Inorganic_1.ppt
- Количество слайдов: 32
Basic concepts and laws of chemistry

lesson plan 1. Basic concept of chemistry: • matter, elements; • atom, molecules; • chemical Reactions; • equivalent mass. 2. Basic laws of chemistry: • Law of conservation of mass; • Law of maltiple proportions; • Law of constant composition; • Avagadro’s Law.
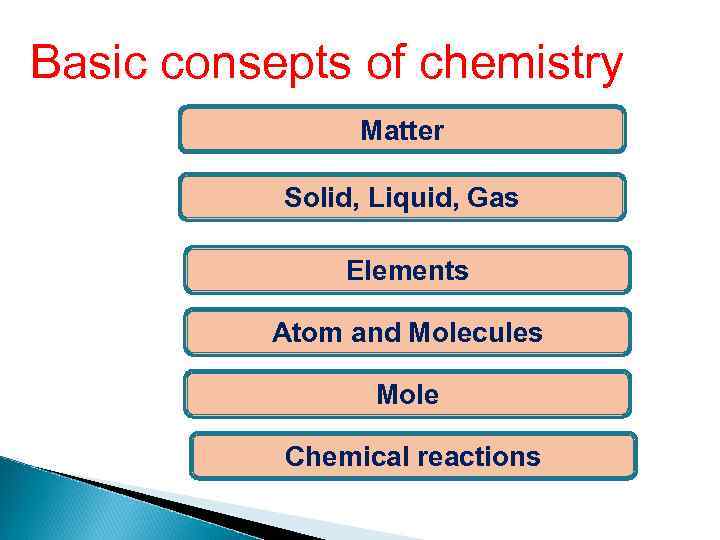
Basic consepts of chemistry Matter Solid, Liquid, Gas Elements Atom and Molecules Mole Chemical reactions

Matter - any material substance with Mass and Volume. Matter comes in 3 phases. Solid Liquid Gas
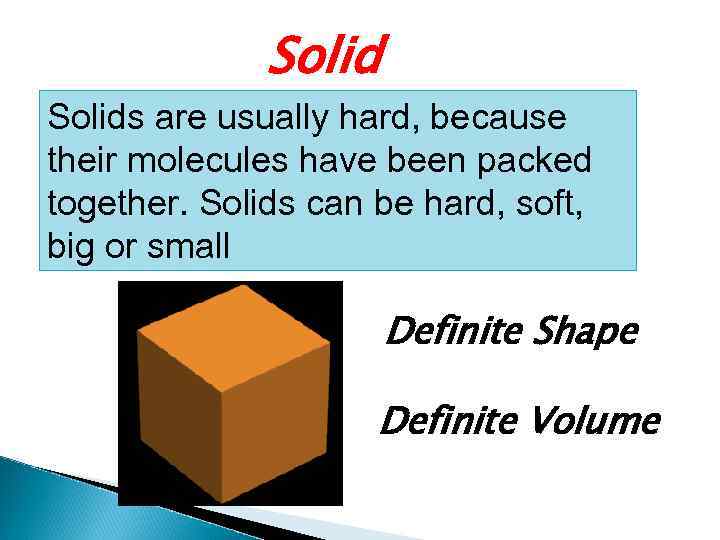
Solids are usually hard, because their molecules have been packed together. Solids can be hard, soft, big or small Definite Shape Definite Volume

Liquid Indefinite Shape – takes the shape of the container Definite Volume

Gas Indefinite Shape – takes the shape of the container Indefinite Volume – can expand be compressed
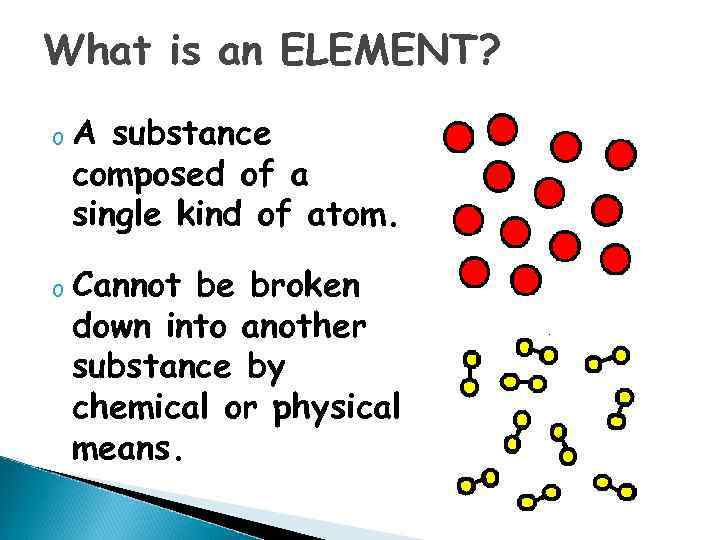
What is an ELEMENT? o A substance composed of a single kind of atom. o Cannot be broken down into another substance by chemical or physical means.
