
B E B S E E S T S 1 T
Laboratory Accreditation Dr Alan G Rowley (c) Alan Rowley Associates 2009 2

Laboratory Accreditation Dr Alan G Rowley ISO 17025 & Family-AHistory ! 1990 - ISO Guide 25 (3 rd) Linked to National Laboratory Accreditation Standards. 1993 - ISO Guide 58 Requirements for Accreditation body and assessors. Basis for MRAs. 1999 - ISO 17025 International Standard. 2003 - ISO 15189 Specific application for medical laboratories. (c) Alan Rowley Associates 2009 3

Laboratory Accreditation Dr Alan G Rowley ISO 17025 & Family-A-History ! 2005 - Minor modification to ISO 17025 to improve consistency with ISO 9001: 2000 2004 - ISO 17011 Requirements for Accreditation body and assessors. Replaces Guide 58. 2005 - ISO 17011 Requirements for Accreditation body and assessors. ‘Corrected’ version. No substantive changes from 2004. (c) Alan Rowley Associates 2009 4

Laboratory Accreditation Dr Alan G Rowley ISO 17025 & Family-A-History ! 2007 - Revised version of ISO 15189 issued. 2012 - ISO 15189 under revision. (c) Alan Rowley Associates 2009 5

Laboratory Accreditation Dr Alan G Rowley ISO 17025 & Family-A-History ! To be replaced ISO/IEC 17025 -CURRENT VERSION 2005 immanently ISO 15189 -CURRENT VERSION 2007. ISO 17011 -CURRENT VERSION 2005. (c) Alan Rowley Associates 2009 6

Laboratory Accreditation Dr Alan G Rowley (c) Alan Rowley Associates 2009 7

Laboratory Accreditation Dr Alan G Rowley (c) Alan Rowley Associates 2009 8

Laboratory Accreditation ISO 9001 -A Comparison ! ISO 9001 Dr Alan G Rowley Deals only with quality management system. ISO 17025 or 15189 ASSESSMENT ISO 9001 External Audit does not establish: - Includes Peer Review of There is evidence that the laboratory’s data is consistent and methods and external quality control. laboratory accurate, e. g. internal and especially staff. ISO 9001 External Auditors are not normally experts in the technical aspects of the laboratory’s operations. (c) Alan Rowley Associates 2009 9

Laboratory Accreditation Dr Alan G Rowley We tell our clients………………. “We have an ISO 17025/15189 Accredited Laboratory” What does this mean to them ? v A buzz phrase word they have heard ! CONFIDENCE IN v This is a reliable laboratory ! LABORATORY v A precise understanding of the nature of quality management in the laboratory. (c) Alan Rowley Associates 2009 10

Accreditation means……………. There is pro-active quality management in the The Laboratory has been laboratory based on a pattern in the standard which anyone can consult. externally approved as meeting defined is audited regularly by The laboratory quality system standards of an external body whose credentials are publicly competence and quality available. management The competence of the laboratory with regard to defined methods is assessed and accredited by the external body. (c) Alan Rowley Associates 2009 11

Laboratory Accreditation Dr Alan G Rowley Following the standard assures clients that … ØTest methods are validated and suitable for purpose. ØWork is performed on equipment which is calibrated Management takes traceably and properly functioning. all necessary steps to assure ØStaff working in the laboratory are appropriately qualified quality conduct. and trained for the work they ! ØThe laboratory is managed so as to maintain quality at an adequate and consistent level. (c) Alan Rowley Associates 2009 12
Laboratory Accreditation Dr Alan G Rowley Following the standard assures clients that … ØThere is internal. Data is quality and functioning/ quality control of data calibration of controlled so it is equipment to provide confidence in results on a routine basis. consistentcontrol globally use of and of data, e. g. ØThere is external quality comparable recognised/certified references, Proficiency Testing ØWhere problems do occur prompt and effective corrective action, which addresses the root cause, is taken to avoid recurrence. (c) Alan Rowley Associates 2009 13

Laboratory Accreditation Dr Alan G Rowley An accredited laboratory is a marketing asset to the whole organisation since it ………… … ØEstablishes that. This is globally any research and development effort which relies on measurements is soundly based. recognised if the ØEnsures that any production quality control based on accrediting body is laboratory measurements has a sound basis. carefully chosen ØProvides external support for any data on products supplied to any client or government agency; accreditation mark. (c) Alan Rowley Associates 2009 14

Laboratory Accreditation Dr Alan G Rowley This means ACTIVELY By a properly validated method. managing quality and Using equipment that was properly functioning and in calibration having records to prove that you do ! You must be able to show that results were produced By staff who are appropriately trained. That quality controls were applied and confirmed results within required performance characteristics. You must have records which allow this to be audited (c) Alan Rowley Associates 2009 15

Laboratory Accreditation Dr Alan G Rowley You must respond to any problems which occur by carrying out corrective action which addresses the ‘root cause’ of the problem and thus improves the quality system The Quality System Continually Improves ! How do we find out about problems ? By auditing the system regularly By being pro-active in looking for weaknesses-Preventive Action Detection of non-conforming work Feedback from clients, both solicited and complaints. (c) Alan Rowley Associates 2009 16
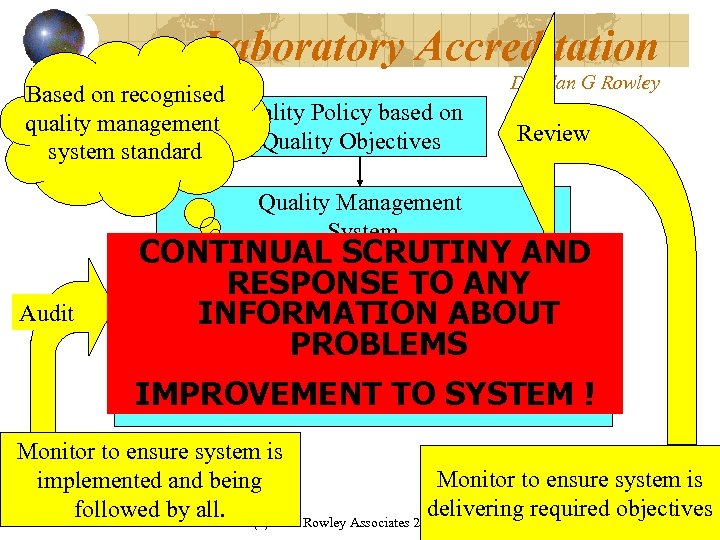
Laboratory Accreditation Based on recognised quality management Quality Policy based on Quality Objectives system standard Dr Alan G Rowley Review Quality Management System Audit CONTINUAL SCRUTINY AND RESPONSE responsibilities Document system, defined. TO ANY INFORMATION ABOUT and procedures PROBLEMS Communicate and Implement IMPROVEMENT TO SYSTEM ! Monitor to ensure system is implemented and being delivering required objectives followed by all. (c) Alan Rowley Associates 2009 17