6f47da57dc5e0585885341b442b14ea2.ppt
- Количество слайдов: 51

Base Excess and Critical Care in the past and in the future Ivar Hejde Gøthgen M. D. , D. M. Sc. IHG 05

Content • • Acid-base basic Scientist in the acid-base story Instruments in the acid-base story The future and Base excess IHG 05
![Acid Base (concept of neutrality) ____________________________________ p. N = [H+] = [OH-] (=neutrality) p. Acid Base (concept of neutrality) ____________________________________ p. N = [H+] = [OH-] (=neutrality) p.](https://present5.com/presentation/6f47da57dc5e0585885341b442b14ea2/image-3.jpg)
Acid Base (concept of neutrality) ____________________________________ p. N = [H+] = [OH-] (=neutrality) p. H = 6. 80 [H+] = [OH-] = 160 nmol/l = neutrality at 37 o. C p. H = 7. 40 [H+] = 40 nmol/l [OH-] = 640 nmol/l = Alkaline offset of 0. 6 p. H unit IHG 05
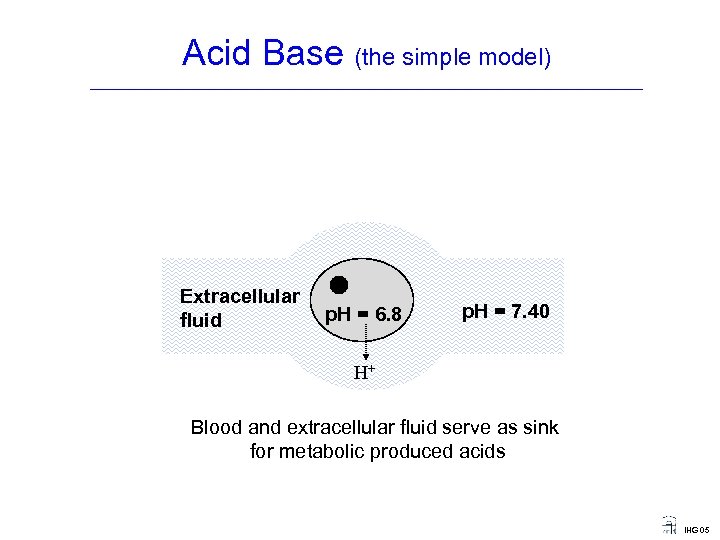
Acid Base (the simple model) ____________________________________ Extracellular fluid p. H = 6. 8 p. H = 7. 40 H+ Blood and extracellular fluid serve as sink for metabolic produced acids IHG 05
![Acid Base (the acid production) ____________________________________ Fixed Acids production [H+] : 60 mmol/24 hour Acid Base (the acid production) ____________________________________ Fixed Acids production [H+] : 60 mmol/24 hour](https://present5.com/presentation/6f47da57dc5e0585885341b442b14ea2/image-5.jpg)
Acid Base (the acid production) ____________________________________ Fixed Acids production [H+] : 60 mmol/24 hour => 700 nmol/sec Volatile Acids production [H+] : 13 mol/24 hour => 150000 nmol/sec > 200 times the fixed acids Total extracellular free [H+] : 40 nmol/l * 15 l => 600 nmol IHG 05
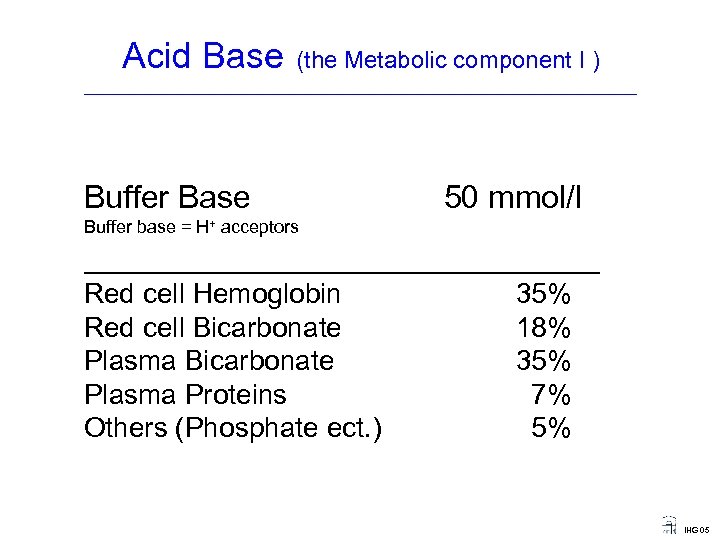
Acid Base (the Metabolic component I ) ____________________________________ Buffer Base 50 mmol/l Buffer base = H+ acceptors _______________ Red cell Hemoglobin Red cell Bicarbonate Plasma Proteins Others (Phosphate ect. ) 35% 18% 35% 7% 5% IHG 05
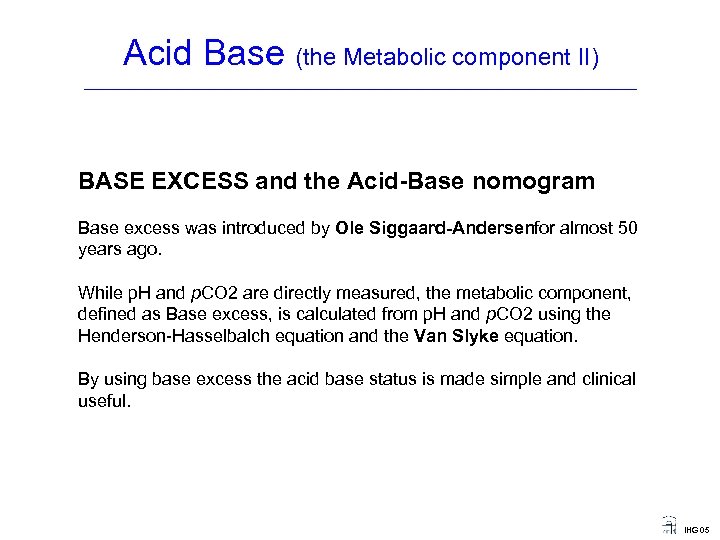
Acid Base (the Metabolic component II) ____________________________________ BASE EXCESS and the Acid Base nomogram Base excess was introduced by Ole Siggaard Andersenfor almost 50 years ago. While p. H and p. CO 2 are directly measured, the metabolic component, defined as Base excess, is calculated from p. H and p. CO 2 using the Henderson-Hasselbalch equation and the Van Slyke equation. By using base excess the acid base status is made simple and clinical useful. IHG 05

