7758098185fefea094bdd869662f0aad.ppt
- Количество слайдов: 53
 AZIENDA OSPEDALIERO-UNIVERSITARIA DI MODENA Highlights in the management of breast cancer: Lapatinib Rome, november 17, 2006 Pier. Franco Conte Dipartimento di Oncologia e Ematologia Università di Modena e Reggio Emilia
AZIENDA OSPEDALIERO-UNIVERSITARIA DI MODENA Highlights in the management of breast cancer: Lapatinib Rome, november 17, 2006 Pier. Franco Conte Dipartimento di Oncologia e Ematologia Università di Modena e Reggio Emilia
 HER 2: beyond Herceptin 1985: human c. DNA cloning 1987: disease validation 1990: MAb 4 D 5 1998: Herceptin® approved by FDA for metastatic breast cancer 2005: Herceptin® becomes an essential component of the adjuvant treatment of HER 2 + early breast cancer 2007: New HER 2 targeted agents are available
HER 2: beyond Herceptin 1985: human c. DNA cloning 1987: disease validation 1990: MAb 4 D 5 1998: Herceptin® approved by FDA for metastatic breast cancer 2005: Herceptin® becomes an essential component of the adjuvant treatment of HER 2 + early breast cancer 2007: New HER 2 targeted agents are available
 “Non esiste vento favorevole per il marinaio che non sa dove andare” (there is no favourable wind for a sailor who does not know where to go) Seneca (5 a. C-65 d. C)
“Non esiste vento favorevole per il marinaio che non sa dove andare” (there is no favourable wind for a sailor who does not know where to go) Seneca (5 a. C-65 d. C)
 Why targeting HER 2 beyond Trastuzumab in Breast Cancer? • • • Efficacy Primary resistance Secondary resistance Cardiac safety HER 2 + molecular subtypes
Why targeting HER 2 beyond Trastuzumab in Breast Cancer? • • • Efficacy Primary resistance Secondary resistance Cardiac safety HER 2 + molecular subtypes
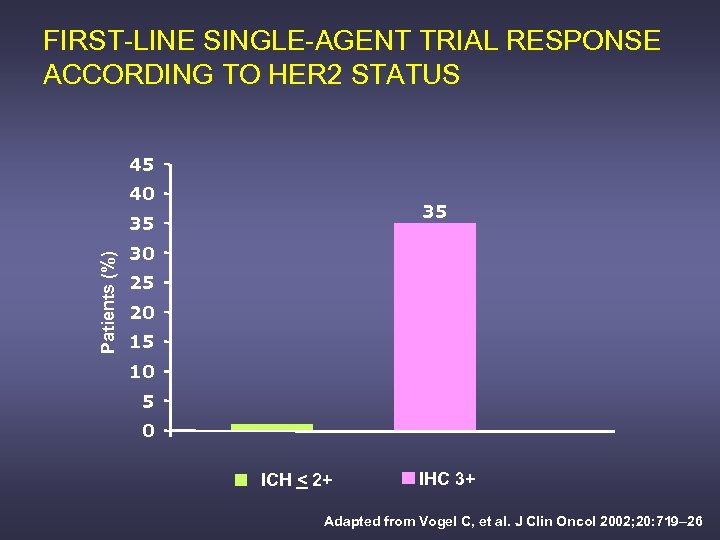 FIRST-LINE SINGLE-AGENT TRIAL RESPONSE ACCORDING TO HER 2 STATUS 45 40 35 Patients (%) 35 30 25 20 15 10 5 0 ICH < 2+ IHC 3+ Adapted from Vogel C, et al. J Clin Oncol 2002; 20: 719– 26
FIRST-LINE SINGLE-AGENT TRIAL RESPONSE ACCORDING TO HER 2 STATUS 45 40 35 Patients (%) 35 30 25 20 15 10 5 0 ICH < 2+ IHC 3+ Adapted from Vogel C, et al. J Clin Oncol 2002; 20: 719– 26
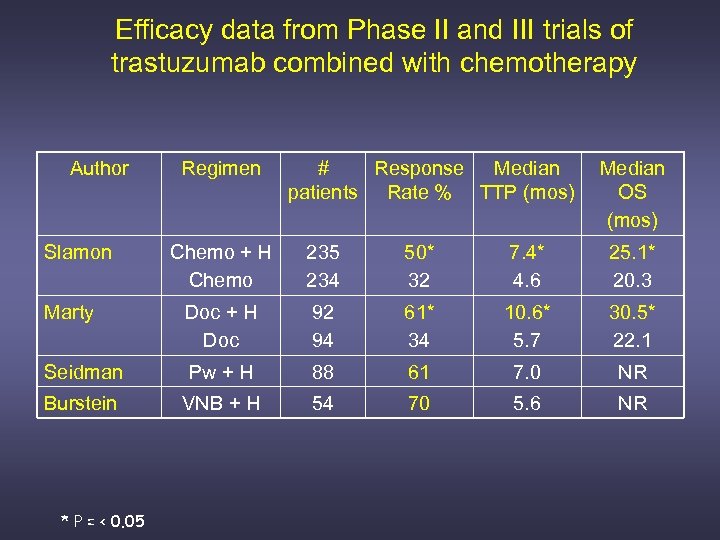 Efficacy data from Phase II and III trials of trastuzumab combined with chemotherapy Author Slamon Regimen # Response Median patients Rate % TTP (mos) Median OS (mos) Chemo + H Chemo 235 234 50* 32 7. 4* 4. 6 25. 1* 20. 3 Marty Doc + H Doc 92 94 61* 34 10. 6* 5. 7 30. 5* 22. 1 Seidman Pw + H 88 61 7. 0 NR Burstein VNB + H 54 70 5. 6 NR * P = < 0. 05
Efficacy data from Phase II and III trials of trastuzumab combined with chemotherapy Author Slamon Regimen # Response Median patients Rate % TTP (mos) Median OS (mos) Chemo + H Chemo 235 234 50* 32 7. 4* 4. 6 25. 1* 20. 3 Marty Doc + H Doc 92 94 61* 34 10. 6* 5. 7 30. 5* 22. 1 Seidman Pw + H 88 61 7. 0 NR Burstein VNB + H 54 70 5. 6 NR * P = < 0. 05
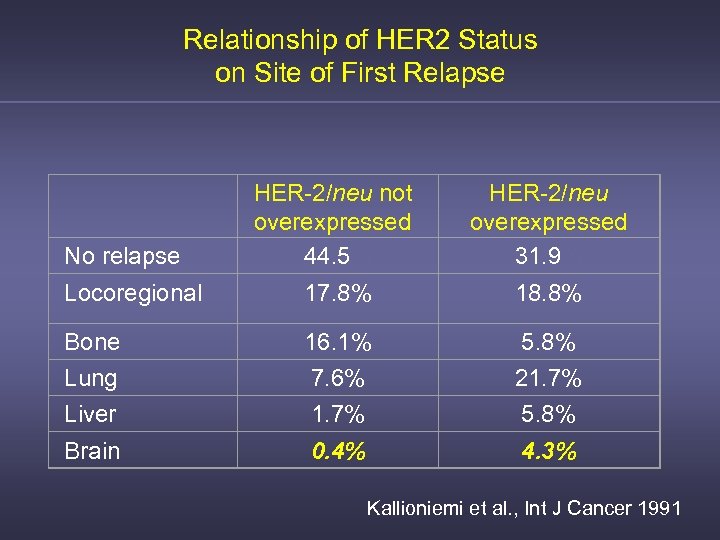 Relationship of HER 2 Status on Site of First Relapse HER-2/neu not overexpressed 44. 5% HER-2/neu overexpressed 31. 9% Locoregional 17. 8% 18. 8% Bone 16. 1% 5. 8% Lung 7. 6% 21. 7% Liver 1. 7% 5. 8% Brain 0. 4% 4. 3% No relapse Kallioniemi et al. , Int J Cancer 1991
