9b5304325624d8ba5e28bf1ebc358472.ppt
- Количество слайдов: 22

Basic Terms Matter • Anything that has mass and occupies space (volume) Properties • Characteristics that distinguish one thing from another Physical Properties • Characteristics of matter that DO NOT involve chemical changes • Examples include: size, shape, color, & texture (sorting and separating)
Terms cont. Chemical properties • Characteristics of matter that DO involve chemical changes Example: BURNING

Physical or Chemical a. Wood burning: Chemical 1. b. plastic can be molded easily into different shapes: Physical c. Oil being used as a lubricant to reduce friction: Physical 1. d. corn being eaten for nutritious reasons: Chemical 1. e. aluminum is used b/c it’s lightweight: Physical

1. f. nickel coating is used b/c it won’t rust: Chemical 1. g. wool is used in clothing b/c it traps heat in: Physical 1. h. coal is used as a fuel: Chemical

States of Matter q There are 3 states of matter: SOLID LIQUID GAS
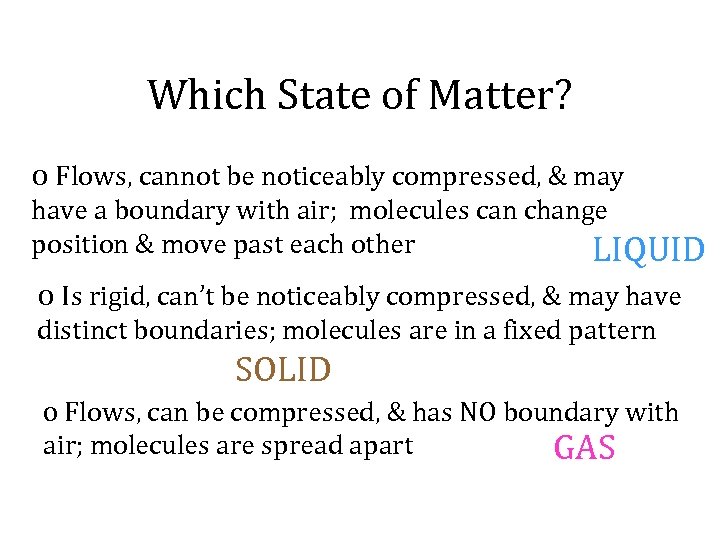
Which State of Matter? o Flows, cannot be noticeably compressed, & may have a boundary with air; molecules can change position & move past each other LIQUID o Is rigid, can’t be noticeably compressed, & may have distinct boundaries; molecules are in a fixed pattern SOLID o Flows, can be compressed, & has NO boundary with air; molecules are spread apart GAS

§ Name 3 things that are solids: ICE, GLASS, ROCK § Name 3 things that are liquids: WATER, GASOLINE, MILK § Name 3 things that are gases: NITROGEN, AIR, HELIUM Ø INTERFACE: Boundary between 2 DIFFERENT substances

A 4 th State of Matter? ? PLASMA is the 4 th state v 2 examples of matter found in this 4 th state: STARS, PLASMA SCREEN TV
Changes of State Physical Change • change in matter that DOES NOT change the identity of matter § Examples: Cutting, grinding, boiling, melting, freezing, etc. Chemical Change § change in matter in which one substance is changed into another § Examples: Rusting, burning

Signs of a Chemical Change ü Color change ü heat given off ü precipitate produced ü Gas (bubbles) produced

Solid to liquid: Melting & liquefaction Liquid to Solid: Freezing & solidification Liquid to Gas: Evaporation, boiling, vaporization

