8ea6a417b238ef08ecba3a800f10f95d.ppt
- Количество слайдов: 53

BASIC PRINCIPLES OF ADAPTIVE IMMUNITY AND IMMUNIZATION CHAPTER 17 Copyright © 2012 John Wiley & Sons, Inc. All rights reserved.

CHAPTER 17 IMMUNOLOGY I: BASIC PRINCIPLES OF ADAPTIVE IMMUNITY AND IMMUNIZATION Immunology and immunity- Immunology is the study of specific immunity and how the immune system responds to specific infectious agents. The immune system consists of various cells, especially lymphocytes and organs such as the thymus that help provide the host with specific immunity to infectious agents.

Types of Immunity Innate immunity- One kind of innate immunity is species immunity, which is common to all members of a species. For example, all humans have immunity to many infectious agents that cause disease in pets and domestic animals, and animals have similar immunity to some human diseases. -determined by genetic and physiological factors is always present and is for a lifetime Adaptive immunity -immunity obtained in some manner other than by heredity. Adaptive immunity. Naturally acquired adaptive immunity- is most often obtained through having a specific disease and is mediated by antigens (disease agent) and antibodies (proteins made by the body to specifically interact with that agent). Typically antibodies form 5 to 14 days following exposure to antigen. Depending on the antigen can last from months to a lifetime Artificially acquired adaptive immunity- produced in response to being vaccinated. Passive immunity- is created when ready-made antibodies are introduced into the body. Typically lasts from days to weeks. Naturally acquired passive immunity- mothers milk-colostrum Artificially acquired passive immunity- antibodies made by other hosts are introduced into a new host (anti-venom, gamma-globulin, tetanus-antibodies)

Panton-Valentine Leukocidin is an important virulence factor for methicillin resistant staphylococcus A. True B. False

How to Develop New Antibiotics THE bacteria are winning. Every year, according to the Centers for Disease Control and Prevention, at least two million people are infected with bacteria that can’t be wiped out with antibiotics, and as a result, 23, 000 people die. Direct health care costs from these illnesses are estimated to be as high as $20 billion annually. Just last week, the U. C. L. A. Health System announced that nearly 180 patients may have been exposed to the CRE superbug that was linked to two deaths in one of its hospitals. Today, 30 percent of severe strep pneumonia infections are resistant to multiple drugs and 30 percent of gonorrhea infections are resistant to all antibiotics. And drug-resistant enterobacteriaceae, enterococcus, acinetobacter and a slew of other unpronounceable bacteria pose serious threats. The development of antibiotics has been glacial. We need a completely new approach. he number of F. D. A. approved antibiotics has decreased steadily in the past two decades. The big pharmaceutical companies have largely stopped work on these drugs. Pfizer, long the leader in developing antibiotics, closed its antibiotic research operations in 2011. Smaller biotech companies now account for 80 percent of antibiotic development. There are now about 40 new antibiotics in development. That might sound promising — but not when compared with the 771 new drugs and vaccines in clinical trials or awaiting F. D. A. review for cancer. And most of these antibiotics are unlikely to come out of the testing process as F. D. A. approved drugs. The big problem is profitability. Unlike drugs for cholesterol or high blood pressure, or insulin for diabetes, which are taken every day for life, antibiotics tend to be given for a short time, a week or at most a few months. So profits have to be made on brief usage. Furthermore, any new antibiotics that might be developed to fight these drug-resistant bacteria are likely to be used very sparingly under highly controlled circumstances, to slow the development of resistant bacteria and extend their usefulness. This also limits the amount that can be sold. Because it costs at least $1 billion to develop a new drug, the prize money could provide a 100 percent return — even before sales. From the government perspective, such a prize would be highly efficient: no payment for research that fizzles. Researchers win only with an approved product. Even if they generated just one new antibiotic class per year, the $2 -billion-per-year payment would be a reasonable investment for a problem that costs the health care system $20 billion per year.

Whole virus vaccine for Ebola found to effectively protect monkeys The vaccine, detailed in the journal Science, was developed using a novel experimental platform at the University of Wisconsin. Madison that allowed the researchers to safely study the virus in laboratory conditions. A group of scientists have developed a whole Ebola virus vaccine that can successfully protect monkeys from the virus and is capable of preparing the immune system with the full range of viral proteins and genes. "In terms of efficacy, this affords excellent protection, " says Prof. Yoshihiro Kawaoka, a professor of pathobiological sciences at the University of Wisconsin-Madison School of Veterinary Medicine. "It is also a very safe vaccine. " Whole virus vaccines have been used in the past to prevent other serious diseases, including hepatitis, human papillomavirus-mediated cervical cancer, influenza and polio. Using inactivated whole viruses provides the immune system with the complete range of viral proteins and genes, improving the likelihood of the virus triggering a strong immune response. Earlier attempts to develop an inactivated whole Ebola virus vaccine, using irradiation and the preservative formalin, were ultimately unsuccessful and failed to protect monkeys from the virus. As a result, these attempts were abandoned. Developing a new experimental platform from which to work with the virus has helped these researchers to be successful this time round. Devised in 2008, the new system enabled the team to work safely with Ebola virus by deleting a key gene called VP 30 which allows the virus to make a protein required for it to reproduce. Using monkey kidney cells engineered by the researchers to express VP 30, the team could safely use the virus as a starting point for the development of treatment for it. The whole virus vaccine devised by Kawaoka and his team was also chemically inactivated with hydrogen peroxide. While the researchers have overcome the problems experienced by previous attempts at developing a whole virus vaccine, it will still be some time before the vaccine is ready to be rolled out. Human trials need to be conducted, and these are both complex and highly expensive.