Relationship of HER 2 Status on Site of First Relapse HER-2/neu not overexpressed 44. 5% HER-2/neu overexpressed 31. 9% Locoregional 17. 8% 18. 8% Bone 16. 1% 5. 8% Lung 7. 6% 21. 7% Liver 1. 7% 5. 8% Brain 0. 4% 4. 3% No relapse Kallioniemi et al. , Int J Cancer 1991
 High incidence of CNS metastases among women treated with trastuzumab Study Incidence Bendell et al ASCO 2002 34% Weitzen et al ASCO 2002 29% Heinrich et al ASCO 2003 43% Clayton et al Br J Cancer 2004 39% Altaha et al ASCO 2004 33% Stemmler et al SABCS 2004 31%
High incidence of CNS metastases among women treated with trastuzumab Study Incidence Bendell et al ASCO 2002 34% Weitzen et al ASCO 2002 29% Heinrich et al ASCO 2003 43% Clayton et al Br J Cancer 2004 39% Altaha et al ASCO 2004 33% Stemmler et al SABCS 2004 31%
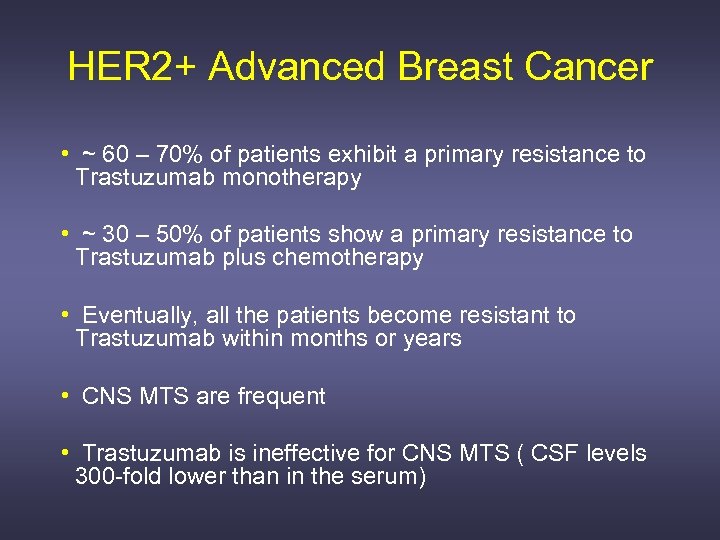 HER 2+ Advanced Breast Cancer • ~ 60 – 70% of patients exhibit a primary resistance to Trastuzumab monotherapy • ~ 30 – 50% of patients show a primary resistance to Trastuzumab plus chemotherapy • Eventually, all the patients become resistant to Trastuzumab within months or years • CNS MTS are frequent • Trastuzumab is ineffective for CNS MTS ( CSF levels 300 -fold lower than in the serum)
HER 2+ Advanced Breast Cancer • ~ 60 – 70% of patients exhibit a primary resistance to Trastuzumab monotherapy • ~ 30 – 50% of patients show a primary resistance to Trastuzumab plus chemotherapy • Eventually, all the patients become resistant to Trastuzumab within months or years • CNS MTS are frequent • Trastuzumab is ineffective for CNS MTS ( CSF levels 300 -fold lower than in the serum)
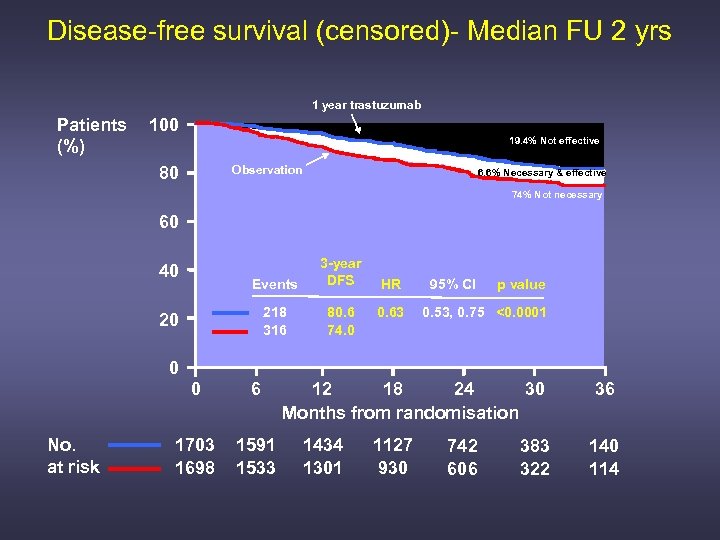 Disease-free survival (censored)- Median FU 2 yrs 1 year trastuzumab Patients (%) 100 19. 4% Not effective Observation 80 6. 6% Necessary & effective 74% Not necessary 60 Events 3 -year DFS 218 316 40 80. 6 74. 0 20 HR 0. 63 95% CI p value 0. 53, 0. 75 <0. 0001 0 0 No. at risk 6 1703 1698 1591 1533 12 18 24 30 Months from randomisation 1434 1301 1127 930 742 606 383 322 36 140 114
Disease-free survival (censored)- Median FU 2 yrs 1 year trastuzumab Patients (%) 100 19. 4% Not effective Observation 80 6. 6% Necessary & effective 74% Not necessary 60 Events 3 -year DFS 218 316 40 80. 6 74. 0 20 HR 0. 63 95% CI p value 0. 53, 0. 75 <0. 0001 0 0 No. at risk 6 1703 1698 1591 1533 12 18 24 30 Months from randomisation 1434 1301 1127 930 742 606 383 322 36 140 114
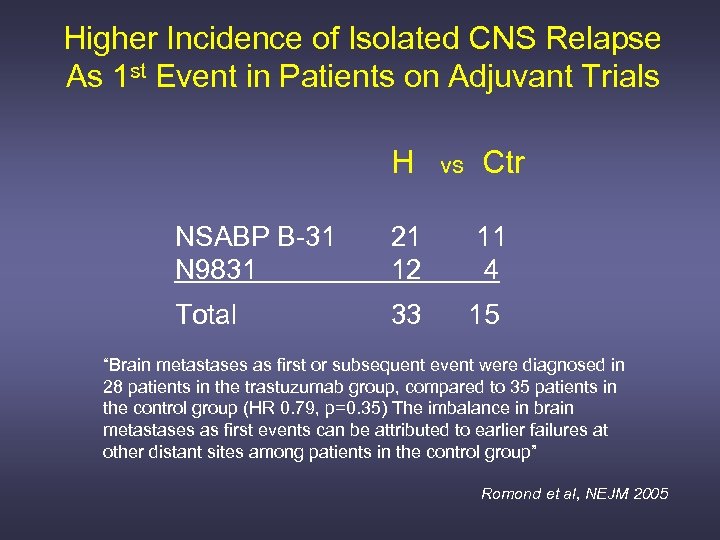 Higher Incidence of Isolated CNS Relapse As 1 st Event in Patients on Adjuvant Trials H vs Ctr NSABP B-31 N 9831 21 11 12 4 Total 33 15 “Brain metastases as first or subsequent event were diagnosed in 28 patients in the trastuzumab group, compared to 35 patients in the control group (HR 0. 79, p=0. 35) The imbalance in brain metastases as first events can be attributed to earlier failures at other distant sites among patients in the control group” Romond et al, NEJM 2005