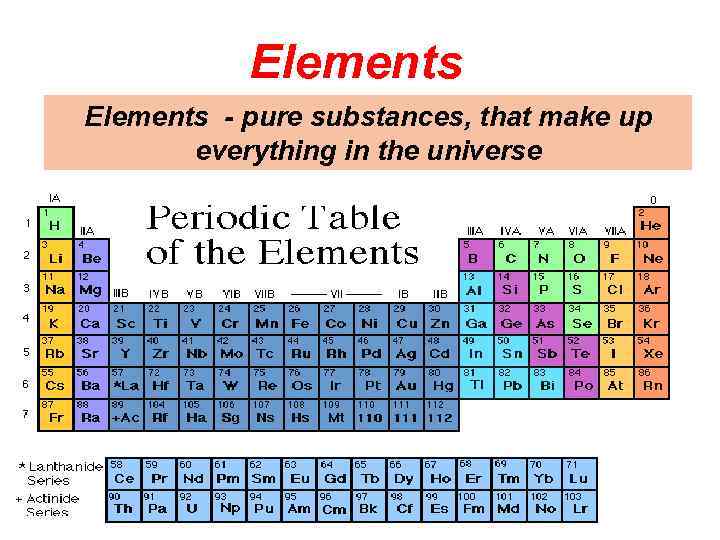
Elements - pure substances, that make up everything in the universe
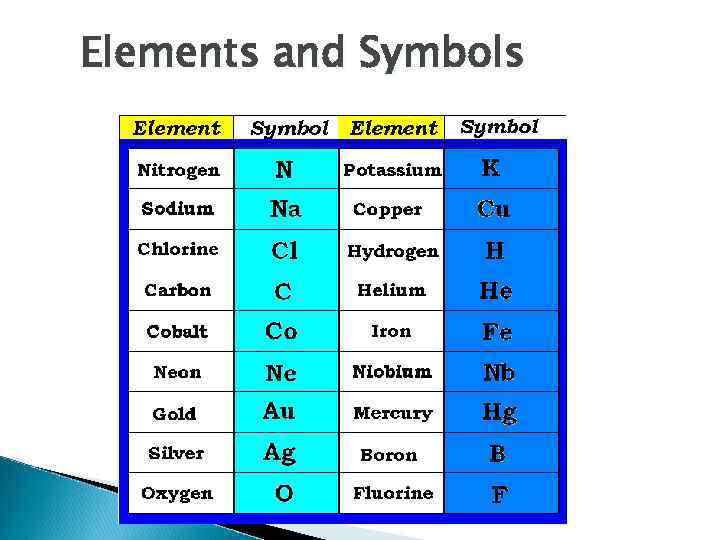
Elements and Symbols
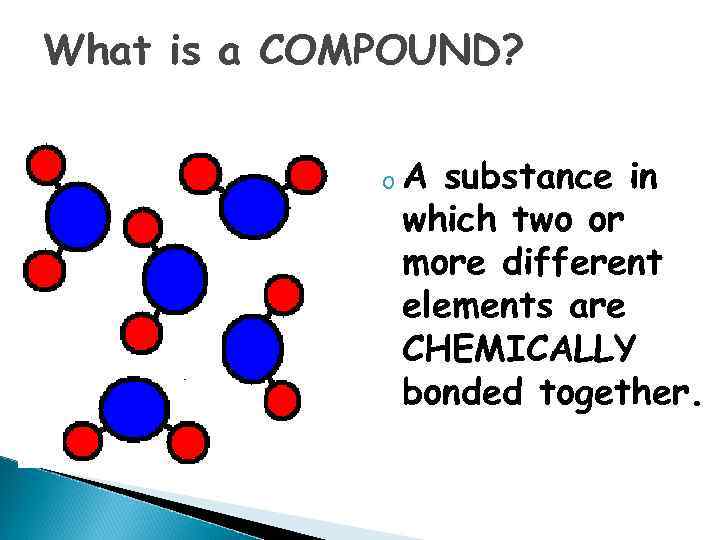
What is a COMPOUND? o. A substance in which two or more different elements are CHEMICALLY bonded together.