Obama Seeks to Double Funding to Fight Antibiotic Resistance. WASHINGTON — President Obama on Friday urged Congress to double the funding to confront the danger of antibiotic-resistant bacteria, calling it a major public health issue that, if left unchecked, would “cause tens of thousands of deaths, millions of illnesses. ” The administration also issued a new plan for attacking the problem, part of a national strategy that Mr. Obama laid out in an executive order in September. The plan calls for improved surveillance of outbreaks, better diagnostic tests and new research on alternative drugs. It also urges government agencies to bolster systems to track the consumption of antibiotics and to reduce inappropriate use in people and animals. The new plan’s strength, experts said, is its actions to curb use in humans. It calls on federal agencies like the Centers for Disease Control and Prevention to create a tougher surveillance system to monitor the use of antibiotics in hospitals and other medical settings. It includes specific steps that hospitals participating in Medicaid and Medicare must take to reduce inappropriate use. “The news here is that the administration is setting specific, annual milestones for tackling the problem, ” said Allan Coukell, the senior director for health programs at the Pew Charitable Trusts, a Washington-based research and advocacy group that contributed to the administration strategy outlined in September. Americans use more antibiotics than people in other industrialized nations, with rates more than twice those in Germany and the Netherlands, according to Pew. The White House said the president wanted to double the amount of federal funding for combating and preventing antibiotic resistance to more than $1. 2 billion. The plan calls for “enhanced summary reports on the sale of distribution and antibiotics” used in food
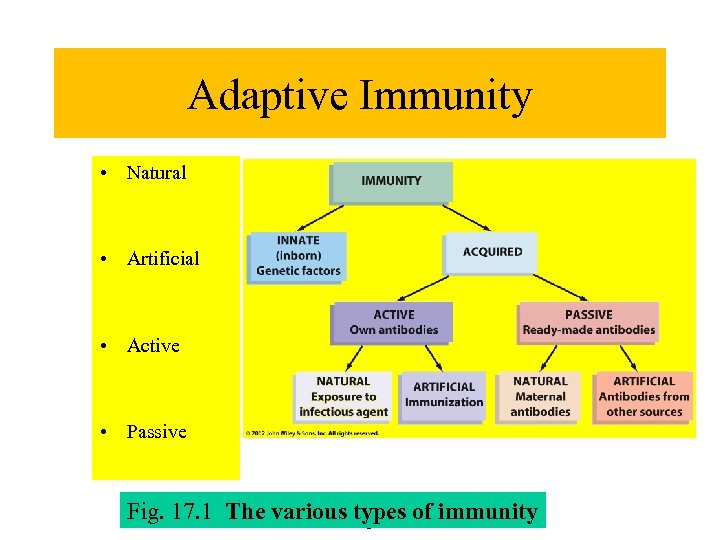
Adaptive Immunity • Natural • Artificial • Active • Passive 2012 John Fig. 17. 1 The Copyright ©Alltypes. Wileyimmunity various rights reserved. & of Sons, Inc.

Antibodies bind to specific chemical groups or structures termed epitopes or antigenic determinants. A single protein may have many epitopes. H-antigen O antigen Gram-negative bacterium with several different antigenic determinants Fig. 17. 2 A typical antigen-antibody reaction

Antigens and Antibodies. Antigen- An antigen is a substance the body recognizes as foreign and against which it mounts an immune response. Most antigens are proteins greater than 10, 000 MW. Some antigens are polysaccharides, and a few are glycoproteins or nucleoproteins Epitopes- large complex proteins can have several antigenic determinants (places where the polyclonal antibody binds). Haptene- haptene can react with an antibody but by itself cannot illicit the production of an antibody (typically because it is too low molecular weight). Antibody- a protein produced in response to an antigen that is capable of binding specifically to the antigen.
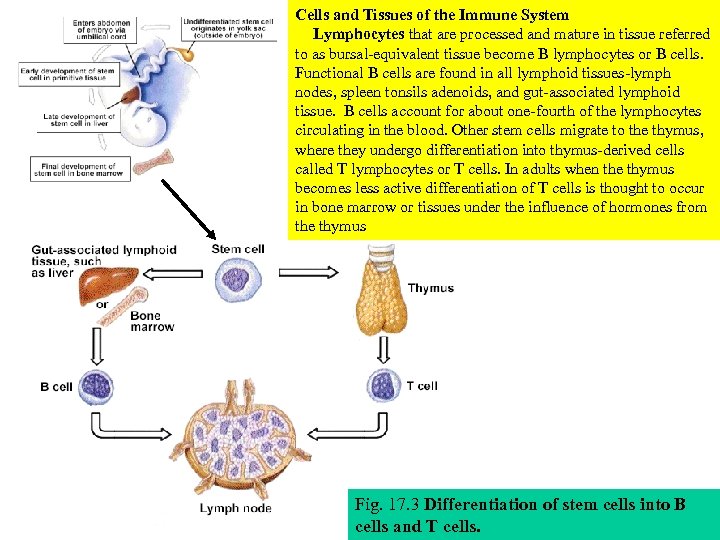
Cells and Tissues of the Immune System Lymphocytes that are processed and mature in tissue referred to as bursal-equivalent tissue become B lymphocytes or B cells. Functional B cells are found in all lymphoid tissues-lymph nodes, spleen tonsils adenoids, and gut-associated lymphoid tissue. B cells account for about one-fourth of the lymphocytes circulating in the blood. Other stem cells migrate to the thymus, where they undergo differentiation into thymus-derived cells called T lymphocytes or T cells. In adults when the thymus becomes less active differentiation of T cells is thought to occur in bone marrow or tissues under the influence of hormones from the thymus Fig. 17. 3 Differentiation of stem cells into B cells and T cells.
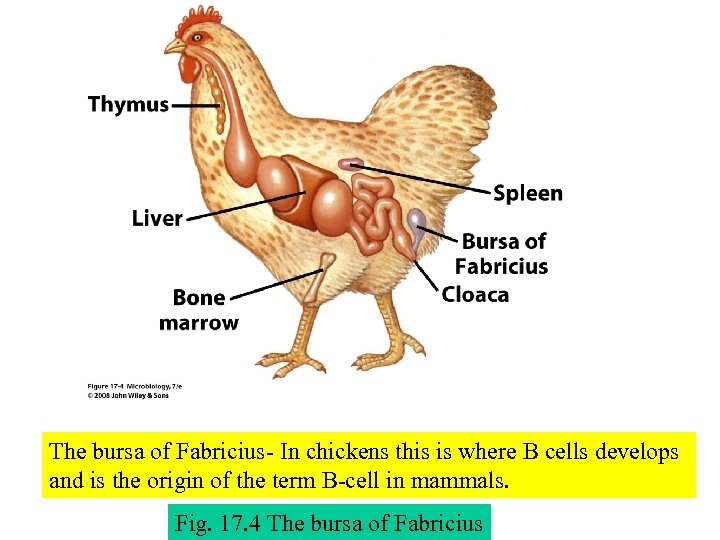
The bursa of Fabricius- In chickens this is where B cells develops and is the origin of the term B-cell in mammals. Fig. 17. 4 The bursa of Fabricius
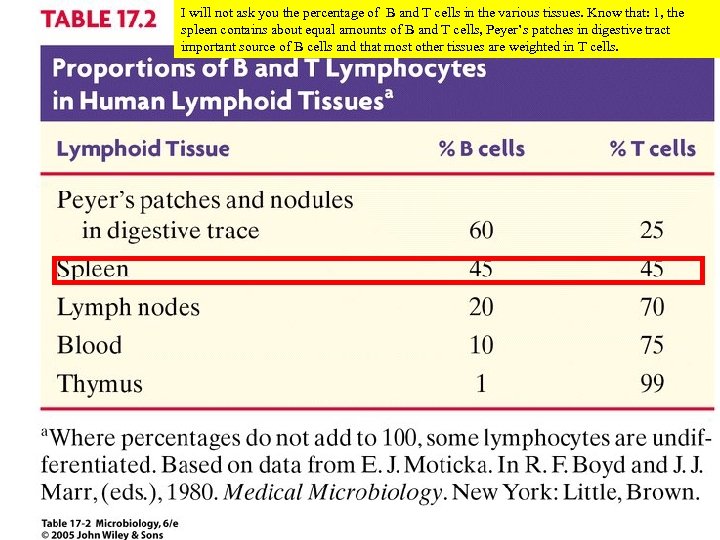
I will not ask you the percentage of B and T cells in the various tissues. Know that: 1, the spleen contains about equal amounts of B and T cells, Peyer’s patches in digestive tract important source of B cells and that most other tissues are weighted in T cells.