Higher Incidence of Isolated CNS Relapse As 1 st Event in Patients on Adjuvant Trials H vs Ctr NSABP B-31 N 9831 21 11 12 4 Total 33 15 “Brain metastases as first or subsequent event were diagnosed in 28 patients in the trastuzumab group, compared to 35 patients in the control group (HR 0. 79, p=0. 35) The imbalance in brain metastases as first events can be attributed to earlier failures at other distant sites among patients in the control group” Romond et al, NEJM 2005
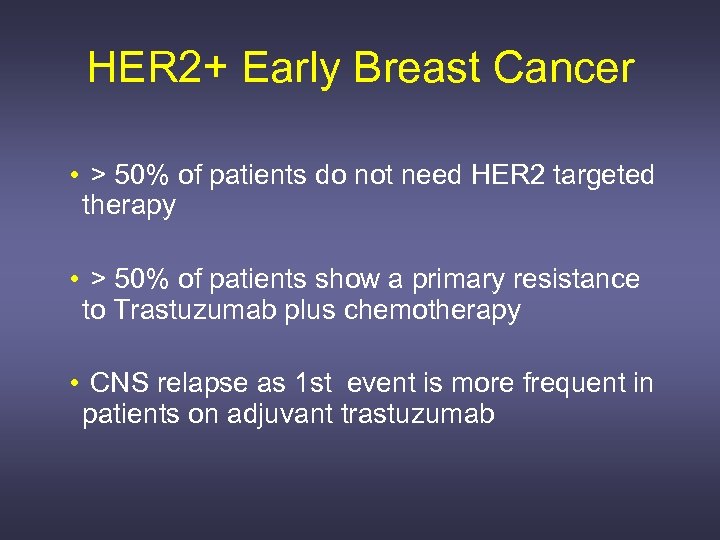 HER 2+ Early Breast Cancer • > 50% of patients do not need HER 2 targeted therapy • > 50% of patients show a primary resistance to Trastuzumab plus chemotherapy • CNS relapse as 1 st event is more frequent in patients on adjuvant trastuzumab
HER 2+ Early Breast Cancer • > 50% of patients do not need HER 2 targeted therapy • > 50% of patients show a primary resistance to Trastuzumab plus chemotherapy • CNS relapse as 1 st event is more frequent in patients on adjuvant trastuzumab
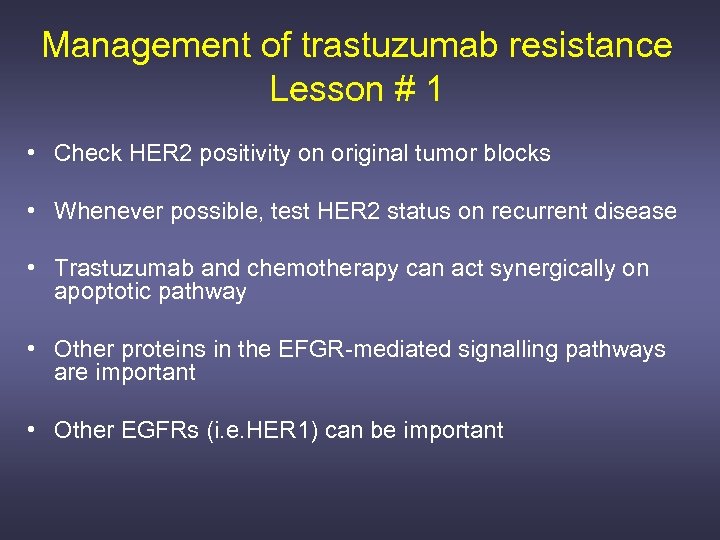 Management of trastuzumab resistance Lesson # 1 • Check HER 2 positivity on original tumor blocks • Whenever possible, test HER 2 status on recurrent disease • Trastuzumab and chemotherapy can act synergically on apoptotic pathway • Other proteins in the EFGR-mediated signalling pathways are important • Other EGFRs (i. e. HER 1) can be important
Management of trastuzumab resistance Lesson # 1 • Check HER 2 positivity on original tumor blocks • Whenever possible, test HER 2 status on recurrent disease • Trastuzumab and chemotherapy can act synergically on apoptotic pathway • Other proteins in the EFGR-mediated signalling pathways are important • Other EGFRs (i. e. HER 1) can be important
 Trastuzumab resistance • Trastuzumab can induce apoptosis through inhibition of PI 3 K/Akt pathway • PTEN normally opposes PI 3 K/Akt signaling • trastuzumab stabilizes PTEN and downregulates Akt signaling • loss of PTEN can induce trastuzumab resistance
Trastuzumab resistance • Trastuzumab can induce apoptosis through inhibition of PI 3 K/Akt pathway • PTEN normally opposes PI 3 K/Akt signaling • trastuzumab stabilizes PTEN and downregulates Akt signaling • loss of PTEN can induce trastuzumab resistance
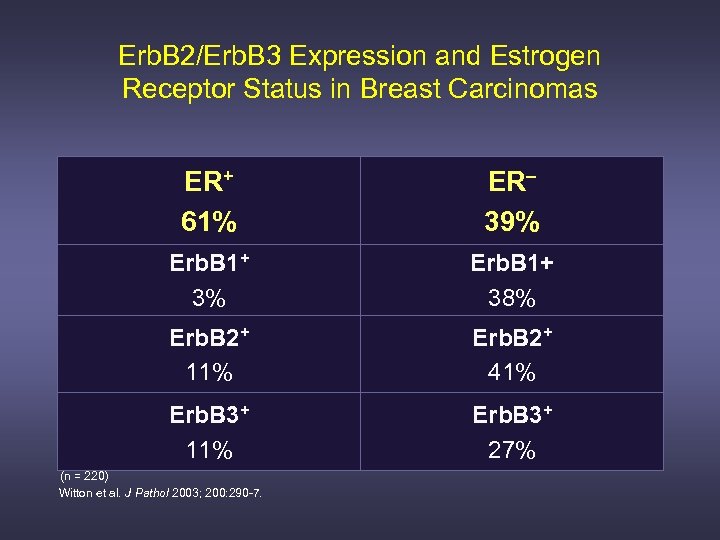 Erb. B 2/Erb. B 3 Expression and Estrogen Receptor Status in Breast Carcinomas ER+ 61% ER– 39% Erb. B 1+ 38% Erb. B 2+ 11% Erb. B 2+ 41% Erb. B 3+ 11% Erb. B 3+ 27% (n = 220) Witton et al. J Pathol 2003; 200: 290 -7.
Erb. B 2/Erb. B 3 Expression and Estrogen Receptor Status in Breast Carcinomas ER+ 61% ER– 39% Erb. B 1+ 38% Erb. B 2+ 11% Erb. B 2+ 41% Erb. B 3+ 11% Erb. B 3+ 27% (n = 220) Witton et al. J Pathol 2003; 200: 290 -7.
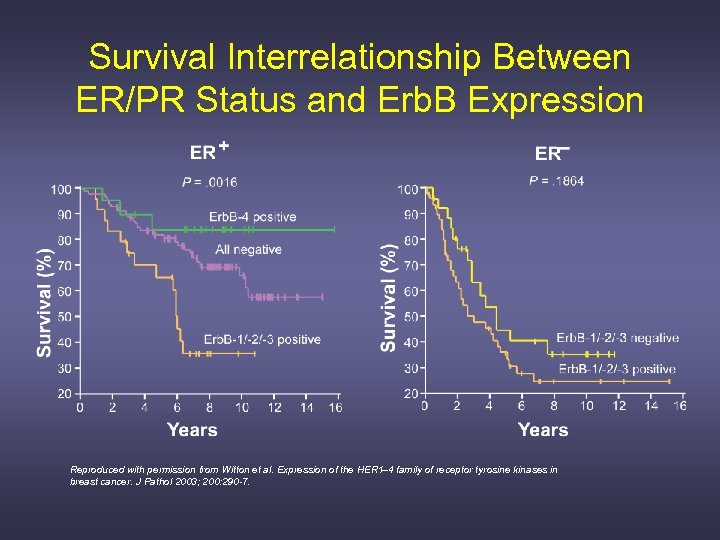 Survival Interrelationship Between ER/PR Status and Erb. B Expression Reproduced with permission from Witton et al. Expression of the HER 1– 4 family of receptor tyrosine kinases in breast cancer. J Pathol 2003; 200: 290 -7.
Survival Interrelationship Between ER/PR Status and Erb. B Expression Reproduced with permission from Witton et al. Expression of the HER 1– 4 family of receptor tyrosine kinases in breast cancer. J Pathol 2003; 200: 290 -7.