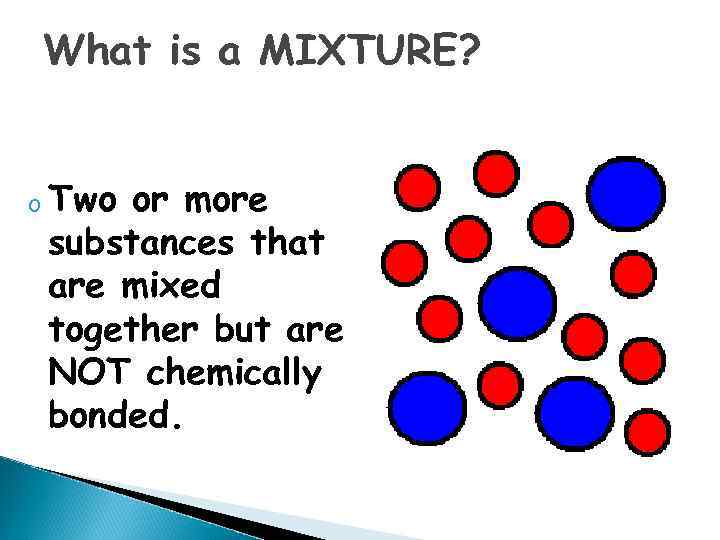
What is a MIXTURE? o Two or more substances that are mixed together but are NOT chemically bonded.
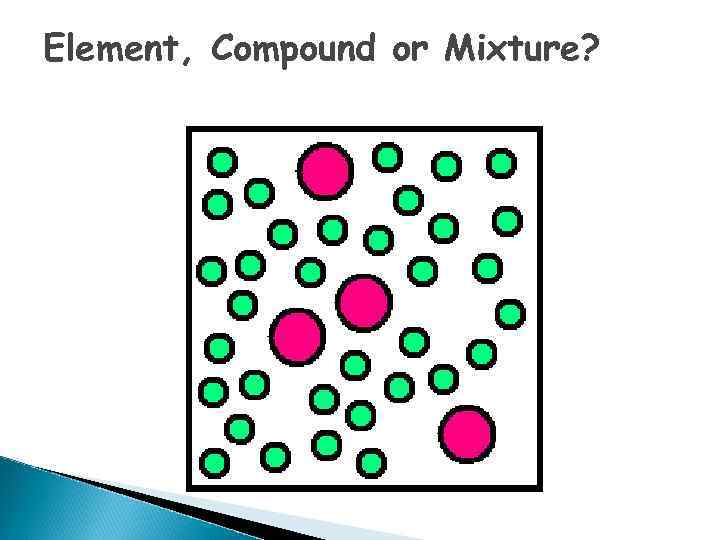
Element, Compound or Mixture?
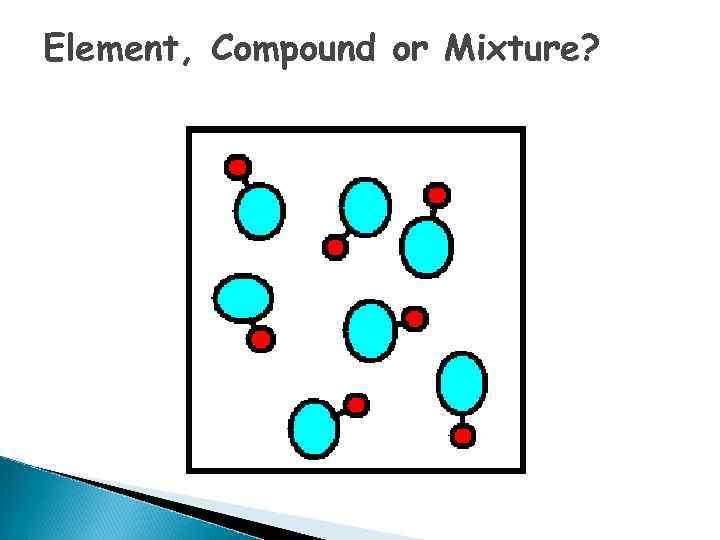
Element, Compound or Mixture?