Dual Nature of the Immune System Humoral immunity- carried out by antibodies, produced by B cells, circulating in the serum. Humoral immunity is most effective in defending the body against bacterial toxins, bacteria, and viruses before they enter cells. Cell-mediated immunity is carried out by T cells. It is most effective in clearing the body of virus-infected cells, but also in defending against fungi and parasites, cancer and foreign tissues such as transplanted organs.

Recognition and self versus non-self. How the body recognizes an antigen How the body distinguishes between a foreign antigen and the body's own antigens

Questions? email the course director: Dr. Sarah D’Orazio (sarah. dorazio@uky. edu) NEW COURSE OFFERING FOR SPRING 2015: MI 495 -G/BIO 495 -G Bacterial Pathogenesis Course topics: u Defining virulence: what is a bacterial pathogen? u Overview of innate and adaptive immune mechanisms u vitro and in vivo approaches to measure infectivity & virulence In u Mechanisms for extracellular survival in the host u Adherence, motility & biofilms u Bacterial toxins and secretion systems u Intracellular bacteria: life in the cytosol vs. escape from the vacuole u How bacteria become resistant to antibiotics u Opportunistic infections u Bacteria as bioweapons Tues. /Thurs. 12: 30 – 1: 45 PM Nursing Bldg. Rm 213 3 credits course prerequisites: BIO 308 (BIO 208 with permission) & BIO 315 BCH 401 recommended
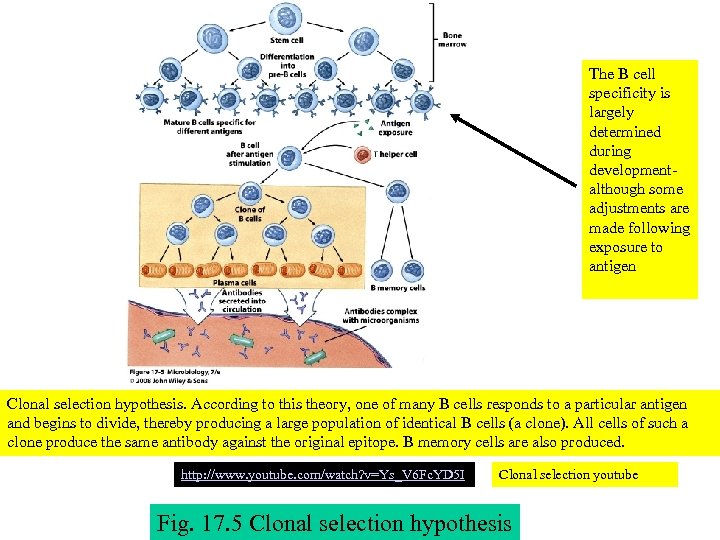
The B cell specificity is largely determined during developmentalthough some adjustments are made following exposure to antigen Clonal selection hypothesis. According to this theory, one of many B cells responds to a particular antigen and begins to divide, thereby producing a large population of identical B cells (a clone). All cells of such a clone produce the same antibody against the original epitope. B memory cells are also produced. http: //www. youtube. com/watch? v=Ys_V 6 Fc. YD 5 I Clonal selection youtube Fig. 17. 5 Clonal selection hypothesis
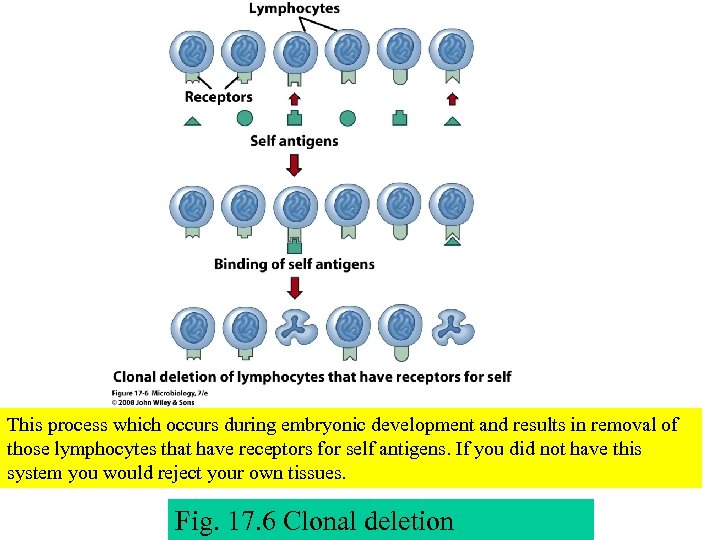
This process which occurs during embryonic development and results in removal of those lymphocytes that have receptors for self antigens. If you did not have this system you would reject your own tissues. Fig. 17. 6 Clonal deletion

Humoral Immunity (antibody production). In a typical immune response, helper T cells facilitate growth and differentiation of plasma cells -which produce antibody (humoral immunity). After about a week this reaction reaches a peak and then subsides, largely because suppressor T cells inhibit further antibody production.

