c167106c317fc8f4246e9aad97ab957d.ppt
- Количество слайдов: 14
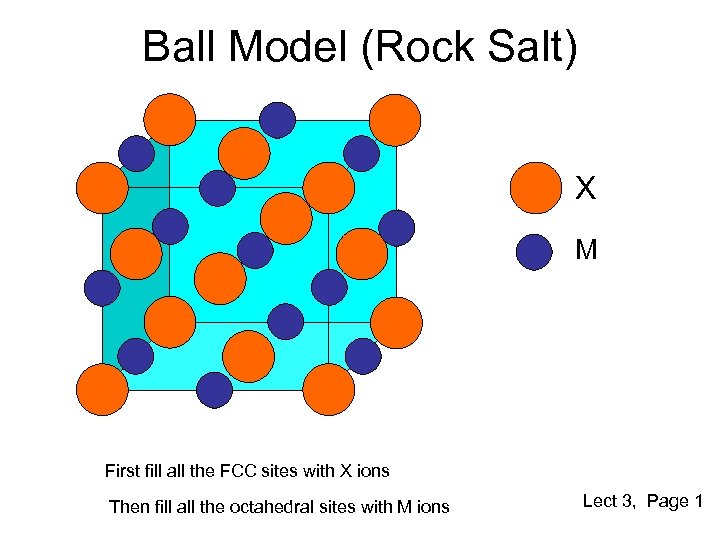
Ball Model (Rock Salt) X M First fill all the FCC sites with X ions Then fill all the octahedral sites with M ions Lect 3, Page 1
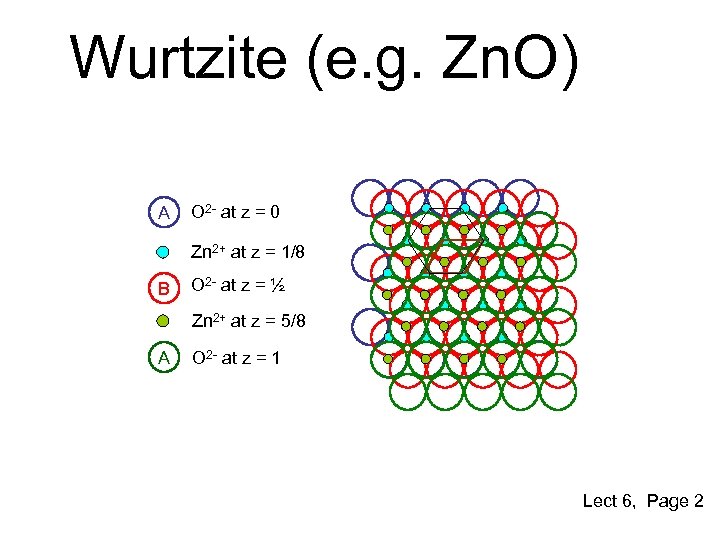
Wurtzite (e. g. Zn. O) A O 2 - at z = 0 Zn 2+ at z = 1/8 B O 2 - at z = ½ Zn 2+ at z = 5/8 A O 2 - at z = 1 Lect 6, Page 2
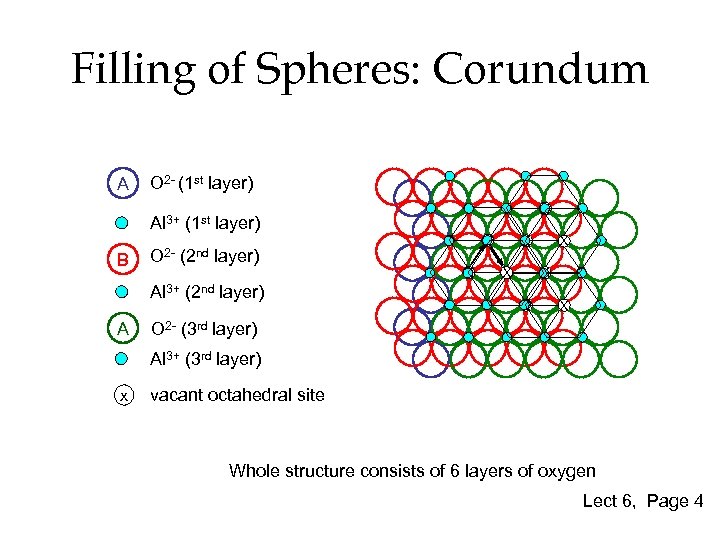
Filling of Spheres: Corundum A O 2 - (1 st layer) X Al 3+ (1 st layer) B X Al 3+ (2 nd layer) A X O 2 - (2 nd layer) X X X O 2 - (3 rd layer) Al 3+ (3 rd layer) x vacant octahedral site Whole structure consists of 6 layers of oxygen Lect 6, Page 4
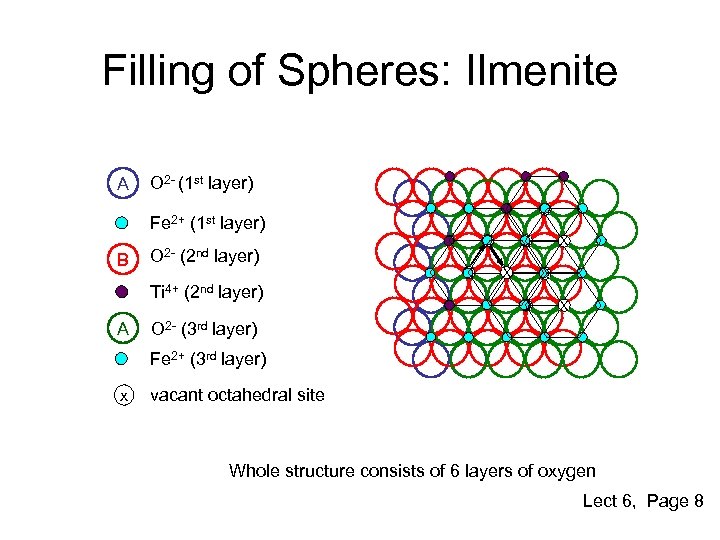
Filling of Spheres: Ilmenite A O 2 - (1 st layer) X Fe 2+ (1 st layer) B X Ti 4+ (2 nd layer) A X O 2 - (2 nd layer) X X X O 2 - (3 rd layer) Fe 2+ (3 rd layer) x vacant octahedral site Whole structure consists of 6 layers of oxygen Lect 6, Page 8