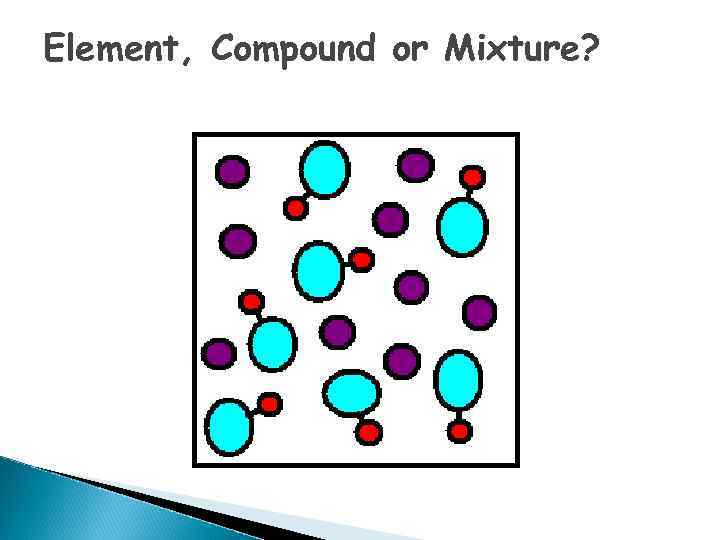
Element, Compound or Mixture?

An atom is the smallest particle of an element. Atoms of elements are measured by their atomic mass. A molecule is the smallest particle of a substance that can have an independent existence. It is formed when two or more atoms join together chemically. Molecules of substances are also measured by relative mass: the unit of comparison being the same – one twelfth of the mass of a carbon atom. It is defined as the average relative mass of a molecule of a substance.
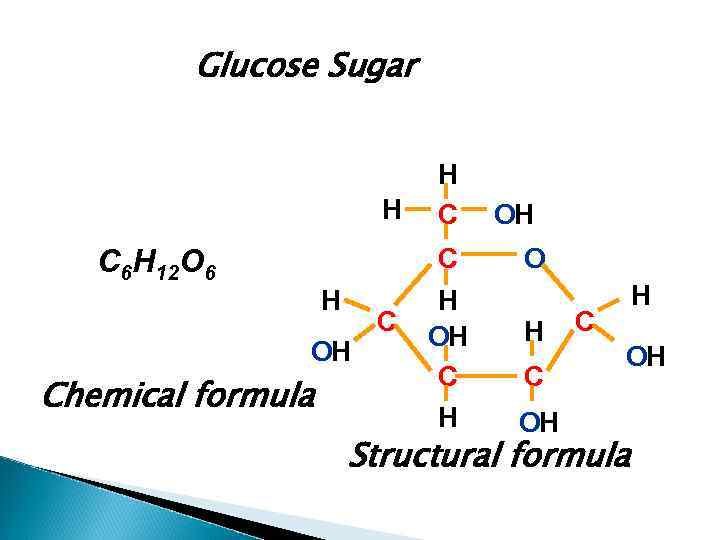
Glucose Sugar H H C C C 6 H 12 O 6 H OH Chemical formula C H OH O H C OH Structural formula

Mole - measure of concentration in chemistry. The Mol unit is based on the number of atoms in exacty 12 grams of pure carbon. Mole: • In terms of particles 6. 23· 10 -23 particles. In terms of particles such as atoms, molecules, ions or electrons, this has a value of 6. 23· 10 -23 of the concerned unit that can be either an atom or a molecule or electrons and ions. This is called Avadadro's number.