Acid Base (the Metabolic component III) ____________________________________ Can you go to a Pharmacy and buy a bottle of Base excess ? BASE EXCES S IHG 05
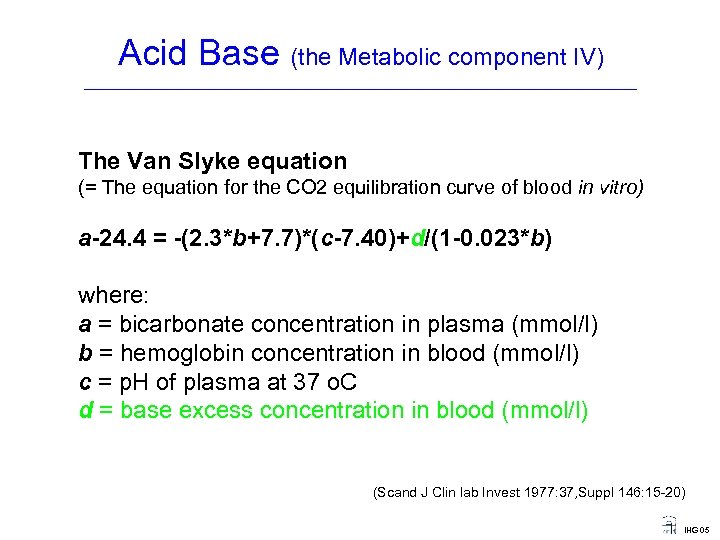
Acid Base (the Metabolic component IV) ____________________________________ The Van Slyke equation (= The equation for the CO 2 equilibration curve of blood in vitro) a 24. 4 = (2. 3*b+7. 7)*(c 7. 40)+d/(1 0. 023*b) where: a = bicarbonate concentration in plasma (mmol/l) b = hemoglobin concentration in blood (mmol/l) c = p. H of plasma at 37 o. C d = base excess concentration in blood (mmol/l) (Scand J Clin lab Invest 1977: 37, Suppl 146: 15 -20) IHG 05
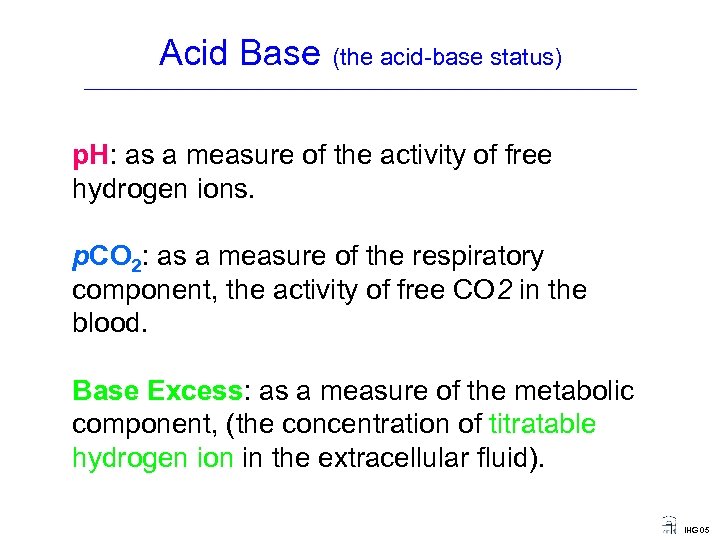