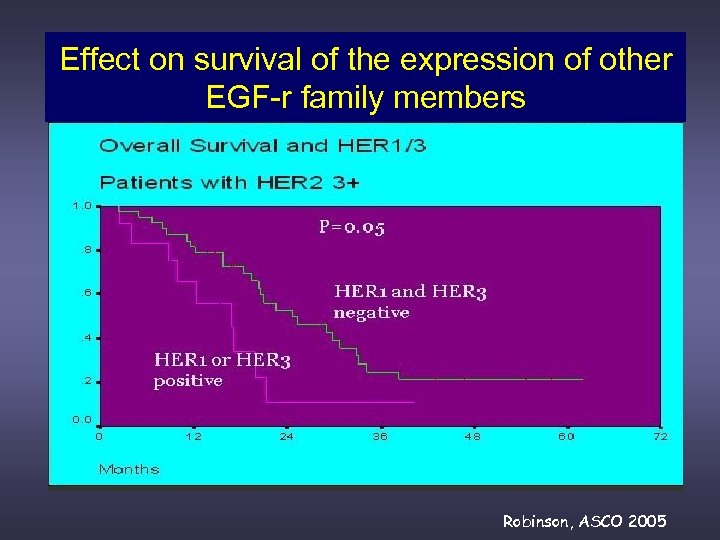 Effect on survival of the expression of other EGF-r family members Robinson, ASCO 2005
Effect on survival of the expression of other EGF-r family members Robinson, ASCO 2005
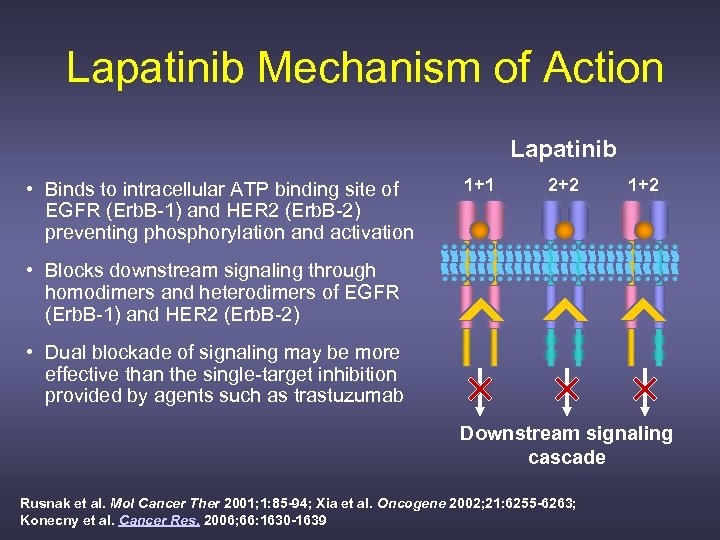 Lapatinib Mechanism of Action Lapatinib • Binds to intracellular ATP binding site of EGFR (Erb. B-1) and HER 2 (Erb. B-2) preventing phosphorylation and activation 1+1 2+2 1+2 • Blocks downstream signaling through homodimers and heterodimers of EGFR (Erb. B-1) and HER 2 (Erb. B-2) • Dual blockade of signaling may be more effective than the single-target inhibition provided by agents such as trastuzumab Downstream signaling cascade Rusnak et al. Mol Cancer Ther 2001; 1: 85 -94; Xia et al. Oncogene 2002; 21: 6255 -6263; Konecny et al. Cancer Res. 2006; 66: 1630 -1639
Lapatinib Mechanism of Action Lapatinib • Binds to intracellular ATP binding site of EGFR (Erb. B-1) and HER 2 (Erb. B-2) preventing phosphorylation and activation 1+1 2+2 1+2 • Blocks downstream signaling through homodimers and heterodimers of EGFR (Erb. B-1) and HER 2 (Erb. B-2) • Dual blockade of signaling may be more effective than the single-target inhibition provided by agents such as trastuzumab Downstream signaling cascade Rusnak et al. Mol Cancer Ther 2001; 1: 85 -94; Xia et al. Oncogene 2002; 21: 6255 -6263; Konecny et al. Cancer Res. 2006; 66: 1630 -1639
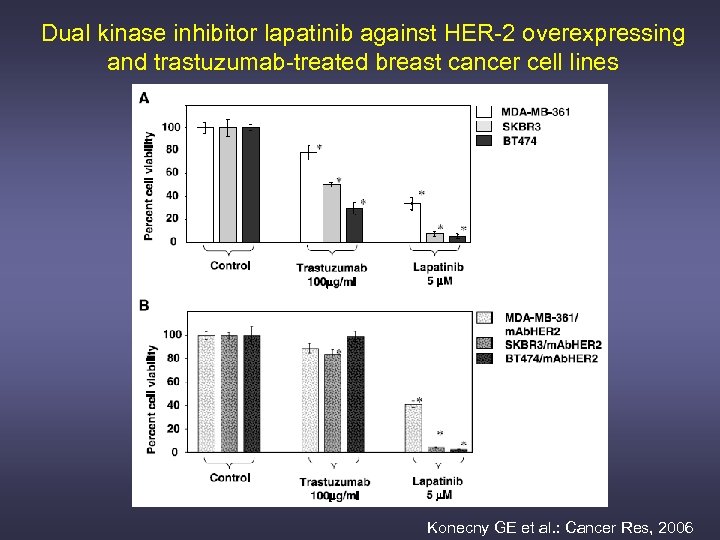 Dual kinase inhibitor lapatinib against HER-2 overexpressing and trastuzumab-treated breast cancer cell lines Konecny GE et al. : Cancer Res, 2006
Dual kinase inhibitor lapatinib against HER-2 overexpressing and trastuzumab-treated breast cancer cell lines Konecny GE et al. : Cancer Res, 2006
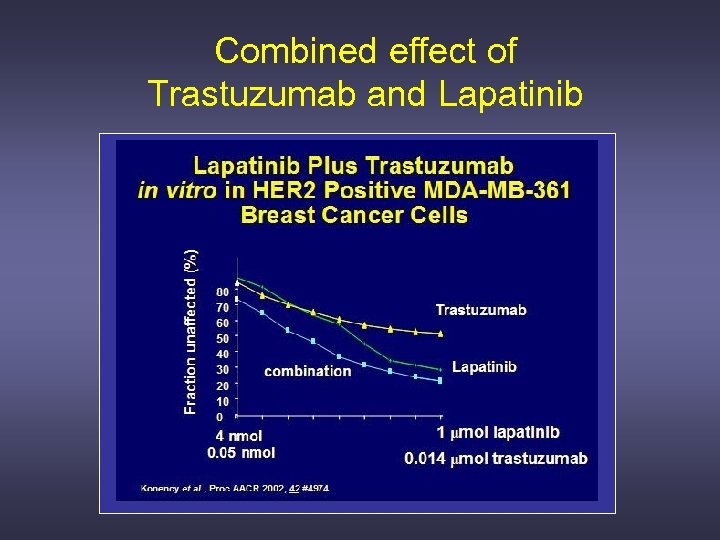 Combined effect of Trastuzumab and Lapatinib
Combined effect of Trastuzumab and Lapatinib
 Phase Ib Trial: EGF 10004 Overview • Study objectives: – Determine a dose or range of biologically active doses – Evaluate safety and tolerability – Examine dose pharmacokinetics and pharmacodynamics • Study design: – Patients randomized to doses of 500, 650, 900, 1200, or 1600 mg/day – Clinical response evaluated every 8 weeks – Biological effects examined by comparing biomarker results from biopsy samples obtained pretreatment and following 21 days of therapy Burris et al. Breast Cancer Res Treat 2003; 82(suppl 1): S 18 (abstract 39).
Phase Ib Trial: EGF 10004 Overview • Study objectives: – Determine a dose or range of biologically active doses – Evaluate safety and tolerability – Examine dose pharmacokinetics and pharmacodynamics • Study design: – Patients randomized to doses of 500, 650, 900, 1200, or 1600 mg/day – Clinical response evaluated every 8 weeks – Biological effects examined by comparing biomarker results from biopsy samples obtained pretreatment and following 21 days of therapy Burris et al. Breast Cancer Res Treat 2003; 82(suppl 1): S 18 (abstract 39).
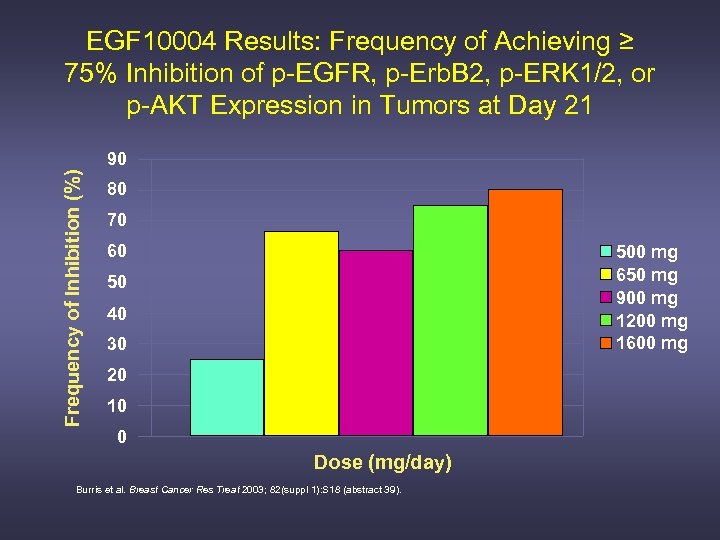 EGF 10004 Results: Frequency of Achieving ≥ 75% Inhibition of p-EGFR, p-Erb. B 2, p-ERK 1/2, or p-AKT Expression in Tumors at Day 21 Frequency of Inhibition (%) 90 80 70 60 500 mg 650 mg 900 mg 1200 mg 1600 mg 50 40 30 20 10 0 Dose (mg/day) Burris et al. Breast Cancer Res Treat 2003; 82(suppl 1): S 18 (abstract 39).
EGF 10004 Results: Frequency of Achieving ≥ 75% Inhibition of p-EGFR, p-Erb. B 2, p-ERK 1/2, or p-AKT Expression in Tumors at Day 21 Frequency of Inhibition (%) 90 80 70 60 500 mg 650 mg 900 mg 1200 mg 1600 mg 50 40 30 20 10 0 Dose (mg/day) Burris et al. Breast Cancer Res Treat 2003; 82(suppl 1): S 18 (abstract 39).