• In terms of volume at NTR 22. 4 L of a gas. In terms of volume at normal temperature pressure this value is 22. 4 litres of a given gas. • In terms of mass 1 gram molecular mass of a substance. In terms of mass, this is equivalent to 1 gram molecular mass of a substance.
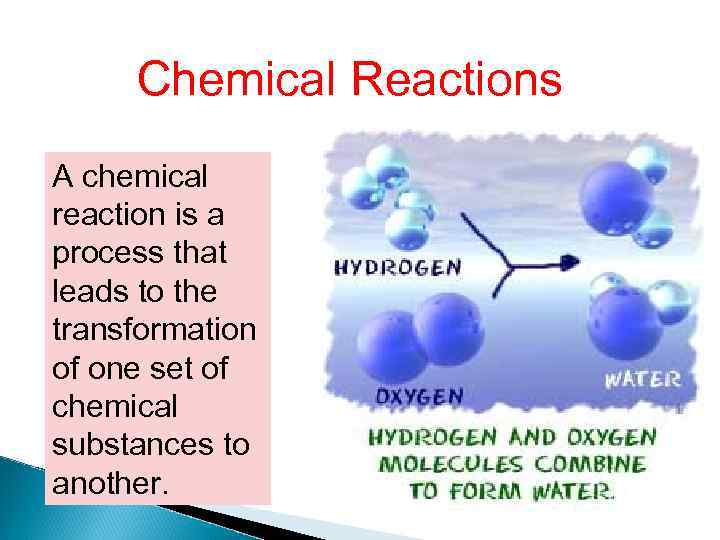
Chemical Reactions A chemical reaction is a process that leads to the transformation of one set of chemical substances to another.
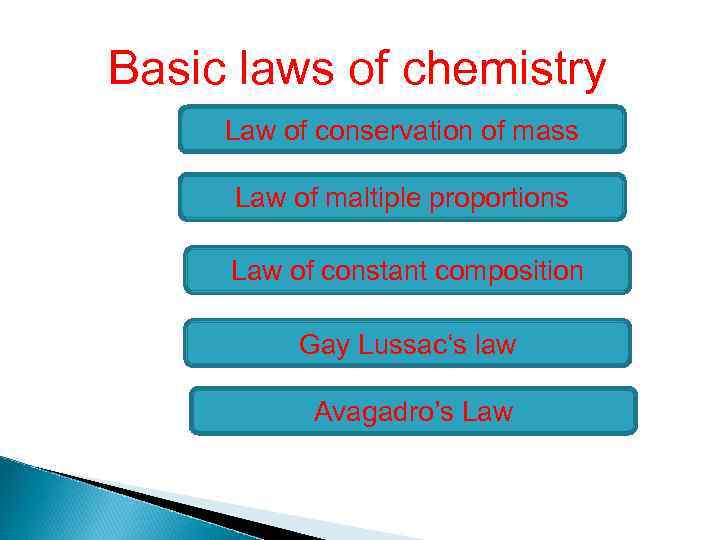
Basic laws of chemistry Law of conservation of mass Law of maltiple proportions Law of constant composition Gay Lussac‘s law Avagadro’s Law

Law of equivalent proportions - law stating that the proportions in which two elements separately combine with a third element are also the proportions in which they combine together. Equivalent Weight of an Element (E): It is defined as the number of parts by weight of the element which combine with or displace from a compund 1 part by weight of Hydrogen, 8 parts by weight of Oxygen or 35. 5 parts by weight of Chlorine. In this method you need not balance the chemical equation. The basic principle is that the equivalents of each reactant Number