Gas to Liquid: Condensation & liquefaction Solid to Gas: Sublimation & Vaporization Gas to Solid: Solidification, sublimation, & crystallization
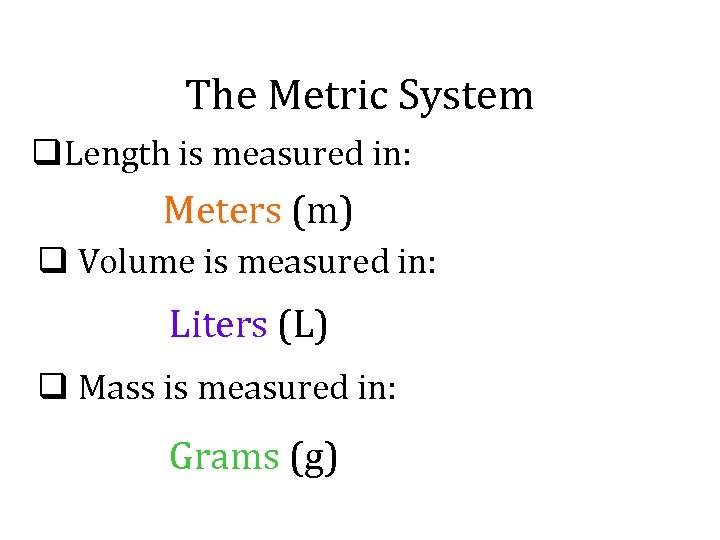
The Metric System q. Length is measured in: Meters (m) q Volume is measured in: Liters (L) q Mass is measured in: Grams (g)
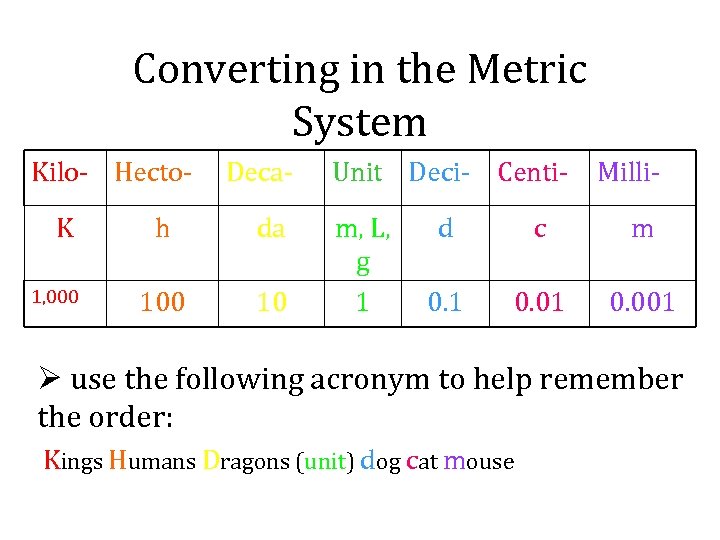
Converting in the Metric System Kilo- Hecto. K 1, 000 Deca- h da 100 10 Unit Deci- Centim, L, g 1 Milli- d c m 0. 1 0. 001 Ø use the following acronym to help remember the order: Kings Humans Dragons (unit) dog cat mouse
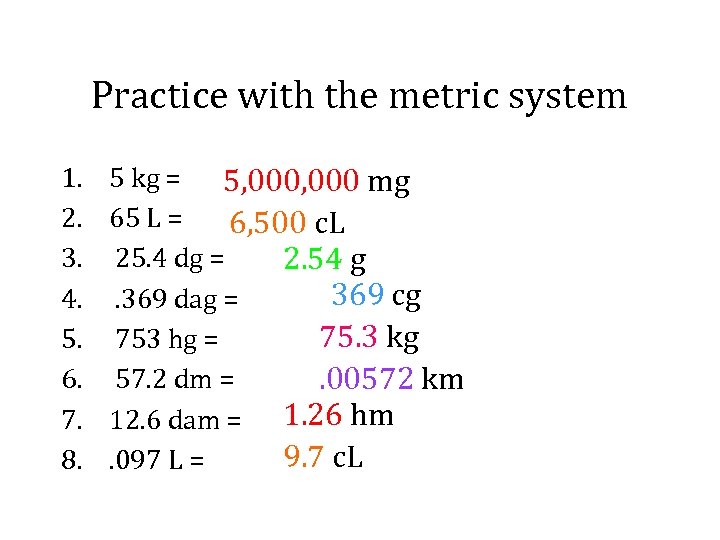
Practice with the metric system 1. 2. 3. 4. 5. 6. 7. 8. 5 kg = 5, 000 mg 65 L = 6, 500 c. L 25. 4 dg = 2. 54 g 369 cg. 369 dag = 75. 3 kg 753 hg = 57. 2 dm =. 00572 km 12. 6 dam = 1. 26 hm 9. 7 c. L. 097 L =
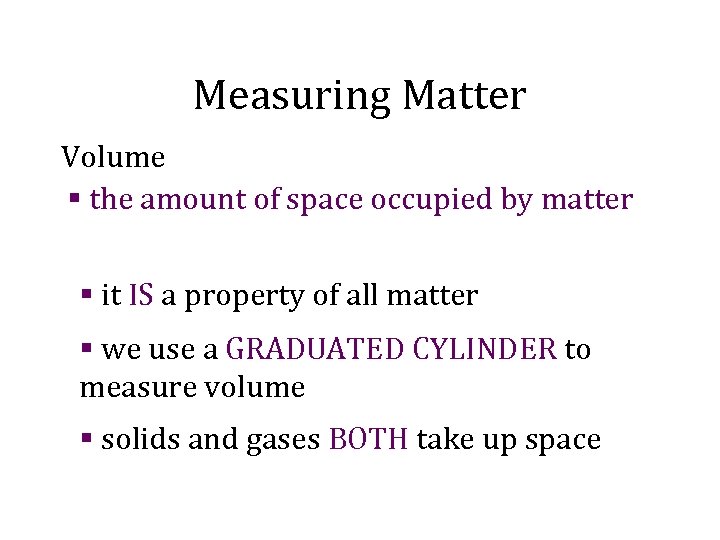
Measuring Matter Volume § the amount of space occupied by matter § it IS a property of all matter § we use a GRADUATED CYLINDER to measure volume § solids and gases BOTH take up space

The Displacement Method ü used when determining volume of irregularly shaped objects ü it works by: submerging a solid in water & measure the amount of water that the solid displaces Do you think you can use this for substances that dissolve in water? NO

Volume True or False a. No 2 objects can occupy the same space at the same time b. A piece of modeling clay has a larger volume when it’s rolled flat than when it’s rolled in a ball c. The volume of sugar can be determined by the displacement method TRUE FALSE e. FALSE a liter An appropriate unit for measuring shampoo is (1000 m. L) f. Gases do not occupy space g. A solid object with a volume of 1 L will displace 1000 m. L of water h. It is more appropriate to buy gasoline by the milliliter than by the liter d. One liter is exactly 500 m. L FALSE TRUE FALSE F

MASS § A measure of the amount of matter in an object § We use a TRIPLE BEAM BALANCE to measure mass § Arrange the following masses from largest to smallest: 18 g, 2 dag, 0. 5 hg, 1000 mg Converting to grams: 18 g = 18 g, 2 dag = 20 g, 0. 5 hg = 50 g, and 1000 mg = 1 g So, in order: 0. 5 hg, 2 dag, 18 g, 1000 mg

Would it be reasonable to measure the mass of an elephant in milligrams? NO WHICH UNIT? a. To measure the distance to China km b. To measure the width of the MS River m c. To measure the volume of a penny m. L

DENSITY • A measurable physical property that can be found by dividing the mass of an object by its volume
9b5304325624d8ba5e28bf1ebc358472.ppt