Acid Base (the acid-base status) ____________________________________ p. H: as a measure of the activity of free hydrogen ions. p. CO 2: as a measure of the respiratory component, the activity of free CO 2 in the blood. Base Excess: as a measure of the metabolic component, (the concentration of titratable hydrogen ion in the extracellular fluid). IHG 05
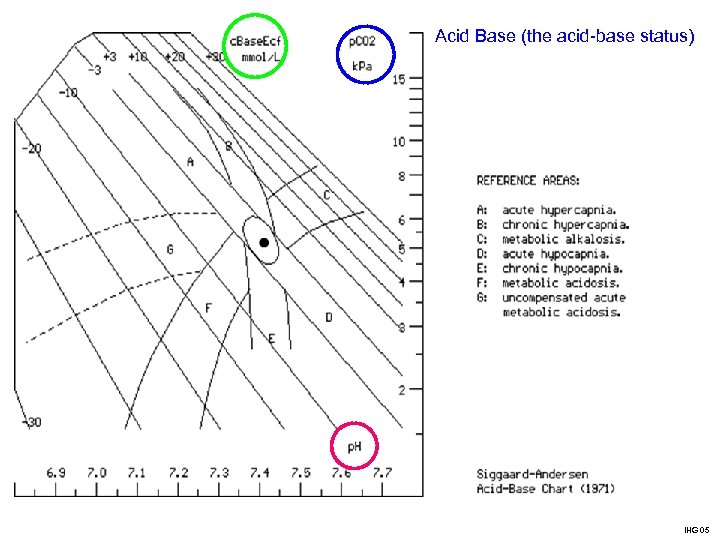
Acid Base (the acid-base status) IHG 05
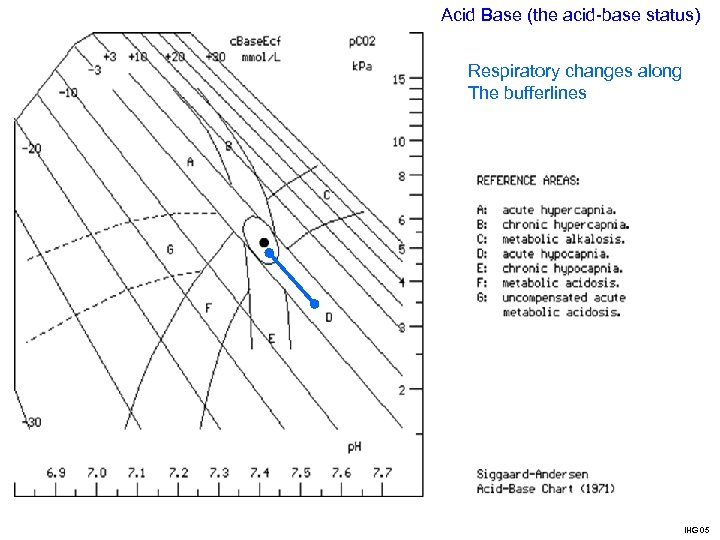
Acid Base (the acid-base status) Respiratory changes along The bufferlines IHG 05
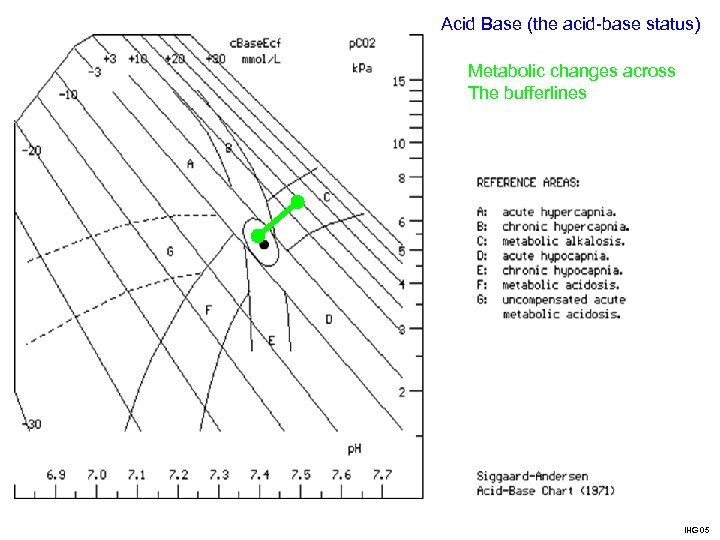
Acid Base (the acid-base status) Metabolic changes across The bufferlines IHG 05
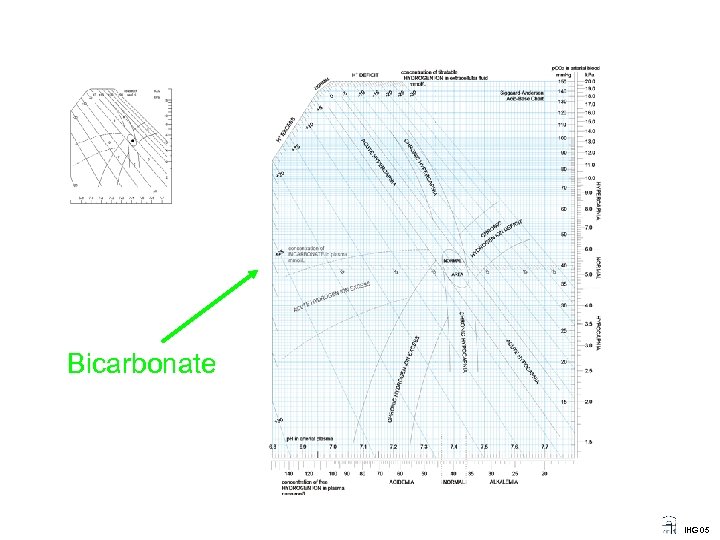
Bicarbonate IHG 05