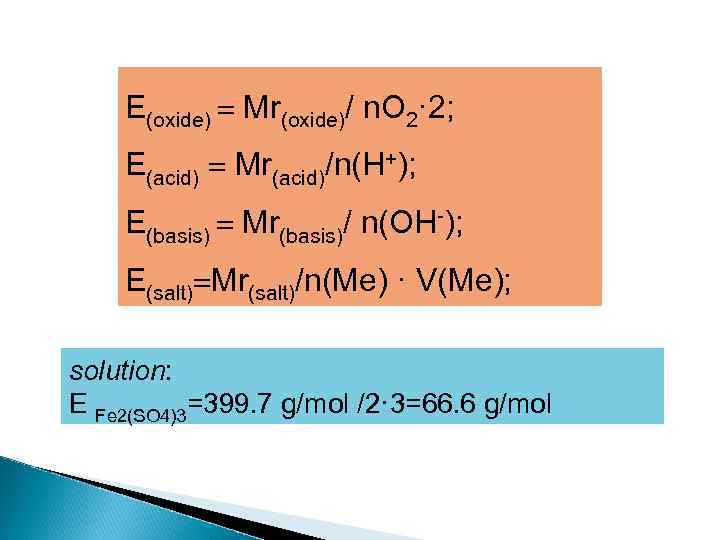
E(oxide) Мr(oxide)/ n. O 2· 2; E(acid) Мr(acid)/n(H+); E(basis) Мr(basis)/ n(OH-); E(salt) Мr(salt)/n(Me) · V(Ме); solution: E Fe 2(SO 4)3=399. 7 g/mol /2· 3=66. 6 g/mol
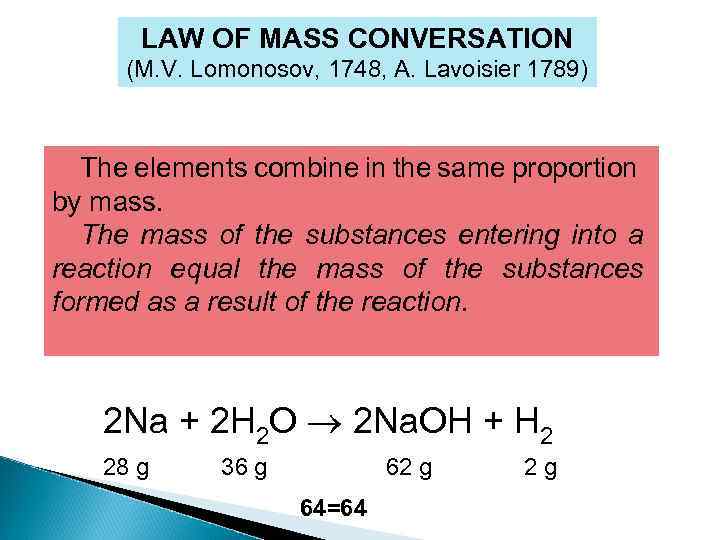
LAW OF MASS CONVERSATION (M. V. Lomonosov, 1748, A. Lavoisier 1789) The elements combine in the same proportion by mass. The mass of the substances entering into a reaction equal the mass of the substances formed as a result of the reaction. 2 Na + 2 H 2 O 2 Na. OH + H 2 28 g 36 g 62 g 64=64 2 g
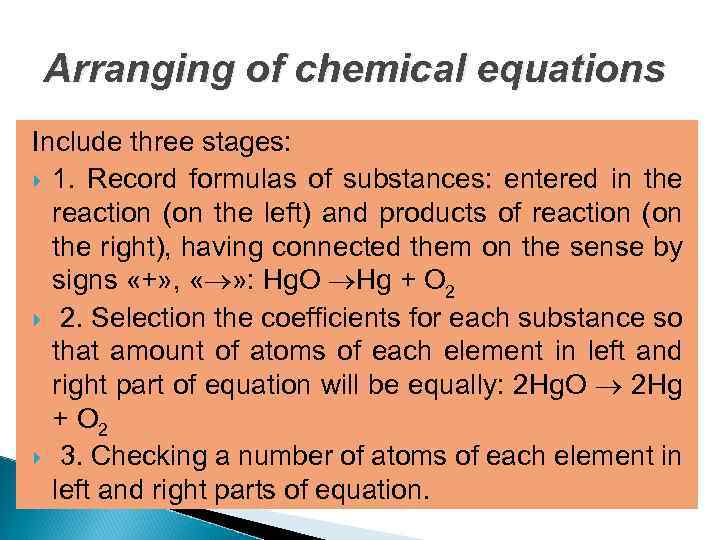
Arranging of chemical equations Include three stages: 1. Record formulas of substances: entered in the reaction (on the left) and products of reaction (on the right), having connected them on the sense by signs «+» , « » : Hg. O Hg + O 2 2. Selection the coefficients for each substance so that amount of atoms of each element in left and right part of equation will be equally: 2 Hg. O 2 Hg + O 2 3. Checking a number of atoms of each element in left and right parts of equation.