![Projection on {1010} Plane: Ilmenite [0001] Lect 6, Page 9 Projection on {1010} Plane: Ilmenite [0001] Lect 6, Page 9](https://present5.com/presentation/c167106c317fc8f4246e9aad97ab957d/image-5.jpg)
Projection on {1010} Plane: Ilmenite [0001] Lect 6, Page 9
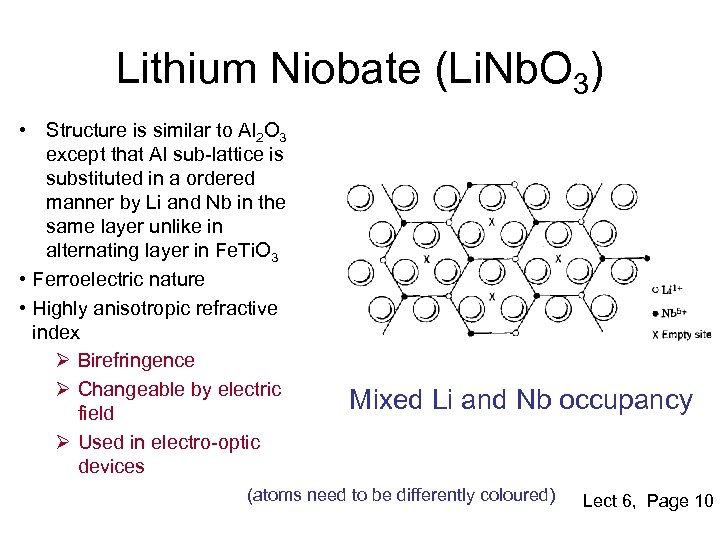
Lithium Niobate (Li. Nb. O 3) • Structure is similar to Al 2 O 3 except that Al sub-lattice is substituted in a ordered manner by Li and Nb in the same layer unlike in alternating layer in Fe. Ti. O 3 • Ferroelectric nature • Highly anisotropic refractive index Ø Birefringence Ø Changeable by electric field Ø Used in electro-optic devices Mixed Li and Nb occupancy (atoms need to be differently coloured) Lect 6, Page 10
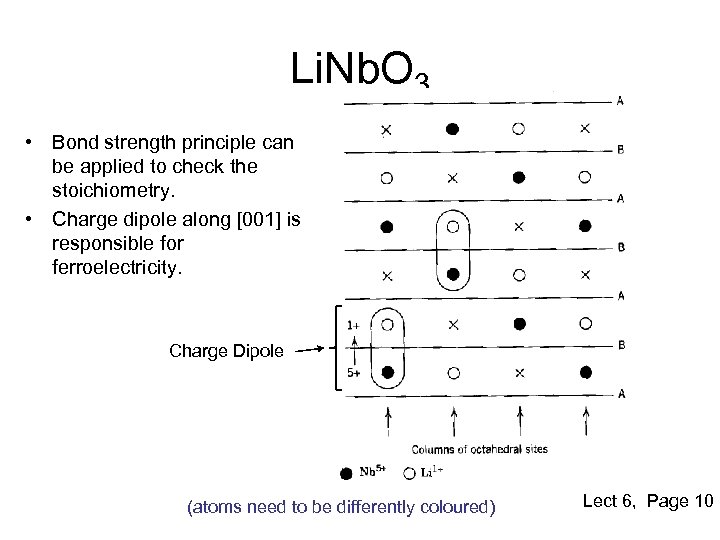