I am a low molecular weight molecule. I cannot by myself illicit the production of antibody. However, if antibody is produced when I am linked to a protein I can react with that antibody. I am a haptene A. True B. False

Enterovirus 68 May Be Linked to Paralysis in Children, Study Says A new strain of a common respiratory virus may be responsible for partly paralyzing scores of children nationwide, researchers reported on Monday. Researchers at the University of California, San Francisco, analyzed genetic sequences of enterovirus 68 cultured from 25 children in Colorado and California with limb paralysis, also called acute flaccid myelitis. The researchers concluded that the viruses were a novel strain of enterovirus 68, which they called B 1. Using a method called “molecular clock analysis, ” the team estimated that the B 1 strain emerged four and half years ago. On balance, he said, that strengthens the case that the B 1 strain of enterovirus — detected in roughly half of the children’s nasal secretions — was linked to their paralysis. One sibling pair — a school-age girl and her younger brother — were both infected with identical B 1 strains of enterovirus 68 and got colds, Dr. Chiu and his colleagues found. The girl suffered paralysis in both arms and her trunk. Her brother experienced no lasting effects. Enterovirus 68 may be a contributor to the children’s paralysis, said Priya Duggal, the director of the genetic epidemiology program at Johns Hopkins Bloomberg School of Public Health, who had nothing to do with the study. “But it must not be acting alone, because children with the same virus and siblings with the same clade have different outcomes. ”

Fecal transplantation for patients with Clostridium difficile infection may be a more effective treatment strategy than previously thought, according to a new study. , , the study reveals that fecal transplantation makes long-term healthy changes to the gut bacteria of patients infected with C. difficile - a finding they say could have important regulatory implications for the procedure. C. difficile infections are a major health concern in the US. According to the Centers for Disease Control and Prevention (CDC), the bacterium caused around half a million infections in 2011 and killed around 29, 000 people within 30 days of diagnosis. While many C. difficile infections can be treated with antibiotics, the infection can keep coming back for some patients. In these cases, fecal microbiota transplantation (FMT) may be recommended. The researchers found that the gut bacteria of patients who underwent FMT was normalized shortly after the procedure. They were surprised to find, however, that while the composition of patients' gut bacteria changed following FMT, it remained healthy for up to 21 weeks.
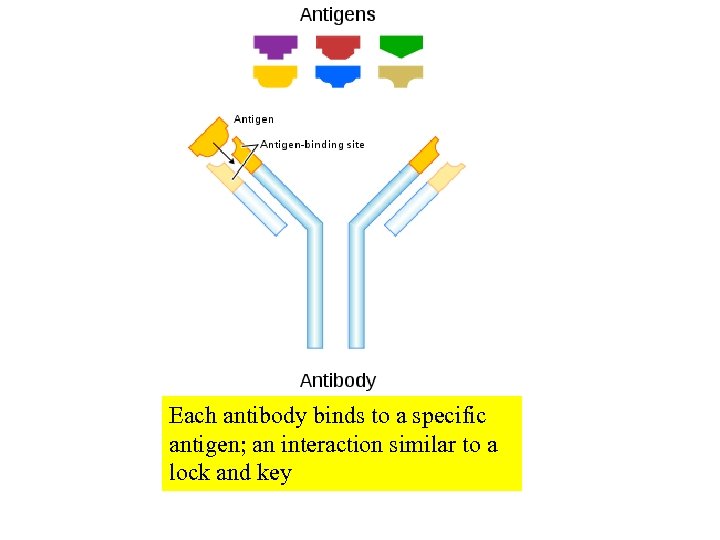
Each antibody binds to a specific antigen; an interaction similar to a lock and key