Content • • Acid-base basic Scientist in the acid-base story Instruments in the acid-base story The future and Base excess IHG 05

Scientist in the acid-base story S. P. L. Sørensen 1868 -1939 L. J. Henderson 1878 -1942 K. A. Hasselbalch 1874 -1962 Donald D van Slyke 1883 -1971 Poul Astrup 1915 -2000 John W. Severinghaus 1922 - Ole Siggaard-Andersen 1932 IHG 05

Scientist in the acid-base story Søren P. L. Sørensen 1868 -1939 Protein Chemist at the Carlsberg brewery. To save having to write that H+ = 0. 000000040 M, he devised the scale of acid in terms of p. H, as the negative log of H+ ion activity. (1907) IHG 05
Scientist in the acid-base story IHG 05

Scientist in the acid-base story Lawrence J Henderson Prof Biochemistry and Physiology. Theory of buffering and of bicarbonate-hydrogen ion-p. CO 2 relationship. The Henderson equation K = [H+][HCO 3 ]/[H 2 CO 3] K is the dissociation constant of carbonic acid, about 10 -3 M A constant 0. 1% of dissolved CO 2 in water is hydrated to carbonic acid. Therefore it can be simplified to H 2 CO 3 = dissolved CO 2 Where K’ is the apparent dissociation constant K’ = 10 -6. 1 IHG 05
Scientist in the acid-base story IHG 05

Scientist in the acid-base story Karl A Hasselbalch 1874 -1962 Agricultural chemist, Denmark. Adapted Henderson’s equation to Sørensen’s logarithmic p. H by replacing H 2 CO 3 with S. p. CO 2 creating the Henderson-Hasselbalch equation: p. H=p. K’ + log[HCO 3 /(S. p. CO 2)] 7. 40 = 6. 10 + log[24/{0. 31. 40}] Where S (solubility) = 0. 031 m. M/liter/mm. Hg at 37 o. C IHG 05
Scientist in the acid-base story IHG 05

Scientist in the acid-base story Donald D Van Slyke 1883 -1971 Major developer of clinical chemistry in the 1910 -50 period. His manometric blood gas apparatus (1924) was used to measure the content in blood of oxygen, carbon dioxide and many other variables. Laboratories calculated p. CO 2 using the Henderson-Hasselbalch equation after measuring p. H of blood and the total plasma CO 2 by the Van Slyke apparatus until the polio epidemics resulted in two new methods: Astrup’s equilibration scheme and Severinghaus modification of the Stow CO 2 electrode. IHG 05
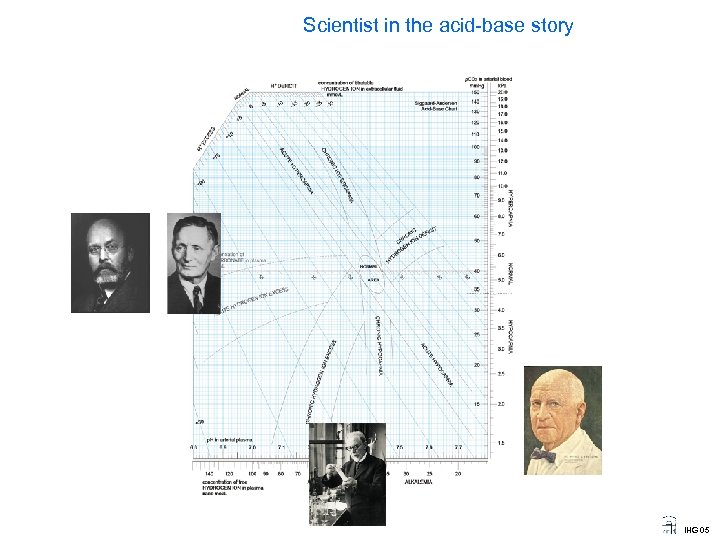
Scientist in the acid-base story IHG 05

Scientist in the acid-base story Poul Astrup 1915 -2000 Prof of Clinical Chemistry Univ. of Copenhagen New method for p. CO 2 To avoid need for the Van. Slyke and Henderson. Hasselbalch method, he devised a method for graphically calculating p. CO 2 by measuring p. H before and again after equilibration of the blood to a known p. CO 2 IHG 05
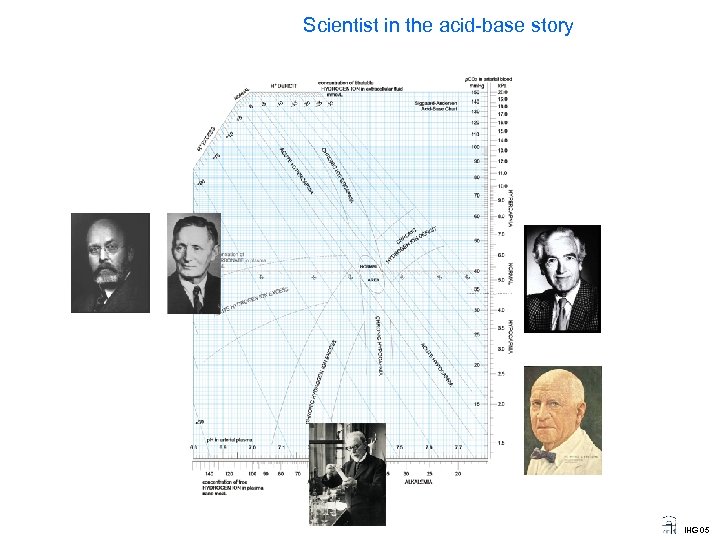
Scientist in the acid-base story IHG 05