Li. Nb. O 3 • Bond strength principle can be applied to check the stoichiometry. • Charge dipole along [001] is responsible for ferroelectricity. Charge Dipole (atoms need to be differently coloured) Lect 6, Page 10
Rutile Structure • • • Polymorph of titanium di-oxide or Ti. O 2 Other forms are Anatase and Brookite It is formed by quasi-HCP packing of anions Half of the octahedral sites filled by cations Resulting structure is tetragonal due to slight distortion Anisotropic diffusion properties of cations in Ti. O 2 Large and anisotropic refractive index High Bi-refringence Used as pigments and is non-toxic Lect 6, Page 11
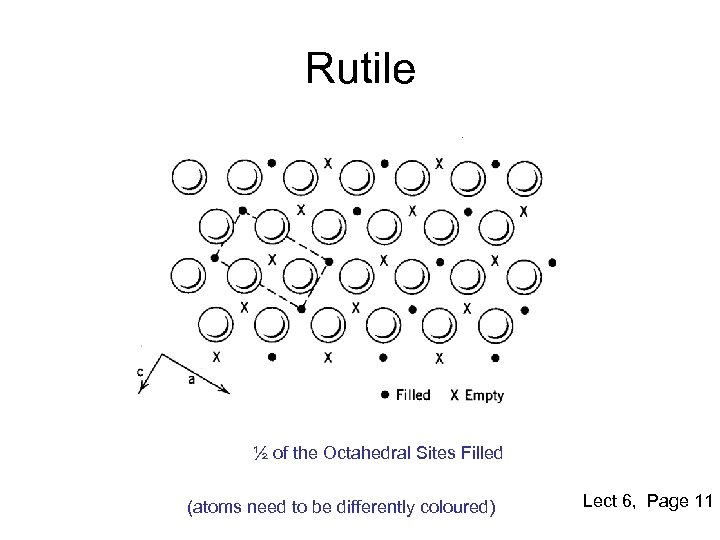
Rutile ½ of the Octahedral Sites Filled (atoms need to be differently coloured) Lect 6, Page 11
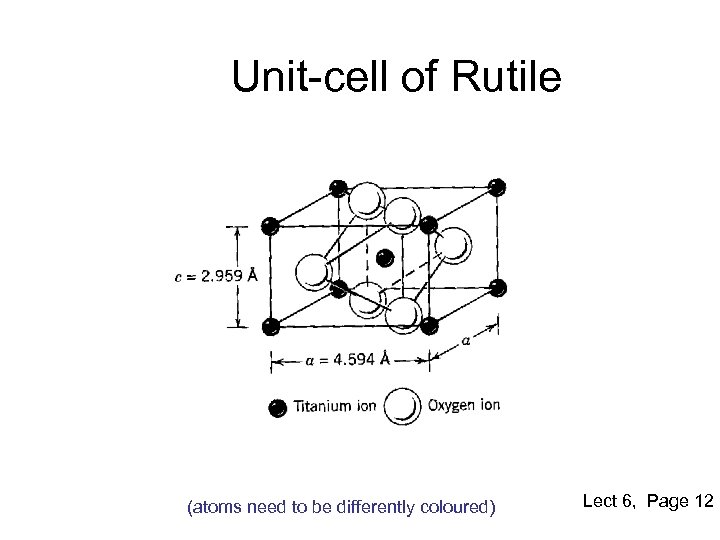
Unit-cell of Rutile (atoms need to be differently coloured) Lect 6, Page 12
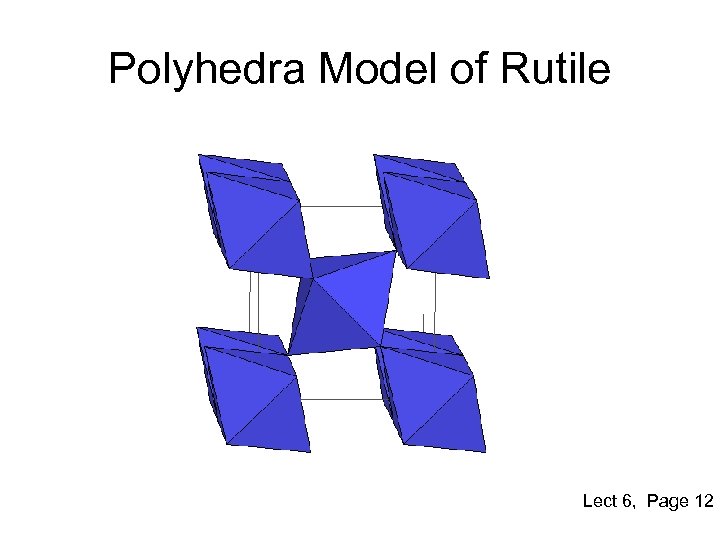
Polyhedra Model of Rutile Lect 6, Page 12
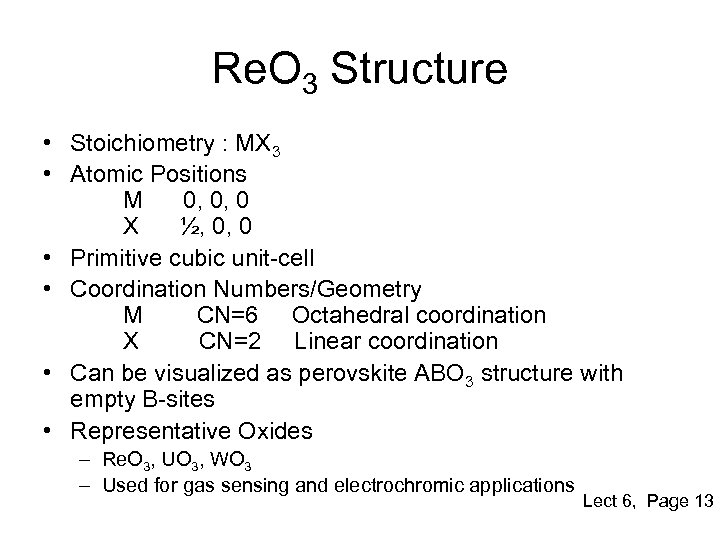