antibody binding site Variable regiondetermines Ab specificity Constant region determines the particular class that an immunoglobuli n belongs to Antibodies or immunoglobulins (Ig), are Y-shaped protein molecules composed of four polypeptide chains- two identical light (L) chains and two identical heavy (H) chains. The chemical structure of the constant region determines the particular class that an immunoglobulin belongs to, . The variable regions of each chain have a particular shape and charge enable the molecule to bind a particular antigen, i. e. , the variable region is what confers the specificity to the antibody. Fig. 17. 7 Antibody structure
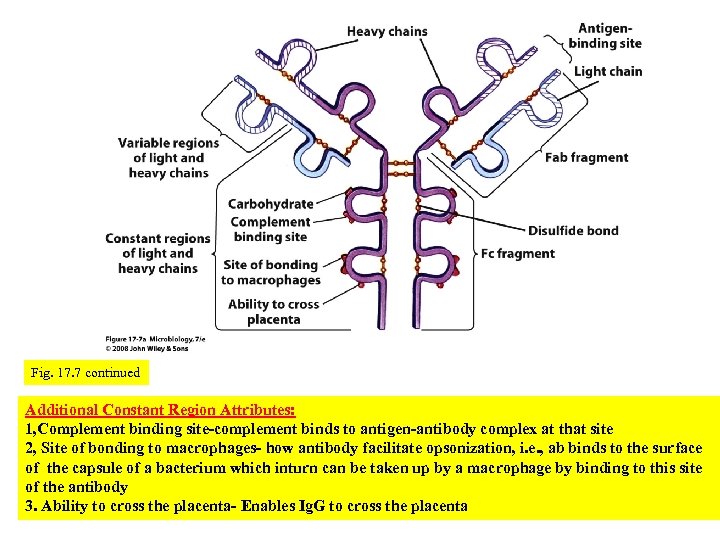
Fig. 17. 7 continued Additional Constant Region Attributes: 1, Complement binding site-complement binds to antigen-antibody complex at that site 2, Site of bonding to macrophages- how antibody facilitate opsonization, i. e. , ab binds to the surface of the capsule of a bacterium which inturn can be taken up by a macrophage by binding to this site of the antibody 3. Ability to cross the placenta- Enables Ig. G to cross the placenta
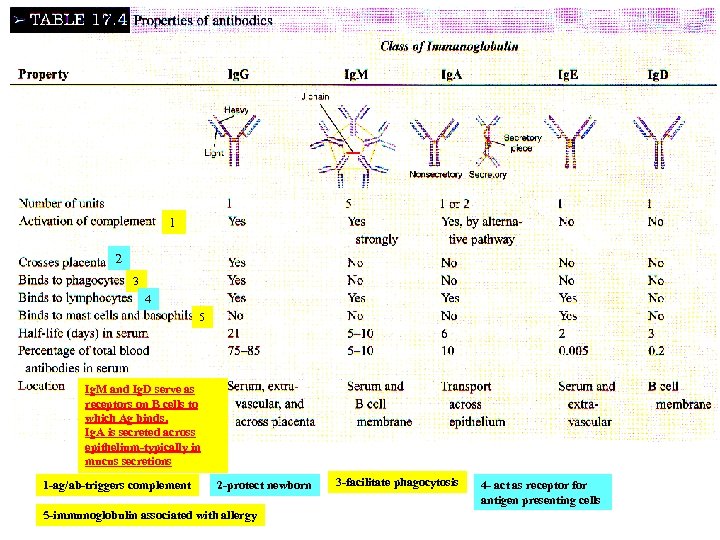
1 2 3 4 5 Ig. M and Ig. D serve as receptors on B cells to which Ag binds. Ig. A is secreted across epithelium-typically in mucus secretions 1 -ag/ab-triggers complement 2 -protect newborn 5 -immunoglobulin associated with allergy 3 -facilitate phagocytosis 4 - act as receptor for antigen presenting cells

Secretory piece associated with Ig. A Confers the unique ability of that antibody to traverse the epithelial layer and be in secretions (mucus) Fig. 17. 8 The structures of the different classes of antibodies

Points of this slide: 1. induction period 2. 3. B cells initially produce Ig. M in higher quantity than Ig. G The peak of Ig. G then follows that of Ig. M (there may be switching from production of Ig. M to production of Ig. G by cytokines) Following second exposure to the same antigen the level of Ig. M roughly the same as the first exposure, however, the level of Ig. G is greatly enhanced because it is generated from a clone of memory cells. There are no memory cells for Ig. M This figure shows the correlation of antibody concentrations with the activities of B cells. Cytokines trigger the class switching from Ig. M to Ig. G Fig. 17. 9 Primary and secondary responses to antigen

T-independent antigens only produce Ig. M hence, there is no “memory” response. Fig. 17. 9 Primary and secondary responses to an antigen
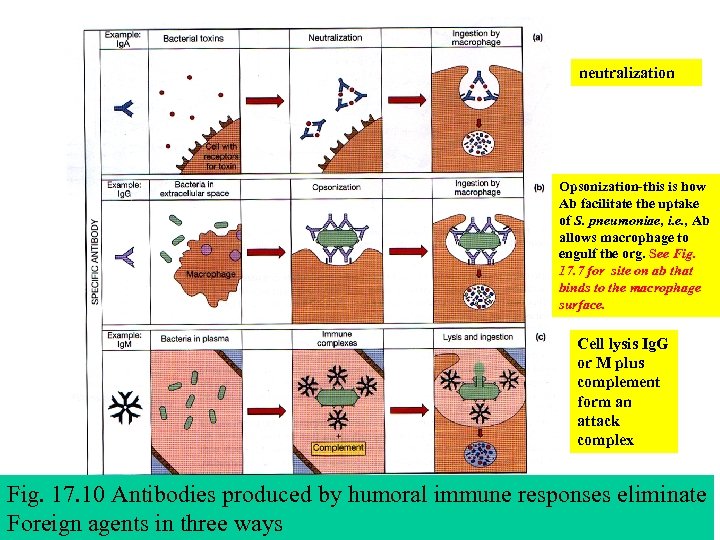
neutralization Opsonization-this is how Ab facilitate the uptake of S. pneumoniae, i. e. , Ab allows macrophage to engulf the org. See Fig. 17. 7 for site on ab that binds to the macrophage surface. Cell lysis Ig. G or M plus complement form an attack complex Fig. 17. 10 Antibodies produced by humoral immune responses eliminate Foreign agents in three ways

lymphoid Fig. 17. 11 Summary of humoral immunity

Figure 17 -10 - Antibodies produced by humoral immune responses eliminate foreign agents in three ways. 1) neutralization of pathogens and toxins by Ig. A or Ig. G 2) opsonization of bacteria by Ig. G and 3) cell lysis initiated by Ig. M or Ig. G immune complexes allows for the formation of membrane attack complexes involving complement proteins.