Scientist in the acid-base story John W Severinghaus 1922 Prof of Anestesia University of California San Francisco Major developer of blood gas measurents since 1950. His modification (invention) of the CO 2 electrode, the first blood gas apparatus (1958), the blood gas ruler, transcutaneous blood gas measurement and pulsoximetry, as well as important work in high altitude respiratory physiology IHG 05
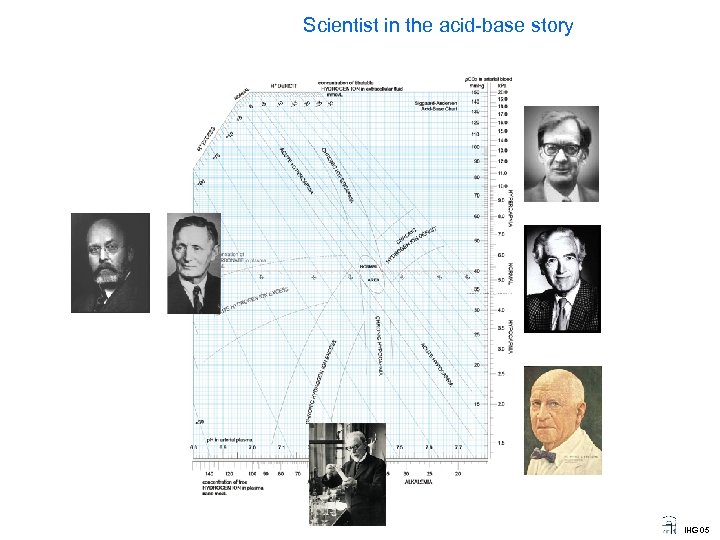
Scientist in the acid-base story IHG 05

Scientist in the acid-base story Ole Siggaard Andersen 1932 Prof of Clinical Chemistry Univ. of Copenhagen A student of Astrup, he devised the micro-method and the concept of base excess and ECF base excess, now called SBE, for standard base excess. His equation for SBE is now used in most blood gas apparatus. Photo 1999 by JWS IHG 05
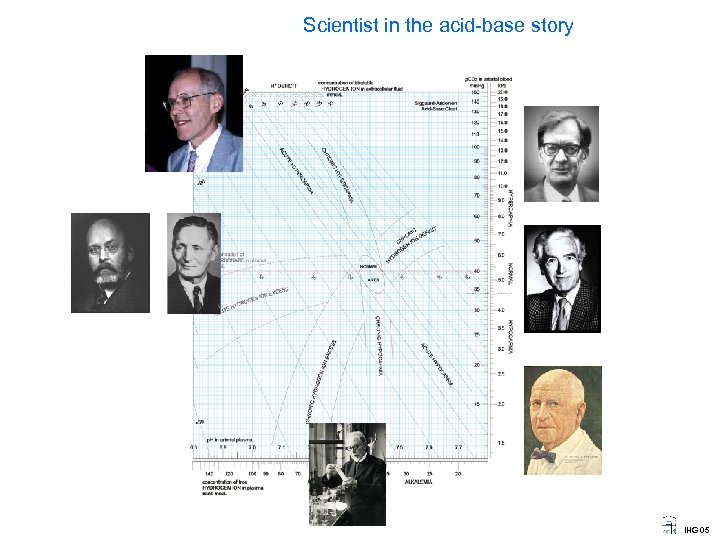
Scientist in the acid-base story IHG 05