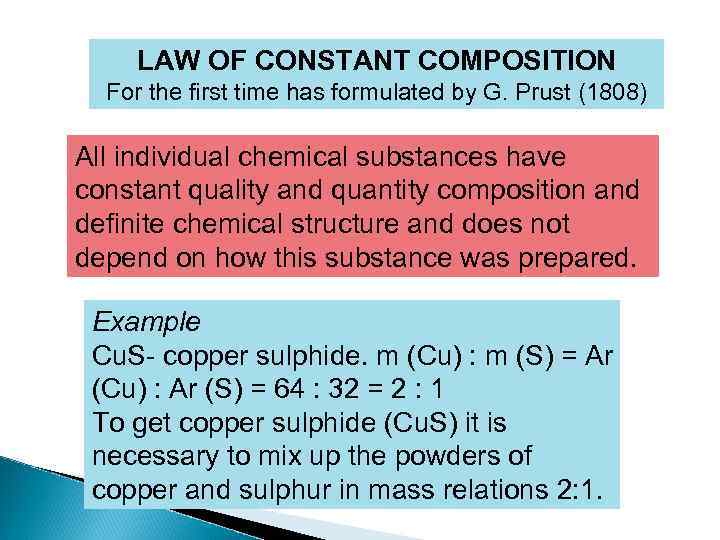
LAW OF CONSTANT COMPOSITION For the first time has formulated by G. Prust (1808) All individual chemical substances have constant quality and quantity composition and definite chemical structure and does not depend on how this substance was prepared. Example Cu. S- copper sulphide. m (Cu) : m (S) = Ar (Cu) : Ar (S) = 64 : 32 = 2 : 1 To get copper sulphide (Cu. S) it is necessary to mix up the powders of copper and sulphur in mass relations 2: 1.
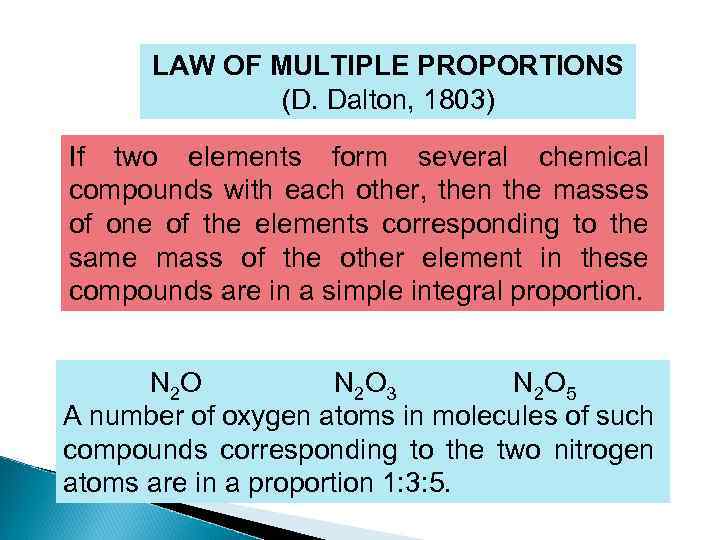
LAW OF MULTIPLE PROPORTIONS (D. Dalton, 1803) If two elements form several chemical compounds with each other, then the masses of one of the elements corresponding to the same mass of the other element in these compounds are in a simple integral proportion. N 2 O 3 N 2 O 5 A number of oxygen atoms in molecules of such compounds corresponding to the two nitrogen atoms are in a proportion 1: 3: 5.

AVOGADRO LAW (1811) Equal volumes of all gases at the same conditions (temperature, pressure) contain the same number of molecules. This law truth for gaseous substances only. Consequences: 1. On mole of any substance in the gaseous state occupies the same volume at the same temperature and pressure. 2. One mole of any gas in standard conditions (0°C = 273°K, 1 atm = 101. 3 k. Pa) occupies a volume of 22. 4 litres.