Below are the numbers of Unassigned Devices 131959 23294 D 57 E 484 595143 5 c 7 B 7 A 7 F 57 F 82 C 91 90 CA 7

T-independent antigens only trigger the production of Ig. G A. True B. False

Growth of global antibiotic use for livestock raises concerns about drug resistance A new study predicts the next 15 years will see a startling increase in worldwide use of antibiotics in livestock, raising serious concerns about the effect this will have on a growing global health problem - drug-resistant pathogens or superbugs. Antibiotics are used widely in the farming of food animals to treat disease and increase productivity. In the US, antibiotic consumption in animals accounts for up to 80% of antibiotic sales. While studies suggest such practice fuels the spread of drug-resistant pathogens in animals and humans, the lack of reliable global data makes it hard to both measure the size of the problem and come up with solutions. n the Proceedings of the National Academy of Sciences, they present their findings in the form of a global map of antibiotic use in livestock, covering a total of 228 countries. Senior study author Dr. Ramanan Laxminarayan, a senior research scholar at the Princeton Environmental Institute at Princeton University, NJ, says: "The invention of antibiotics was a major public health revolution of the 20 th century. Their effectiveness - and the lives of millions of people around the world - are now in danger due to the increasing global problem of antibiotic resistance, which is being driven by antibiotic consumption. "The researchers estimate "conservatively" that the total global consumption of antibiotics by livestock in 2010 was 63, 151 tons, and that by 2030, this figure will be 67% larger overall. They suggest most of the growth (66%) will be due to increases in the number of animals raised for food driven mostly by rising demand in middle-income countries, and partly (34%) due to a shift toward largescale, intensive or "factory" farming where antibiotics are used routinely. In Brazil, China, India, Russia and South Africa the increase will be dramatic, mostly because of these two factors. These five countries will see a 99% increase in antibiotic consumption but only a 13% growth in their human populations over the same period. "Antibiotic resistance is a dangerous and growing global public health threat that isn't showing any signs of slowing down. Our findings advance our understanding of the consequences of the rampant growth of livestock antibiotic use and its effects on human health - a crucial step towards addressing the problem of resistance. "

Common bacteria on verge of becoming antibiotic-resistant superbugs Antibiotic resistance is poised to spread globally among bacteria frequently implicated in respiratory and urinary infections in hospital settings, according to new research at Washington University School of Medicine in St. Louis. The study shows that two genes that confer resistance against a particularly strong class of antibiotics can be shared easily among a family of bacteria responsible for a significant portion of hospital-associated infections. Drug-resistant germs in the same family of bacteria recently infected several patients at two Los Angeles hospitals. The infections have been linked to medical scopes believed to have been contaminated with bacteria that can resist carbapenems, potent antibiotics that are supposed to be used only in gravely ill patients or those infected by resistant bacteria. "Carbapenems are one of our last resorts for treating bacterial infections, what we use when nothing else works, " said senior author Gautam Dantas, Ph. D, associate professor of pathology and immunology. "Given what we know now, I don't think it's overstating the case to say that for certain types of infections, we may be looking at the start of the post-antibiotic era, a time when most of the antibiotics we rely on to treat bacterial infections are no longer effective. " The researchers studied a family of bacteria called Enterobacteriaceae, which includes E. coli, Klebsiella pneumoniae and Enterobacter. Some strains of these bacteria do not cause illness and can help keep the body healthy. But in people with weakened immune systems, infections with carbapenem-resistant versions of these bacteria can be deadly. Two genes are primarily responsible for carbapenem-resistant versions of these disease-causing bacteria. One gene, KPC, was detected in New York in 2001 and quickly spread around most of the world, with the exception of India, Pakistan and other South Asian countries. This gene was present in the bacteria that recently contaminated medical equipment in a Los Angeles hospital where two patients died. A second carbapenem resistance gene, NDM-1, was identified in 2006 in New Delhi, India. It was soon detected throughout South Asia, and most patients infected by bacteria with NDM-1 have had an epidemiological link to South Asian countries. The researchers identified a few key instances in which the plasmids carrying NDM-1 or KPC were nearly identical, meaning they easily could facilitate the spread of antibiotic resistance between disease-causing bacteria found in the United States and South Asia. Recent evidence suggests that this intermingling already may be happening in parts of China.

Why the Flu Sometimes Kills Many people catch the flu every year and, after several days, recover. But for some, the disease becomes life threatening. Researchers now know one reason why. In 2011, the parents of a 2 -year-old girl with labored breathing carried their daughter into a French hospital. She was admitted, tested, and eventually diagnosed with the flu. Influenza is dangerous in children because of their still-developing immune systems, but some cases like this one, can be more severe, requiring hospitalization. Now, using next -generation sequencing and stem cell technologies, researchers from The Rockefeller University have found the Achilles heel in this child’s immune system that allowed for such a serious first encounter with the flu. When Casanova and his colleagues learned of this girl's condition, they focused on identifying genes that might be responsible for her particular vulnerability to flu by first sequencing both her and her parents' protein-coding DNA. By focusing on mutations in genes related to the immune system that were unique to the girl, the researchers identified interferon regulatory factor 7 (IRF 7) as the culprit. In this case, she had inherited a different mutation from each parent, whose heterozygous status protected them from the same response to the flu she experienced but made her susceptible to flu. Normally, the transcription factor IRF 7 drives expression of genes essential to the anti-viral response. To investigate IRF 7 function in the patient's cells, the team exposed her blood samples to different viruses and confirmed that they did not produce interferons in response. In contrast, her parents' cells produced a healthy response, showing that only one copy of the gene is necessary. The research team then collected skin cells from the child, reprogrammed them into i. PS cells, and differentiated these into lung cells where they could study the effects of influenza. They found that the virus replicated significantly more in the child's cells than in cells from healthy donors. The study not only identified the genetic underpinnings of this severe influenza infection in the young girl, but also suggested a possible treatment, advancing physicians one step closer towards personalized medicine for influenza. "Had we known the patient had IRF 7 deficiency when she was admitted, perhaps she would have benefited from recombinant alpha interferon, in addition to the Tamiflu and oxygen that she was given, " said Casanova.
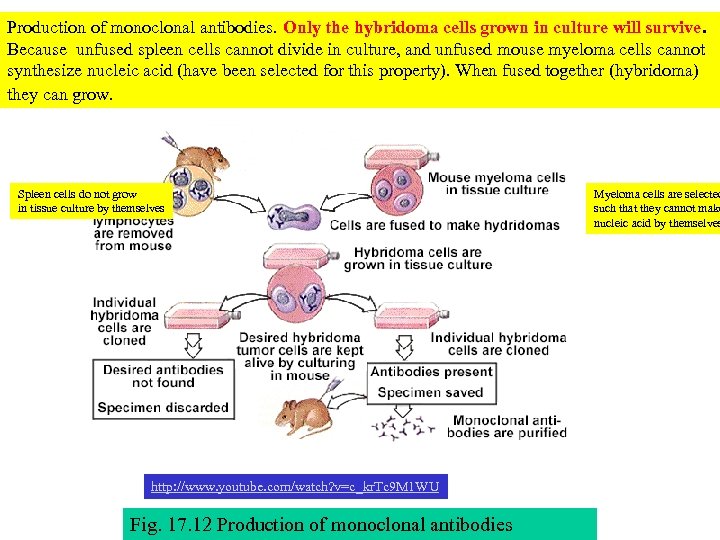
Production of monoclonal antibodies. Only the hybridoma cells grown in culture will survive. Because unfused spleen cells cannot divide in culture, and unfused mouse myeloma cells cannot synthesize nucleic acid (have been selected for this property). When fused together (hybridoma) they can grow. Spleen cells do not grow in tissue culture by themselves http: //www. youtube. com/watch? v=c_kr. Tc 9 M 1 WU Fig. 17. 12 Production of monoclonal antibodies Myeloma cells are selected such that they cannot make nucleic acid by themselves