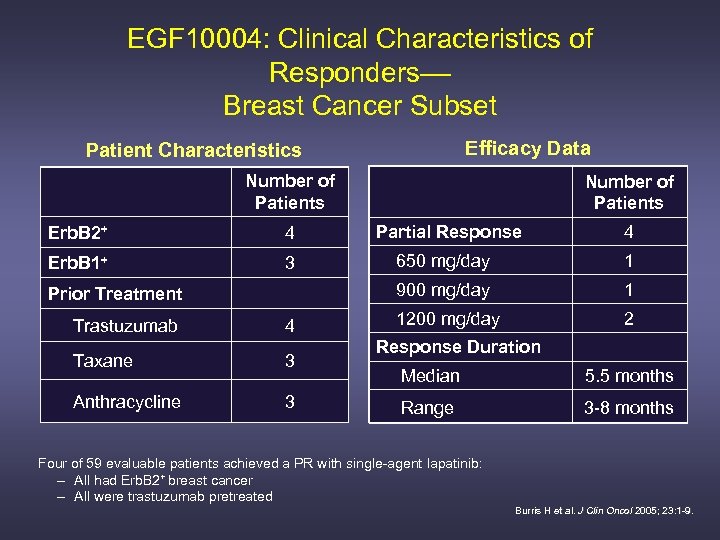 EGF 10004: Clinical Characteristics of Responders–– Breast Cancer Subset Efficacy Data Patient Characteristics Number of Patients Erb. B 2+ 4 Partial Response 4 Erb. B 1+ 3 650 mg/day 1 900 mg/day 1 1200 mg/day 2 Prior Treatment Trastuzumab 4 Taxane 3 Anthracycline 3 Response Duration Median 5. 5 months Range 3 -8 months Four of 59 evaluable patients achieved a PR with single-agent lapatinib: – All had Erb. B 2+ breast cancer – All were trastuzumab pretreated Burris H et al. J Clin Oncol 2005; 23: 1 -9. Burris H et al. J Oncol 2005; 23: 1 -9.
EGF 10004: Clinical Characteristics of Responders–– Breast Cancer Subset Efficacy Data Patient Characteristics Number of Patients Erb. B 2+ 4 Partial Response 4 Erb. B 1+ 3 650 mg/day 1 900 mg/day 1 1200 mg/day 2 Prior Treatment Trastuzumab 4 Taxane 3 Anthracycline 3 Response Duration Median 5. 5 months Range 3 -8 months Four of 59 evaluable patients achieved a PR with single-agent lapatinib: – All had Erb. B 2+ breast cancer – All were trastuzumab pretreated Burris H et al. J Clin Oncol 2005; 23: 1 -9. Burris H et al. J Oncol 2005; 23: 1 -9.
 EGF 20009: A Phase II, Randomized Trial Using the Small Molecule Tyrosine Kinase Inhibitor Lapatinib as a First-Line Treatment in Patients with FISH Positive Advanced or Metastatic Breast Cancer H. L. Gomez, M. A. Chavez, D. C. Doval, L. W. Chow, B. Newstat, S. H. Stein, M. S. Berger, G. W. Sledge ASCO 2005
EGF 20009: A Phase II, Randomized Trial Using the Small Molecule Tyrosine Kinase Inhibitor Lapatinib as a First-Line Treatment in Patients with FISH Positive Advanced or Metastatic Breast Cancer H. L. Gomez, M. A. Chavez, D. C. Doval, L. W. Chow, B. Newstat, S. H. Stein, M. S. Berger, G. W. Sledge ASCO 2005
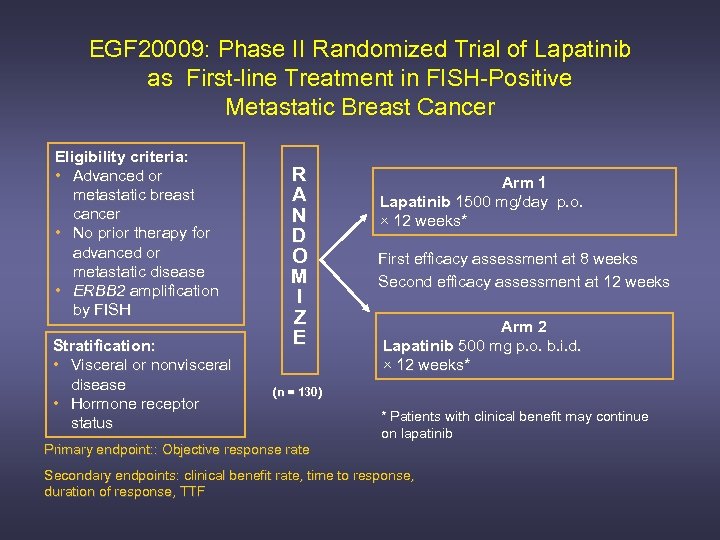 EGF 20009: Phase II Randomized Trial of Lapatinib as First-line Treatment in FISH-Positive Metastatic Breast Cancer Eligibility criteria: • Advanced or metastatic breast cancer • No prior therapy for advanced or metastatic disease • ERBB 2 amplification by FISH Stratification: • Visceral or nonvisceral disease • Hormone receptor status R A N D O M I Z E Arm 1 Lapatinib 1500 mg/day p. o. × 12 weeks* First efficacy assessment at 8 weeks Second efficacy assessment at 12 weeks Arm 2 Lapatinib 500 mg p. o. b. i. d. × 12 weeks* (n = 130) * Patients with clinical benefit may continue on lapatinib Primary endpoint: : Objective response rate Secondary endpoints: clinical benefit rate, time to response, duration of response, TTF
EGF 20009: Phase II Randomized Trial of Lapatinib as First-line Treatment in FISH-Positive Metastatic Breast Cancer Eligibility criteria: • Advanced or metastatic breast cancer • No prior therapy for advanced or metastatic disease • ERBB 2 amplification by FISH Stratification: • Visceral or nonvisceral disease • Hormone receptor status R A N D O M I Z E Arm 1 Lapatinib 1500 mg/day p. o. × 12 weeks* First efficacy assessment at 8 weeks Second efficacy assessment at 12 weeks Arm 2 Lapatinib 500 mg p. o. b. i. d. × 12 weeks* (n = 130) * Patients with clinical benefit may continue on lapatinib Primary endpoint: : Objective response rate Secondary endpoints: clinical benefit rate, time to response, duration of response, TTF
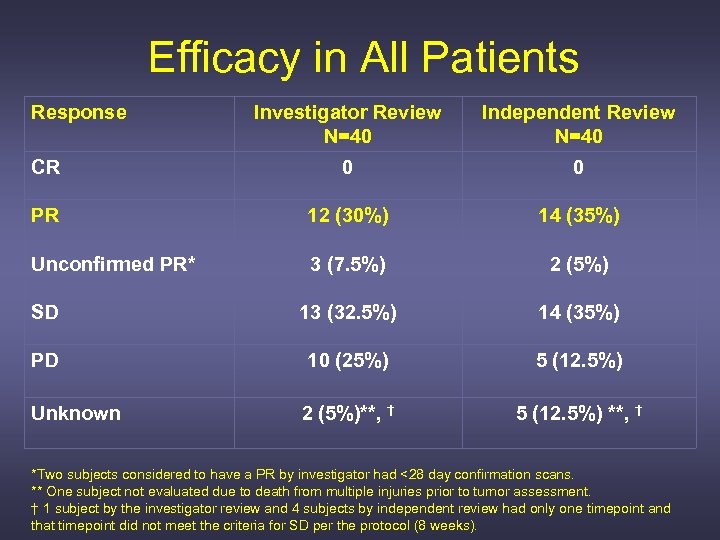 Efficacy in All Patients Response Investigator Review N=40 Independent Review N=40 CR 0 0 PR 12 (30%) 14 (35%) Unconfirmed PR* 3 (7. 5%) 2 (5%) SD 13 (32. 5%) 14 (35%) PD 10 (25%) 5 (12. 5%) Unknown 2 (5%)**, † 5 (12. 5%) **, † *Two subjects considered to have a PR by investigator had <28 day confirmation scans. ** One subject not evaluated due to death from multiple injuries prior to tumor assessment. † 1 subject by the investigator review and 4 subjects by independent review had only one timepoint and that timepoint did not meet the criteria for SD per the protocol (8 weeks).