example What volume of hydrogen at s. t. p. would be evolved at dissolution 4. 8 g magnesium in excess of hydrochloric acid? solution Mg + 2 HCl Mg. Cl 2 + H 2 At dissolution of 24 g (1 g-mol) magnesium in HCl –– 22. 4 l (1 g-mol) of hydrogen is evaluated; at dissolution of 4. 8 g of magnesium –– X l of hydrogen. X = = 4. 48 l of hydrogen
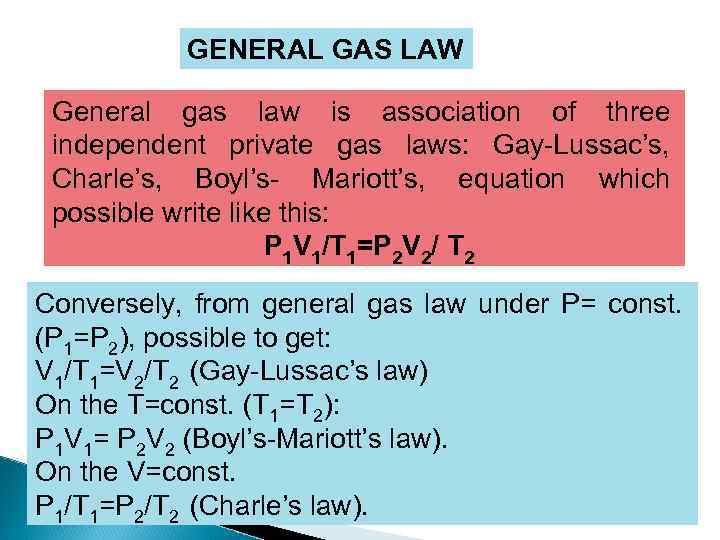
GENERAL GAS LAW General gas law is association of three independent private gas laws: Gay-Lussac’s, Charle’s, Boyl’s- Mariott’s, equation which possible write like this: P 1 V 1/T 1=P 2 V 2/ T 2 Conversely, from general gas law under P= const. (P 1=P 2), possible to get: V 1/T 1=V 2/T 2 (Gay-Lussac’s law) On the T=const. (T 1=T 2): P 1 V 1= P 2 V 2 (Boyl’s-Mariott’s law). On the V=const. P 1/T 1=P 2/T 2 (Charle’s law).
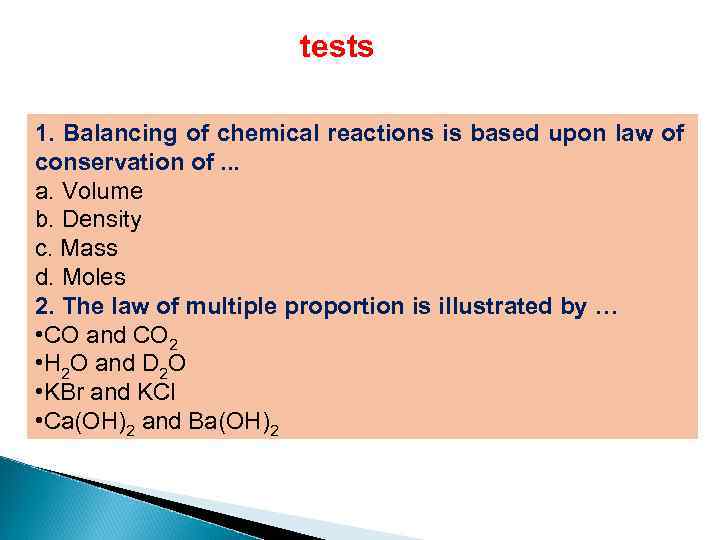
tests 1. Balancing of chemical reactions is based upon law of conservation of. . . a. Volume b. Density c. Mass d. Moles 2. The law of multiple proportion is illustrated by … • CO and CO 2 • H 2 O and D 2 O • KBr and KCl • Ca(OH)2 and Ba(OH)2

3. Matter can neither be created not destroyed. . а. True в. False 4. All individual chemical substances have constant quality and quantity composition and definite chemical structure and does not depend on how this substance was prepared. What is law it? a. Law of mass conversation b. Law of combining volumes c. Avogadro law d. General gas law 5. Equal volumes of gases at the same temperature and pressure contain unequal number of molecules. a. True b. False
Inorganic_1.ppt