Cell-Mediated Immunity- In contrast to humoral immunity, which involves B cells and immunoglobulins, cell-mediated immunity involves the direct actions of T cells. In cellmediated immunity, T cells interact directly with other cells that display foreign antigens. The cell-mediated immune response involves the differentiation and actions of different types of T cells and the production of chemical mediators called lymphokines.

The cell mediated immune reaction-
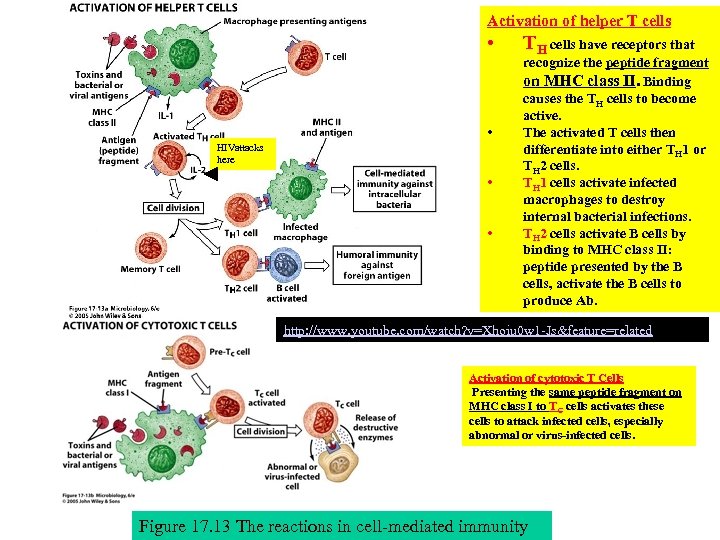
Activation of helper T cells • • HIVattacks here • • TH cells have receptors that recognize the peptide fragment on MHC class II. Binding causes the TH cells to become active. The activated T cells then differentiate into either TH 1 or TH 2 cells. TH 1 cells activate infected macrophages to destroy internal bacterial infections. TH 2 cells activate B cells by binding to MHC class II: peptide presented by the B cells, activate the B cells to produce Ab. http: //www. youtube. com/watch? v=Xhoiu 0 w 1 -Js&feature=related Activation of cytotoxic T Cells Presenting the same peptide fragment on MHC class I to TC cells activates these cells to attack infected cells, especially abnormal or virus-infected cells. Figure 17. 13 The reactions in cell-mediated immunity
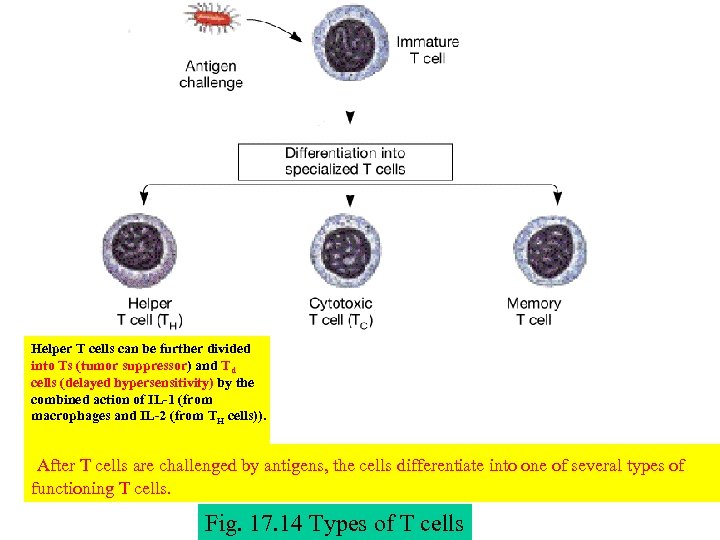
Helper T cells can be further divided into Ts (tumor suppressor) and Td cells (delayed hypersensitivity) by the combined action of IL-1 (from macrophages and IL-2 (from TH cells)). After T cells are challenged by antigens, the cells differentiate into one of several types of functioning T cells. Fig. 17. 14 Types of T cells

Macrophages that have processed an antigen secrete the lymphokine IL-1 which activates helper T cells (TH). Activated TH cells produce another cytokine, Il-2, which activate other T cells suppressor T (TS) cells, delayed hypersensitivity T cells (TD), and cytotoxic (killer) T cells (TC). Fig. 17. 15 Summary of cell-mediated immunity

Activated TD cells also release various lymphokines including: 1. Macrophage chemotactic factor, which helps macrophage find microbes 2. Macrophage activating factor, which stimulates phagocytic activity and the production of anti -bacterial compounds, e. g. , H 2 O 2, O 23. Migration inhibiting factor, which prevents macrophage from leaving sites of infection 4. Macrophage aggregation factor, which causes macrophages to congregate at such sites TD cells also participate in delayed hypersensitivity which will be discussed in chapter 18.
How killer cells kill. Cytotoxic T cells act mainly on virally infected cells, whereas NK cells act mainly on tumor cells, cells of transplanted tissues, and possible on cells infected with intracellular agents such as rickettsias and chlamydias. Cytotoxic T cells bind to antigens presented by macrophages and then attack virus-infected cells. In contrast, NK cells bind directly to malignant or other target cells without the help of macrophages. Both kinds of killer cells contain granules of a lethal protein, perforin, which is released when they bind to a target cell. Perforin bores holes in the target cell.