Efficacy in All Patients Response Investigator Review N=40 Independent Review N=40 CR 0 0 PR 12 (30%) 14 (35%) Unconfirmed PR* 3 (7. 5%) 2 (5%) SD 13 (32. 5%) 14 (35%) PD 10 (25%) 5 (12. 5%) Unknown 2 (5%)**, † 5 (12. 5%) **, † *Two subjects considered to have a PR by investigator had <28 day confirmation scans. ** One subject not evaluated due to death from multiple injuries prior to tumor assessment. † 1 subject by the investigator review and 4 subjects by independent review had only one timepoint and that timepoint did not meet the criteria for SD per the protocol (8 weeks).
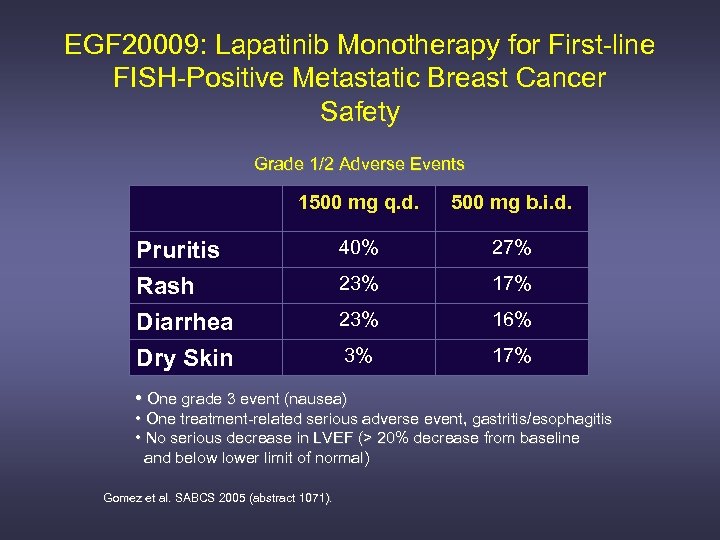 EGF 20009: Lapatinib Monotherapy for First-line FISH-Positive Metastatic Breast Cancer Safety Grade 1/2 Adverse Events 1500 mg q. d. 500 mg b. i. d. Pruritis Rash Diarrhea 40% 27% 23% 16% Dry Skin 3% 17% • One grade 3 event (nausea) • One treatment-related serious adverse event, gastritis/esophagitis • No serious decrease in LVEF (> 20% decrease from baseline and below lower limit of normal) Gomez et al. SABCS 2005 (abstract 1071).
EGF 20009: Lapatinib Monotherapy for First-line FISH-Positive Metastatic Breast Cancer Safety Grade 1/2 Adverse Events 1500 mg q. d. 500 mg b. i. d. Pruritis Rash Diarrhea 40% 27% 23% 16% Dry Skin 3% 17% • One grade 3 event (nausea) • One treatment-related serious adverse event, gastritis/esophagitis • No serious decrease in LVEF (> 20% decrease from baseline and below lower limit of normal) Gomez et al. SABCS 2005 (abstract 1071).
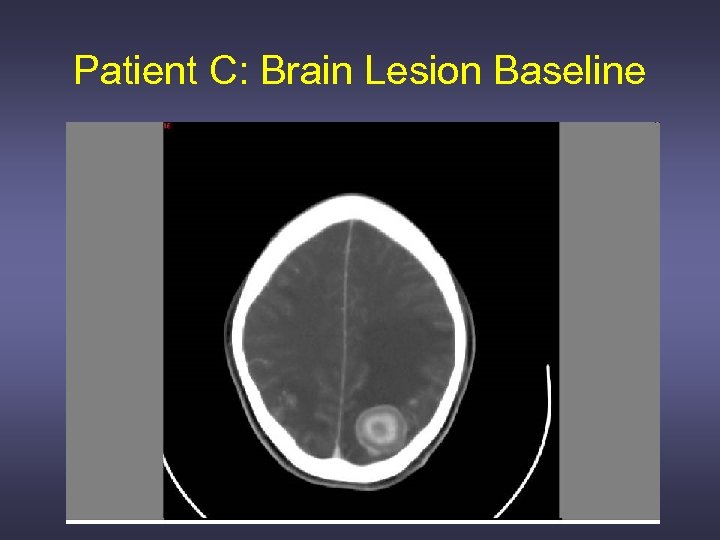 Patient C: Brain Lesion Baseline
Patient C: Brain Lesion Baseline
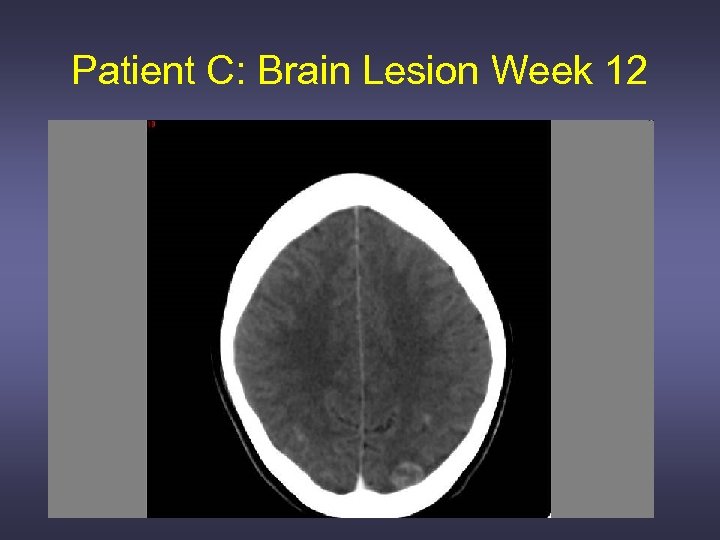 Patient C: Brain Lesion Week 12
Patient C: Brain Lesion Week 12
 Lapatinib + Trastuzumab in Advanced Pretreated Metastatic Breast Cancer Phase I Study: EGF 10023 • Study objectives: – – Safety of lapatinib in combination with trastuzumab Optimally tolerated regimen of combination Pharmacokinetic parameters Clinical activity • Eligibility criteria: – Advanced Erb. B 2+ MBC – Prior treatment with trastuzumab allowed but not required • Treatment consisted of escalating doses of lapatinib 750, 1000, 1250, or 1500 mg/day with trastuzumab (4 -mg/kg loading dose; 2 mg/kg/week) Storniolo et al. ECCO 2005 (abstract 278); SABCS 2005 (abstract 1075).
Lapatinib + Trastuzumab in Advanced Pretreated Metastatic Breast Cancer Phase I Study: EGF 10023 • Study objectives: – – Safety of lapatinib in combination with trastuzumab Optimally tolerated regimen of combination Pharmacokinetic parameters Clinical activity • Eligibility criteria: – Advanced Erb. B 2+ MBC – Prior treatment with trastuzumab allowed but not required • Treatment consisted of escalating doses of lapatinib 750, 1000, 1250, or 1500 mg/day with trastuzumab (4 -mg/kg loading dose; 2 mg/kg/week) Storniolo et al. ECCO 2005 (abstract 278); SABCS 2005 (abstract 1075).