Content • • Acid-base basic Scientist in the acid-base story Instruments in the acid-base story The future and Base excess IHG 05
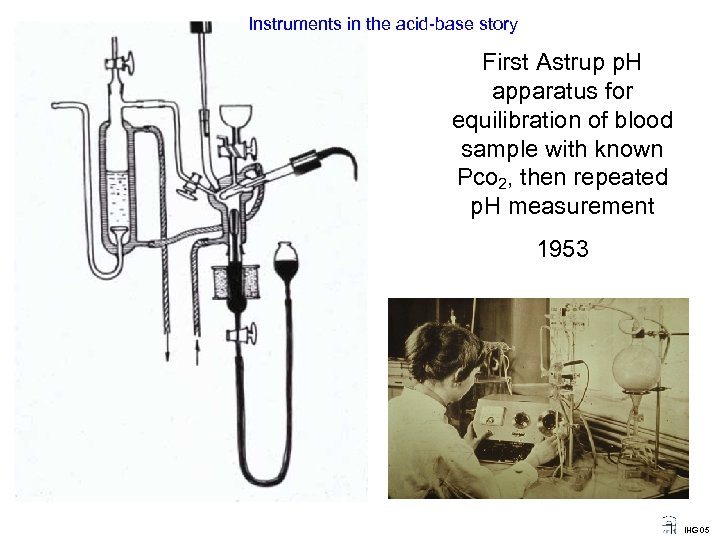
Instruments in the acid-base story First Astrup p. H apparatus for equilibration of blood sample with known Pco 2, then repeated p. H measurement 1953 IHG 05
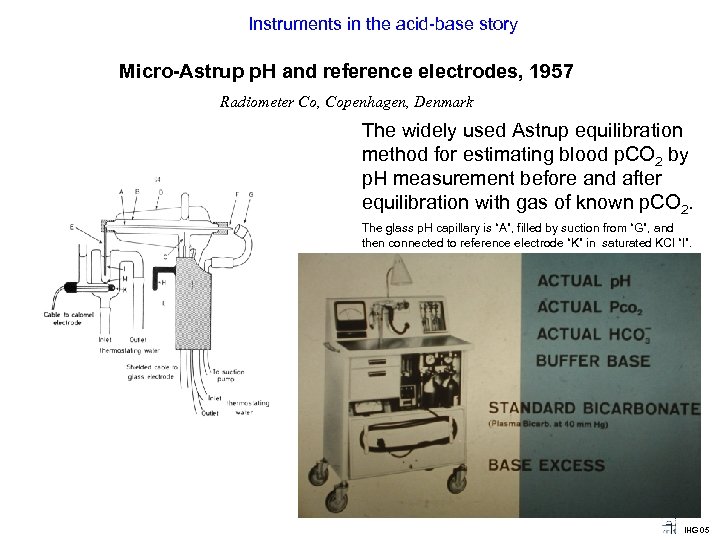
Instruments in the acid-base story Micro Astrup p. H and reference electrodes, 1957 Radiometer Co, Copenhagen, Denmark The widely used Astrup equilibration method for estimating blood p. CO 2 by p. H measurement before and after equilibration with gas of known p. CO 2. The glass p. H capillary is “A”, filled by suction from “G”, and then connected to reference electrode “K” in saturated KCl “I”. IHG 05
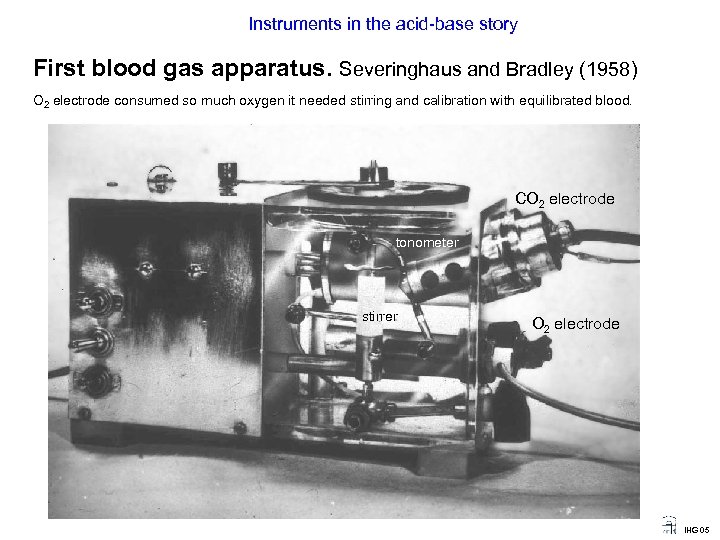
Instruments in the acid-base story First blood gas apparatus. Severinghaus and Bradley (1958) O 2 electrode consumed so much oxygen it needed stirring and calibration with equilibrated blood. CO 2 electrode tonometer stirrer O 2 electrode IHG 05
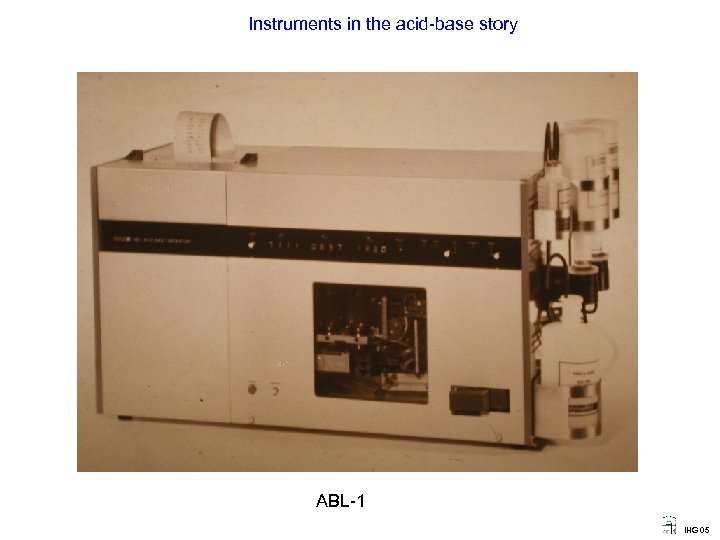
Instruments in the acid-base story ABL-1 IHG 05

Instruments in the acid-base story BMS-2 , OSM-3 and ABL-4 ( incl. Ole Siggaard-Andersen at work) IHG 05

