added: G 0430, G 0431, S 3713, 83987, 86305, 86780, 86825, 86826, 87150, 87153, 87493, 88387, 88388, 89398 – The following Pathology and Laboratory codes have been deleted: 82307, 86781 See: June 2010 GM Bulletin 432 11
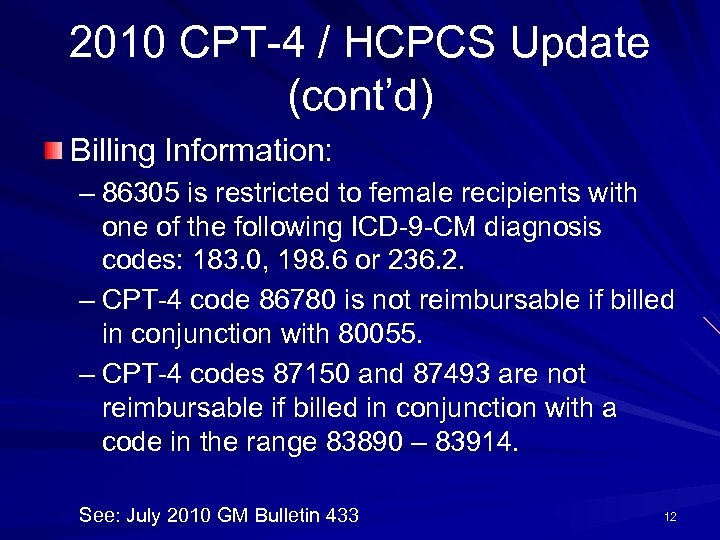
2010 CPT-4 / HCPCS Update (cont’d) Billing Information: – 86305 is restricted to female recipients with one of the following ICD-9 -CM diagnosis codes: 183. 0, 198. 6 or 236. 2. – CPT-4 code 86780 is not reimbursable if billed in conjunction with 80055. – CPT-4 codes 87150 and 87493 are not reimbursable if billed in conjunction with a code in the range 83890 – 83914. See: July 2010 GM Bulletin 433 12

2010 CPT-4 / HCPCS Update (cont’d) – CPT-4 code 89398 (unlisted reproductive medicine laboratory procedure) requires an approved TAR. – HCPCS Level II code S 3713 is reimbursable once in a lifetime and is restricted to the following diagnoses: 153. 0 – 153. 4, 153. 6 – 154. 0, 159. 0, 230. 4, 235. 2, 239. 0. See: July 2010 GM Bulletin 433 13
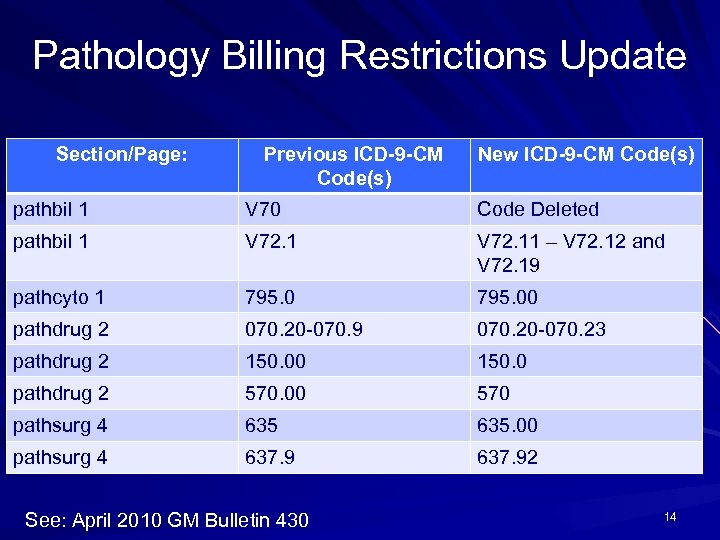
Pathology Billing Restrictions Update Section/Page: Previous ICD-9 -CM Code(s) New ICD-9 -CM Code(s) pathbil 1 V 70 Code Deleted pathbil 1 V 72. 11 – V 72. 12 and V 72. 19 pathcyto 1 795. 00 pathdrug 2 070. 20 -070. 9 070. 20 -070. 23 pathdrug 2 150. 00 150. 0 pathdrug 2 570. 00 570 pathsurg 4 635. 00 pathsurg 4 637. 92 See: April 2010 GM Bulletin 430 14
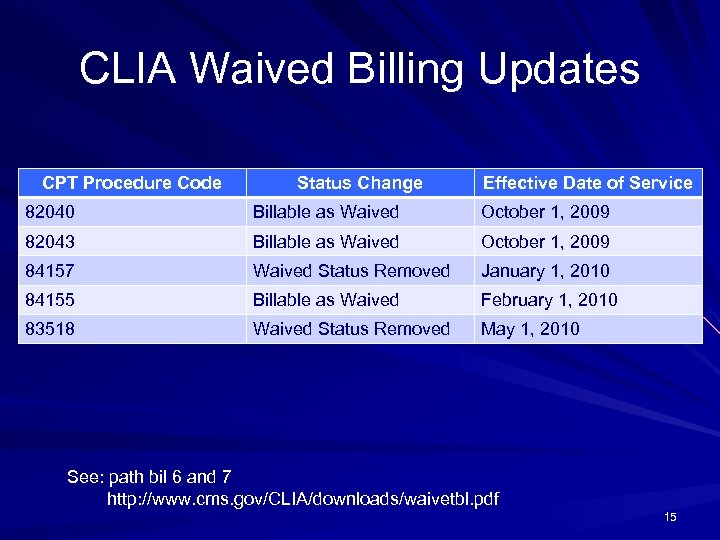
CLIA Waived Billing Updates CPT Procedure Code Status Change Effective Date of Service 82040 Billable as Waived October 1, 2009 82043 Billable as Waived October 1, 2009 84157 Waived Status Removed January 1, 2010 84155 Billable as Waived February 1, 2010 83518 Waived Status Removed May 1, 2010 See: path bil 6 and 7 http: //www. cms. gov/CLIA/downloads/waivetbl. pdf 15