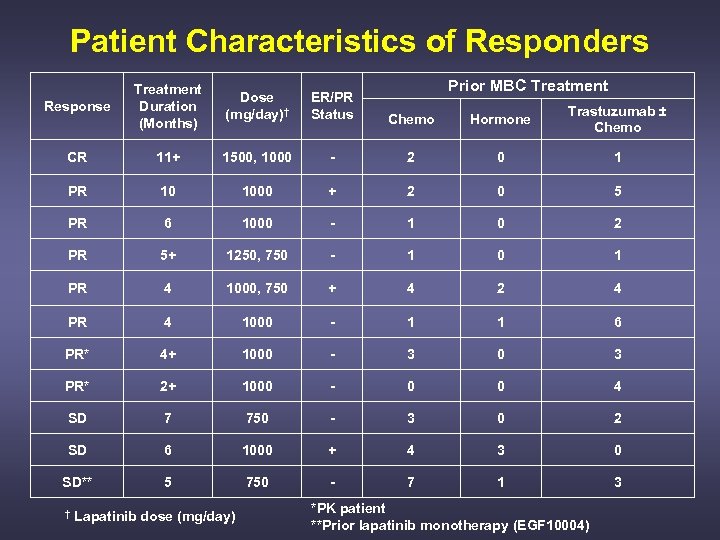 Patient Characteristics of Responders Prior MBC Treatment Response Treatment Duration (Months) Dose (mg/day)† ER/PR Status Chemo Hormone Trastuzumab ± Chemo CR 11+ 1500, 1000 - 2 0 1 PR 10 1000 + 2 0 5 PR 6 1000 - 1 0 2 PR 5+ 1250, 750 - 1 0 1 PR 4 1000, 750 + 4 2 4 PR 4 1000 - 1 1 6 PR* 4+ 1000 - 3 0 3 PR* 2+ 1000 - 0 0 4 SD 7 750 - 3 0 2 SD 6 1000 + 4 3 0 SD** 5 750 - 7 1 3 † Lapatinib dose (mg/day) *PK patient **Prior lapatinib monotherapy (EGF 10004)
Patient Characteristics of Responders Prior MBC Treatment Response Treatment Duration (Months) Dose (mg/day)† ER/PR Status Chemo Hormone Trastuzumab ± Chemo CR 11+ 1500, 1000 - 2 0 1 PR 10 1000 + 2 0 5 PR 6 1000 - 1 0 2 PR 5+ 1250, 750 - 1 0 1 PR 4 1000, 750 + 4 2 4 PR 4 1000 - 1 1 6 PR* 4+ 1000 - 3 0 3 PR* 2+ 1000 - 0 0 4 SD 7 750 - 3 0 2 SD 6 1000 + 4 3 0 SD** 5 750 - 7 1 3 † Lapatinib dose (mg/day) *PK patient **Prior lapatinib monotherapy (EGF 10004)
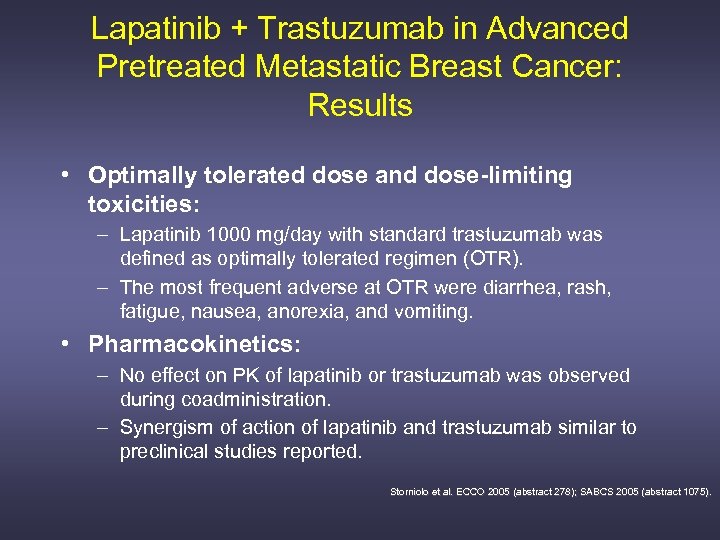 Lapatinib + Trastuzumab in Advanced Pretreated Metastatic Breast Cancer: Results • Optimally tolerated dose and dose-limiting toxicities: – Lapatinib 1000 mg/day with standard trastuzumab was defined as optimally tolerated regimen (OTR). – The most frequent adverse at OTR were diarrhea, rash, fatigue, nausea, anorexia, and vomiting. • Pharmacokinetics: – No effect on PK of lapatinib or trastuzumab was observed during coadministration. – Synergism of action of lapatinib and trastuzumab similar to preclinical studies reported. Storniolo et al. ECCO 2005 (abstract 278); SABCS 2005 (abstract 1075).
Lapatinib + Trastuzumab in Advanced Pretreated Metastatic Breast Cancer: Results • Optimally tolerated dose and dose-limiting toxicities: – Lapatinib 1000 mg/day with standard trastuzumab was defined as optimally tolerated regimen (OTR). – The most frequent adverse at OTR were diarrhea, rash, fatigue, nausea, anorexia, and vomiting. • Pharmacokinetics: – No effect on PK of lapatinib or trastuzumab was observed during coadministration. – Synergism of action of lapatinib and trastuzumab similar to preclinical studies reported. Storniolo et al. ECCO 2005 (abstract 278); SABCS 2005 (abstract 1075).
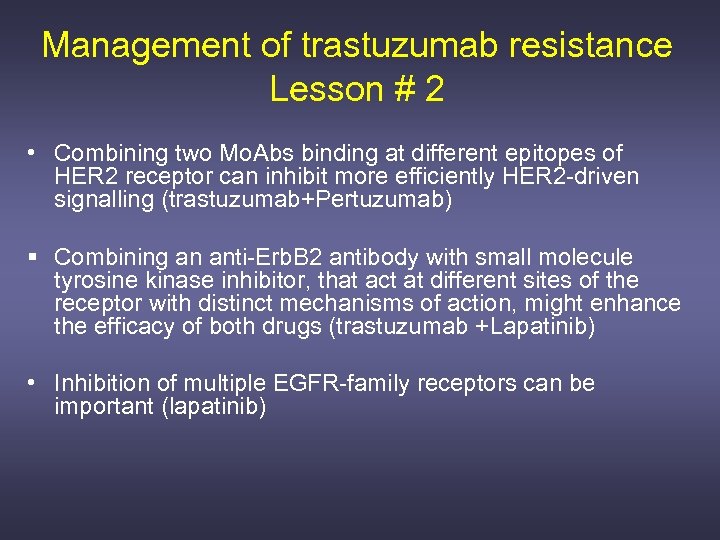 Management of trastuzumab resistance Lesson # 2 • Combining two Mo. Abs binding at different epitopes of HER 2 receptor can inhibit more efficiently HER 2 -driven signalling (trastuzumab+Pertuzumab) § Combining an anti-Erb. B 2 antibody with small molecule tyrosine kinase inhibitor, that act at different sites of the receptor with distinct mechanisms of action, might enhance the efficacy of both drugs (trastuzumab +Lapatinib) • Inhibition of multiple EGFR-family receptors can be important (lapatinib)
Management of trastuzumab resistance Lesson # 2 • Combining two Mo. Abs binding at different epitopes of HER 2 receptor can inhibit more efficiently HER 2 -driven signalling (trastuzumab+Pertuzumab) § Combining an anti-Erb. B 2 antibody with small molecule tyrosine kinase inhibitor, that act at different sites of the receptor with distinct mechanisms of action, might enhance the efficacy of both drugs (trastuzumab +Lapatinib) • Inhibition of multiple EGFR-family receptors can be important (lapatinib)
 A Phase III Randomized, Open-Label, International Study Comparing Lapatinib and Capecitabine vs. Capecitabine in Women with Refractory Advanced or Metastatic Breast Cancer (EGF 100151) C. E. Geyer, D. Cameron, D. Lindquist, S. Chan, T. Pienkowski, C. G. Romieu, A. Jagiello-Gruszfeld, J. Crown, B. Kaufman, A. Chan, J. K. Forster Allegheny General Hospital, Pittsburgh, PA; Western General Hospital, Edinburgh, UK; US Oncolgy Research Network, Houston, TX; Nottingham City Hospital, Nottingham, UK; Cancer Center, Warsaw, Poland; CRCC Val d’Aurelle Paul Lamarque, Montpellier, France; ZOZ MSWi. A, Olsztyn, Poland; St. Vincent’s University Hospital, Dublin, Ireland; Sheba Medical Center, Tel Hashomer, Israel; Mount Medical Centre, Perth, Australia; Glaxo. Smith. Kline, Greenford, UK
A Phase III Randomized, Open-Label, International Study Comparing Lapatinib and Capecitabine vs. Capecitabine in Women with Refractory Advanced or Metastatic Breast Cancer (EGF 100151) C. E. Geyer, D. Cameron, D. Lindquist, S. Chan, T. Pienkowski, C. G. Romieu, A. Jagiello-Gruszfeld, J. Crown, B. Kaufman, A. Chan, J. K. Forster Allegheny General Hospital, Pittsburgh, PA; Western General Hospital, Edinburgh, UK; US Oncolgy Research Network, Houston, TX; Nottingham City Hospital, Nottingham, UK; Cancer Center, Warsaw, Poland; CRCC Val d’Aurelle Paul Lamarque, Montpellier, France; ZOZ MSWi. A, Olsztyn, Poland; St. Vincent’s University Hospital, Dublin, Ireland; Sheba Medical Center, Tel Hashomer, Israel; Mount Medical Centre, Perth, Australia; Glaxo. Smith. Kline, Greenford, UK