Instruments in the acid-base story ABL-700 IHG 05

Critical care treatment and the acid-base story Polio victim in Copenhagen epidemic being ventilated manually by medical student through tracheostomy, 1952. Invasive ventilation IHG 05
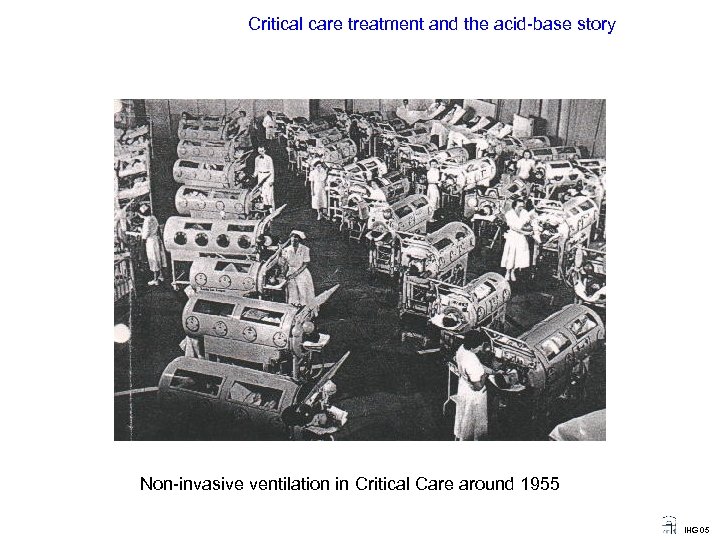
Critical care treatment and the acid-base story Non-invasive ventilation in Critical Care around 1955 IHG 05
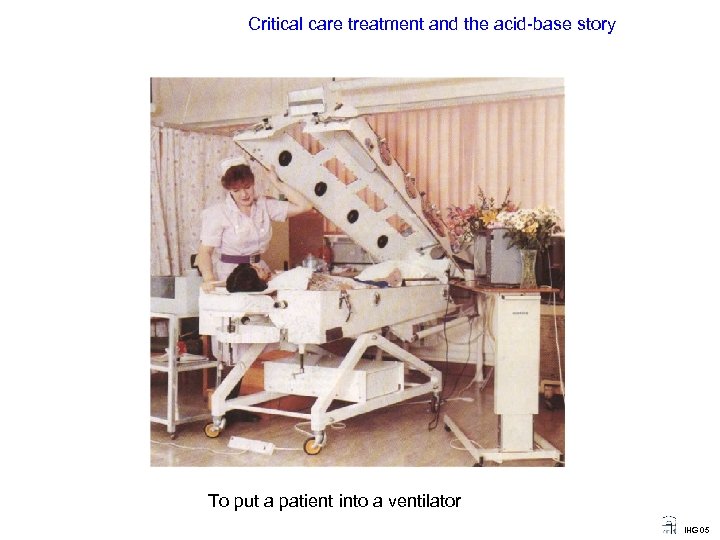
Critical care treatment and the acid-base story To put a patient into a ventilator IHG 05
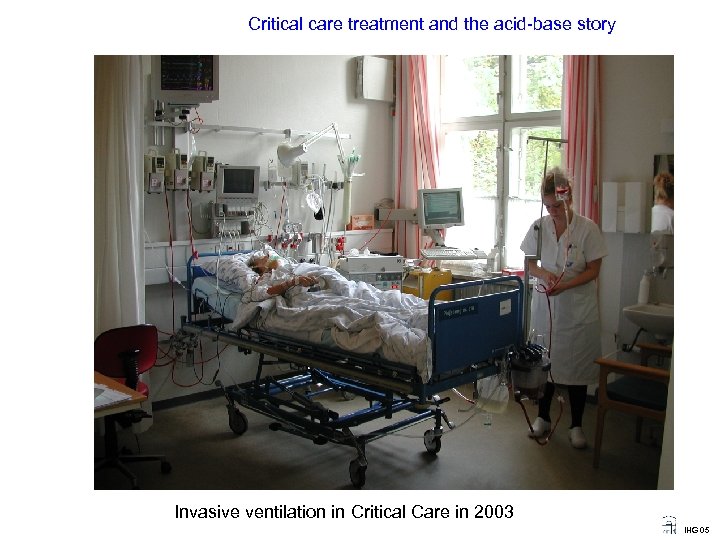
Critical care treatment and the acid-base story Invasive ventilation in Critical Care in 2003 IHG 05

Content • • Acid-base basic Scientist in the acid-base story Instruments in the acid-base story The future and Base excess IHG 05