Genital Wart Surgical Pathology Effective for Dates of Service on or after March 1, 2010 – For FPACT Claims – Use CPT-4 88305 (88304 will no longer be reimbursed) – Required secondary ICD-9 -CM 078. 0 078. 11 See: FPACT Manual, lab 19 16
BRCA 1 and BRCA 2 Gene Sequence Analysis Payable on or after DOS May 1, 2010: – Requires a Treatment Authorization Request (TAR) – Bill with HCPCS code S 3820 – Once In a Lifetime Procedure See: path bil 15 and 16 17

H 1 N 1 Testing Retroactive for Dates of Service on or after July 24, 2009. – H 1 N 1 RT-PCR testing is a Medi-Cal benefit Use CPT-4 87798 ICD-9 -CM 488. 1 Reimbursable for 2 units with modifier 59 See: path immun 1 18

HIV Drug Resistance Testing Effective for Dates of Service on or after September 1, 2010 Will no longer require Medical Justification CPT-4 Codes affected: – 87901 – 87903 – 87904 See: path micro 8 - 10 19
Trofile Testing Initial Testing – Reimbursement is made with Documentation According to the guidelines of The Department of Health and Human Services Use of a CCR 5 Inhibitor is Being Considered or Patient Exhibits Virologic Failure on a CCR 5 Inhibitor See: path micro 10 20

Trofile Testing (cond’t) Subsequent Testing – Requires a TAR All claims must be accompanied by a Report Bill with CPT-4 87999 See: path micro 11 21

Compatibility Testing CPT-4 codes 86920, 86921, 86922 and 86923 are all “By Report” codes – Crossmatch documentation must be submitted with the claim in order to be reimbursed See: path chem 7 22

Cystic Fibrosis Reimbursement Issues with Underpayment and Overpayment problems are being researched Corrections will be forthcoming 23

Fiscal Intermediary (FI) Transition Will be Complete in June 2011 Will Appear Seamless to Providers 24

National Correct Coding Initiative (NCCI) Is owned by CMS Automated System of Edits Controls specific Code Pairs that cannot be reported on the same day Legislation has Mandated Implementation by all Medicaids Implementation set by CMS for Medicaids was October 1, 2010 25

NCCI and Medi-Cal has received a Waiver for the October 1, 2010 Implementation Date Many Edits have been implemented Look for Medi-Cal implementation late 2011 26

ICD-10 Two components – ICD-10 -CM (Clinical Modification) Replaces ICD-9 -CM Volumes 1 and 2 For all US healthcare treatment settings – ICD-10 -PCS (Procedural Classification System) Replaces ICD-9 -CM Volume 3 For reporting Hospital Inpatient procedures only Is not Applicable to Clinical Laboratories 27

ICD-10 Has been the Standard In other countries for up to 15 Years Offers significant Improvements through greater Information Specificity Expandable to capture Advancements in Medicine Includes updated Terminology and Classification of Diseases Provides more Extensive Data 28

ICD-10 -CM (cont’d) Increased Information for Public Health, Bio-surveillance, Quality Measurement ICD-9 -CM running out of Codes CPT-4 remains the procedure coding standard regardless of whether the services were provided in the inpatient or outpatient setting. 29

ICD-9 -CM vs ICD-10 -CM ICD-9 -CM 5 Position (one alpha) 13, 000 Possible Codes ICD-10 -CM 7 Position (all alphanumeric) 68, 000 Possible Codes No clear mapping between the Two – General Equivalence Tables (GEMS) from The National Center for Health Statistics (NCHS) 30

ASX X 12 N Version 5010 Is an Electronic Transaction Standard Is Required to accommodate ICD-10 -CM Corrects Many of the Flaws of the Current 4010 A 1 Version Increases the Field Size for ICD Codes from 5 bytes to 7 bytes 31

5010 (cond’t) Adds a Version Indicator to the ICD Code to Indicate Version 9 or 10 Increases the Number of Diagnosis Codes Allowed on a Claim Includes Data Modifications in the Standards Adopted by CMS 32

5010 / ICD-10 -CM & Medi-Cal Both are Enhancements Required in the New FI Contract Mandated Compliance Dates: – All Claims Must Compliant with 5010 on January 1, 2012 – All Claims Must Use ICD-10 -CM Diagnosis Codes on October 1, 2013 – Medi-Cal Will Not be Directly Accepting 5010 Formatted Claims until January 1, 2012 33

5010 / ICD-10 -CM & Medi-Cal (cont’d) Crossover Claims will be Converted by Group Health Incorporated (GHI) to the 4010 A 1 Format before they Reach Medi. Cal Implementation is Expected to allow Providers as Much Testing Time as Possible. 34

5010 / ICD-10 -CM & Medi-Cal Questions should be directed to the Telephone Service Center (1 -800 -5415555) For Updates Watch the Medi-Cal Web Site and the Medi-Cal Updates 35

Electronic Health Records (EHR) Efforts to Standardize Transport of Health Data Including Laboratory Results at the National and State Levels is ongoing Two Approaches: – Standard Format Transmitted from Laboratories – Network Transforms Data into Standardized Format Ref: CAe. Health. org 36

Bernie Betlach CLS, MT(ASCP) Medical Lab Consultant HP Enterprise Services 3215 Prospect Park Drive Rancho Cordova, CA 95670 916 -636 -1025 bernie. betlach@hp. com 37

38
a602788315a6171a837be2692bb0f561.ppt