Given their strong cytolytic activity and the potential for auto-reactivity, NK cell activity is tightly regulated. NK cells must receive an activating signal, which can come in a variety of forms, the most important of which are listed below. • 1. Cytokines • The cytokines play a crucial role in NK cell activation. As these are stress molecules released by cells upon viral infection, they serve to signal to the NK cell the presence of viral pathogens. Cytokines involved in NK activation include IL-12, IL-15, IL-18, IL-2, and CCL 5. • 2. Fc receptor • NK cells, along with macrophages and several other cell types, express the Fc receptor (Fc. R) molecule (FC-gamma-RIII = CD 16), an activating biochemical receptor that binds the Fc portion of antibodies. This allows NK cells to target cells against which a humoral response has been mobilized and to lyse cells through Antibody-depdendent cellular cytotoxicity (ADCC). • 3. Activating and inhibitory receptors Aside from the Fc receptor, NK cells express a variety of receptors that serve either to activate or to suppress their cytolytic activity. These receptors bind to various ligands on target cells, both endogenous and exogenous, and have an important role in regulating the NK cell response.

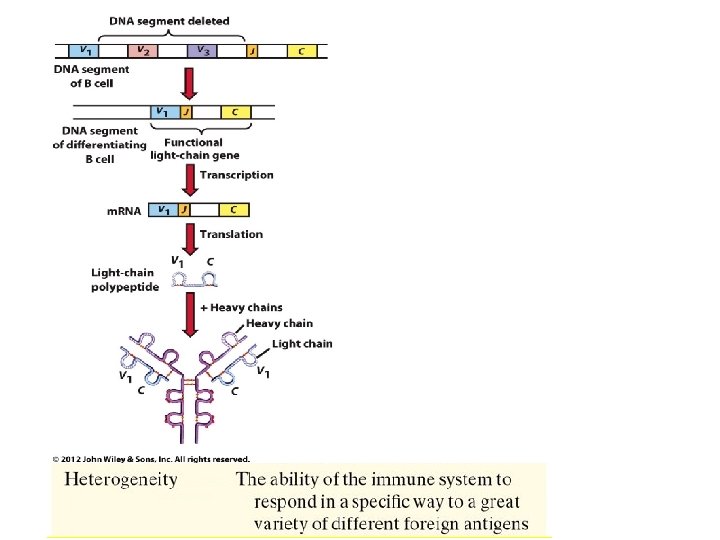
The action of cytokines may autocrine, paracrine and endocrine. Cytokines are critical to the development and functioning of both the innate and adaptive immune response, although not limited to just the immune system. They are often secreted by immune cells that have encountered a pathogen, thereby activating and recruiting further immune cells to increase the system's response to the pathogen. Cytokines are also involved in several developmental processes during embryogenesis. Interleukins are a group of cytokines (secreted signaling molecules) Table 17. 5 Characteristics of B cells, T cells and Macrophages

Immunization Active immunization- is the process of inducing active immunity (vaccines or toxoids). A vaccine in a substance that contains an antigen to which the immune system responds.

Passive Immunization- Antiserum- serum containing antibodies- gamma globulin, or hyperimmune serum, Can also use animals, such as a horse, for preformed tetanus antibodies that have been raised commercially by injections of toxoid. (serum sickness is one danger of this type of immunization (will discuss in the next chapter). Immune serum globulin (gamma globulin)- pooled gamma globulin fraction from many individualsprovides passive immunity to a number of common disease such as mumps, measles, hepatitis B, tetanus, rabies, and pertusis (whopping cough). hyperimmune sera contains especially high titers against a particular agent obtained from donors with a high titer of antibody against that agent. Antitoxins- to combat snake or spider venoms

Immunity to Various Kinds of Pathogens Bacteria- Antibodies can work with complement to opsonize bacteria (coat with antibody) for later phagocytosis or lysis by other cells of the immune system. Or they can neutralize bacterial toxins or inactivate bacterial enzymes.
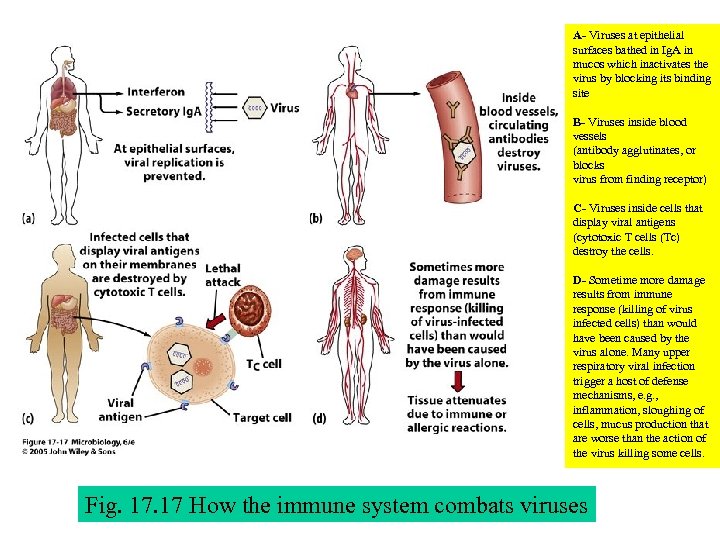
A- Viruses at epithelial surfaces bathed in Ig. A in mucos which inactivates the virus by blocking its binding site B- Viruses inside blood vessels (antibody agglutinates, or blocks virus from finding receptor) C- Viruses inside cells that display viral antigens (cytotoxic T cells (Tc) destroy the cells. D- Sometime more damage results from immune response (killing of virus infected cells) than would have been caused by the virus alone. Many upper respiratory viral infection trigger a host of defense mechanisms, e. g. , inflammation, sloughing of cells, mucus production that are worse than the action of the virus killing some cells. Fig. 17 How the immune system combats viruses
8ea6a417b238ef08ecba3a800f10f95d.ppt