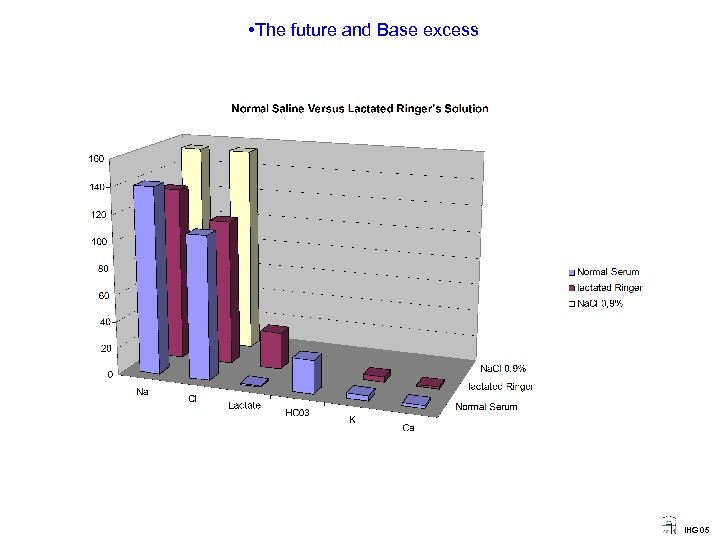
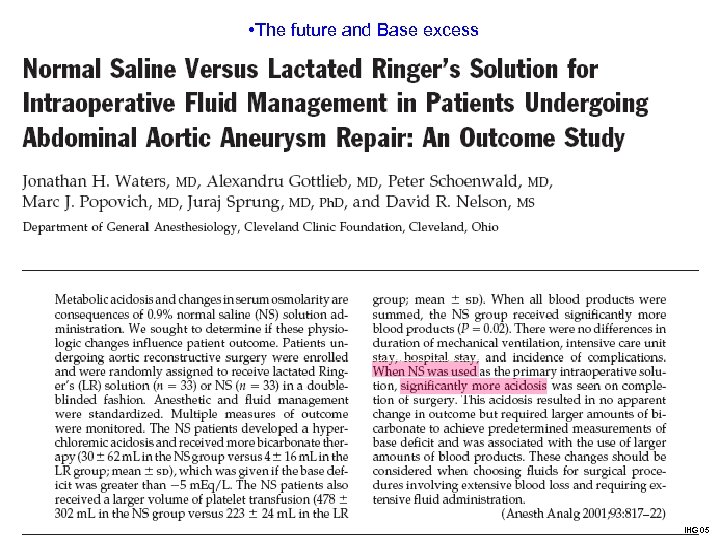
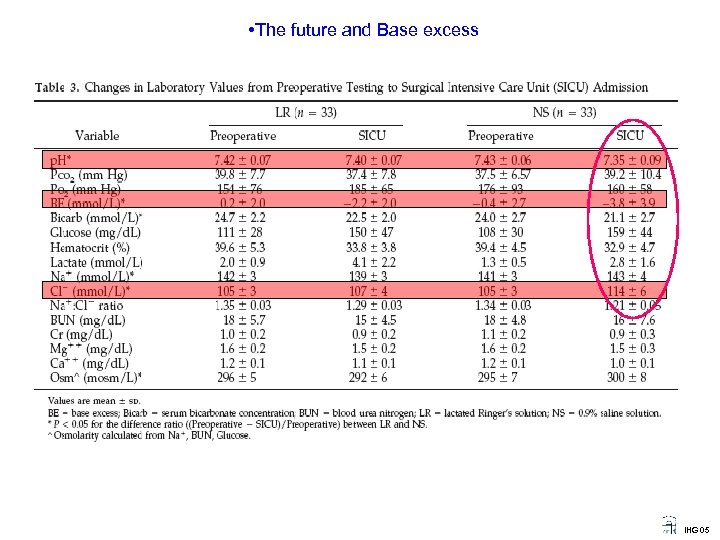
• The future and Base excess Acid-Base status and Normal Saline versus Lactated Ringer’s solution ? IHG 05
• The future and Base excess IHG 05
• The future and Base excess IHG 05
• The future and Base excess IHG 05
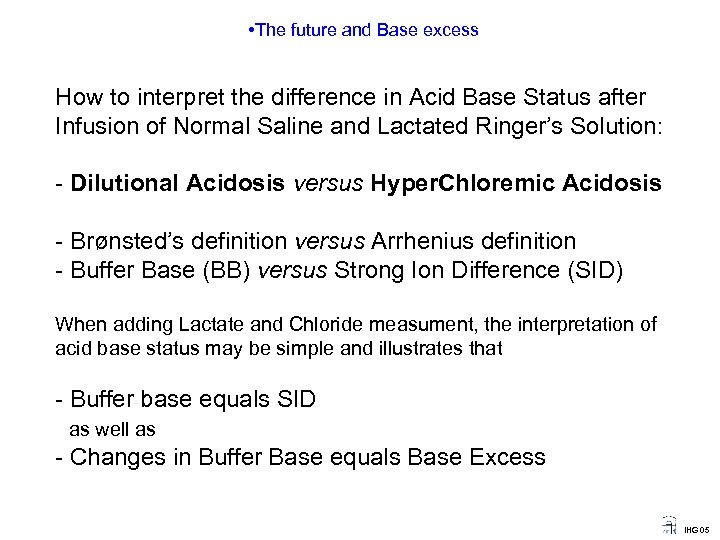
• The future and Base excess How to interpret the difference in Acid Base Status after Infusion of Normal Saline and Lactated Ringer’s Solution: - Dilutional Acidosis versus Hyper. Chloremic Acidosis - Brønsted’s definition versus Arrhenius definition - Buffer Base (BB) versus Strong Ion Difference (SID) When adding Lactate and Chloride measument, the interpretation of acid base status may be simple and illustrates that - Buffer base equals SID as well as - Changes in Buffer Base equals Base Excess IHG 05
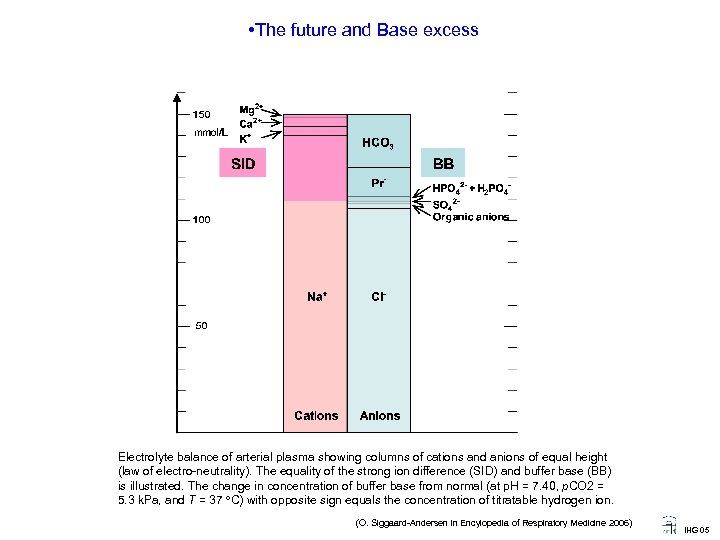
• The future and Base excess Electrolyte balance of arterial plasma showing columns of cations and anions of equal height (law of electro-neutrality). The equality of the strong ion difference (SID) and buffer base (BB) is illustrated. The change in concentration of buffer base from normal (at p. H = 7. 40, p. CO 2 = 5. 3 k. Pa, and T = 37 C) with opposite sign equals the concentration of titratable hydrogen ion. (O. Siggaard-Andersen in Encylopedia of Respiratory Medicine 2006) IHG 05
• The future and Base excess Base Excess is a virtual parameter, making some very complex matter simple and clinical useful Base Excess may continue to exist as the metabolic parameter in Acid-Base status IHG 05

Thanks to John W Severinghaus for his kind advice and help Reykjavik june 2005 Thanks to Ole Siggaard-Andersen for his kind advice and help Gilleleje 2003 IHG 05

Advice from your brain and body: ”Use me or loose me” IHG 05
6f47da57dc5e0585885341b442b14ea2.ppt