Re. O 3 Structure • Stoichiometry : MX 3 • Atomic Positions M 0, 0, 0 X ½, 0, 0 • Primitive cubic unit-cell • Coordination Numbers/Geometry M CN=6 Octahedral coordination X CN=2 Linear coordination • Can be visualized as perovskite ABO 3 structure with empty B-sites • Representative Oxides – Re. O 3, UO 3, WO 3 – Used for gas sensing and electrochromic applications Lect 6, Page 13
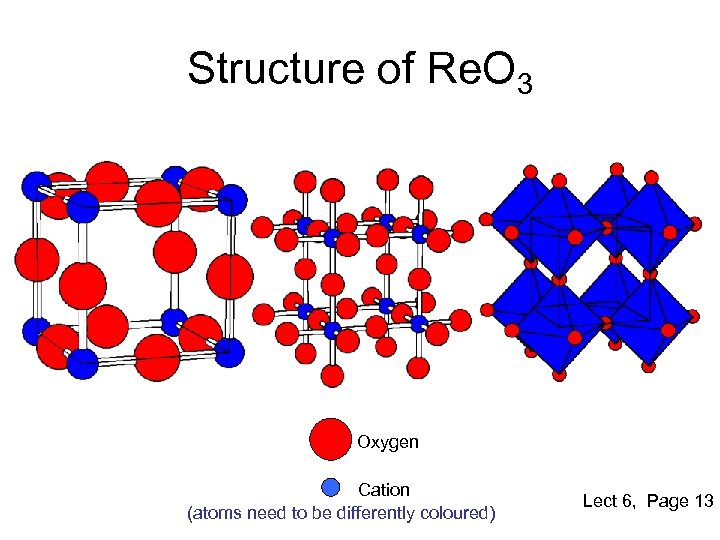
Structure of Re. O 3 Oxygen Cation (atoms need to be differently coloured) Lect 6, Page 13

Summary • Anions form the base lattice • Interstices can be completely or partially filled • Pauling’s rules play important role in structure determination • Deviations lead to structural distortions • Most compounds follow three common structures – FCC packing of anions – HCP packing of anions – Primitive cubic structures Lect 6, Page 14
c167106c317fc8f4246e9aad97ab957d.ppt