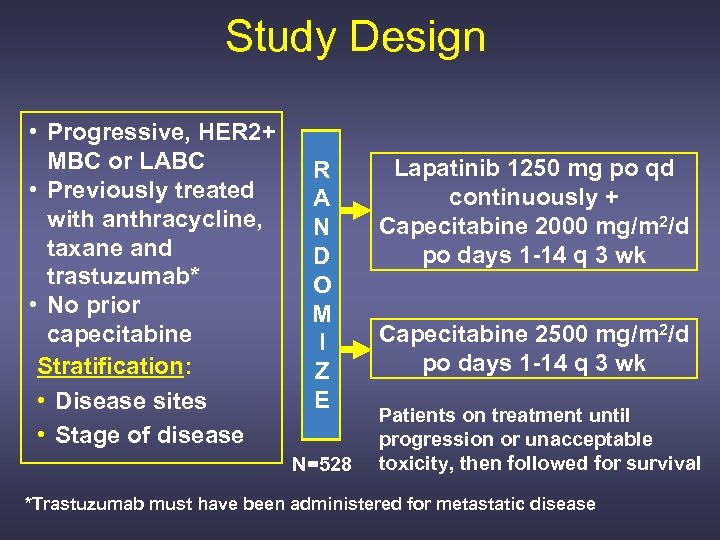 Study Design • Progressive, HER 2+ MBC or LABC • Previously treated with anthracycline, taxane and trastuzumab* • No prior capecitabine Stratification: • Disease sites • Stage of disease R A N D O M I Z E N=528 Lapatinib 1250 mg po qd continuously + Capecitabine 2000 mg/m 2/d po days 1 -14 q 3 wk Capecitabine 2500 mg/m 2/d po days 1 -14 q 3 wk Patients on treatment until progression or unacceptable toxicity, then followed for survival *Trastuzumab must have been administered for metastatic disease
Study Design • Progressive, HER 2+ MBC or LABC • Previously treated with anthracycline, taxane and trastuzumab* • No prior capecitabine Stratification: • Disease sites • Stage of disease R A N D O M I Z E N=528 Lapatinib 1250 mg po qd continuously + Capecitabine 2000 mg/m 2/d po days 1 -14 q 3 wk Capecitabine 2500 mg/m 2/d po days 1 -14 q 3 wk Patients on treatment until progression or unacceptable toxicity, then followed for survival *Trastuzumab must have been administered for metastatic disease
 EGF 100151 - Study Objectives • Primary – TTP • Secondary – Overall survival – Progression free survival – 6 -month progression free survival – Overall tumor response rate – Clinical benefit (complete, partial response or stable disease for at least 6 months) – Time to response – Duration of response
EGF 100151 - Study Objectives • Primary – TTP • Secondary – Overall survival – Progression free survival – 6 -month progression free survival – Overall tumor response rate – Clinical benefit (complete, partial response or stable disease for at least 6 months) – Time to response – Duration of response
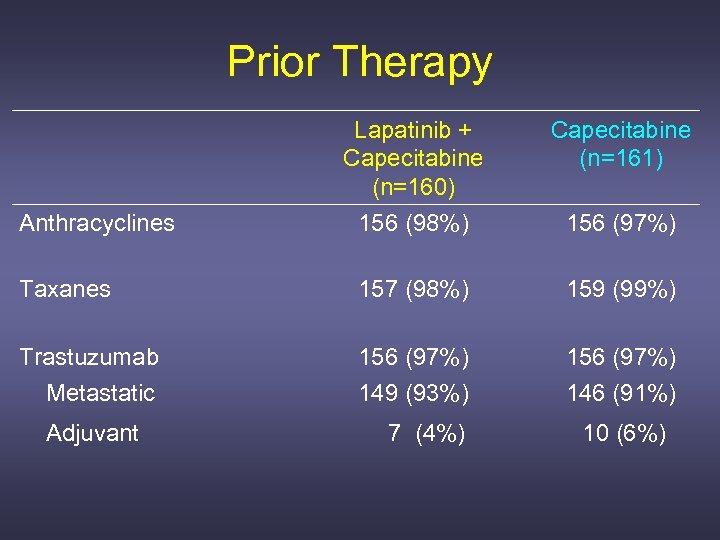 Prior Therapy Lapatinib + Capecitabine (n=160) 156 (98%) Capecitabine (n=161) Taxanes 157 (98%) 159 (99%) Trastuzumab Metastatic 156 (97%) 149 (93%) 156 (97%) 146 (91%) Adjuvant 7 (4%) 10 (6%) Anthracyclines 156 (97%)
Prior Therapy Lapatinib + Capecitabine (n=160) 156 (98%) Capecitabine (n=161) Taxanes 157 (98%) 159 (99%) Trastuzumab Metastatic 156 (97%) 149 (93%) 156 (97%) 146 (91%) Adjuvant 7 (4%) 10 (6%) Anthracyclines 156 (97%)
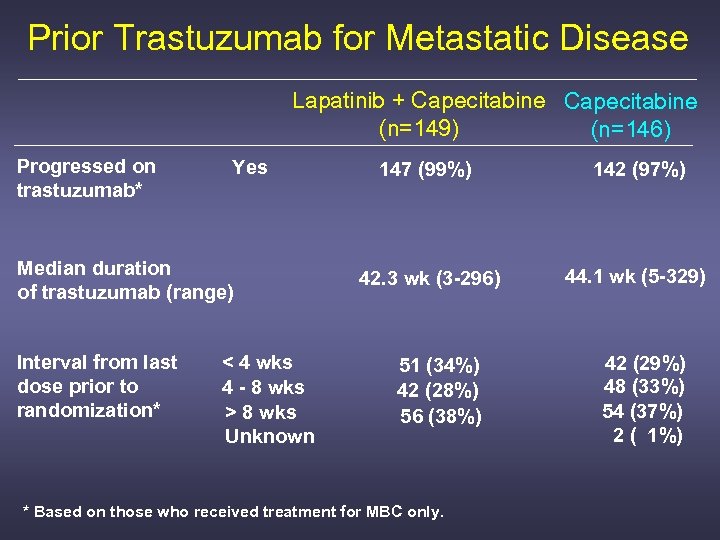 Prior Trastuzumab for Metastatic Disease Lapatinib + Capecitabine (n=149) (n=146) Progressed on trastuzumab* Yes Median duration of trastuzumab (range) Interval from last dose prior to randomization* < 4 wks 4 - 8 wks > 8 wks Unknown 147 (99%) 142 (97%) 42. 3 wk (3 -296) 44. 1 wk (5 -329) 51 (34%) 42 (28%) 56 (38%) * Based on those who received treatment for MBC only. 42 (29%) 48 (33%) 54 (37%) 2 ( 1%)
Prior Trastuzumab for Metastatic Disease Lapatinib + Capecitabine (n=149) (n=146) Progressed on trastuzumab* Yes Median duration of trastuzumab (range) Interval from last dose prior to randomization* < 4 wks 4 - 8 wks > 8 wks Unknown 147 (99%) 142 (97%) 42. 3 wk (3 -296) 44. 1 wk (5 -329) 51 (34%) 42 (28%) 56 (38%) * Based on those who received treatment for MBC only. 42 (29%) 48 (33%) 54 (37%) 2 ( 1%)
 Issued – Monday 3 April 2006, London, UK & Philadelphia, US LSE Announcement Glaxo. Smith. Kline Receives Positive Data and Halts Enrolment in Phase III Trial of Tykerb® (Lapatinib) in Advanced Breast Cancer First Regulatory Filings now Planned for 2 nd Half of 2006 Based on the unanimous recommendation of an Independent Data Monitoring Committee (IDMC), Glaxo. Smith. Kline (GSK) announced today that it has halted enrolment in its Phase III clinical trial evaluating the combination of Tykerb (lapatinib ditosylate) and capecitabine (Xeloda®) versus capecitabine alone. The trial evaluated women with refractory advanced or metastatic breast cancer who have documented Erb. B 2 (HER 2) overexpression and whose disease progressed following treatment with trastuzumab (Herceptin ) as well as other cancer therapies. A pre-planned interim analysis of 321 patients in the study yielded statistically significant results, exceeding the primary endpoint.
Issued – Monday 3 April 2006, London, UK & Philadelphia, US LSE Announcement Glaxo. Smith. Kline Receives Positive Data and Halts Enrolment in Phase III Trial of Tykerb® (Lapatinib) in Advanced Breast Cancer First Regulatory Filings now Planned for 2 nd Half of 2006 Based on the unanimous recommendation of an Independent Data Monitoring Committee (IDMC), Glaxo. Smith. Kline (GSK) announced today that it has halted enrolment in its Phase III clinical trial evaluating the combination of Tykerb (lapatinib ditosylate) and capecitabine (Xeloda®) versus capecitabine alone. The trial evaluated women with refractory advanced or metastatic breast cancer who have documented Erb. B 2 (HER 2) overexpression and whose disease progressed following treatment with trastuzumab (Herceptin ) as well as other cancer therapies. A pre-planned interim analysis of 321 patients in the study yielded statistically significant results, exceeding the primary endpoint.
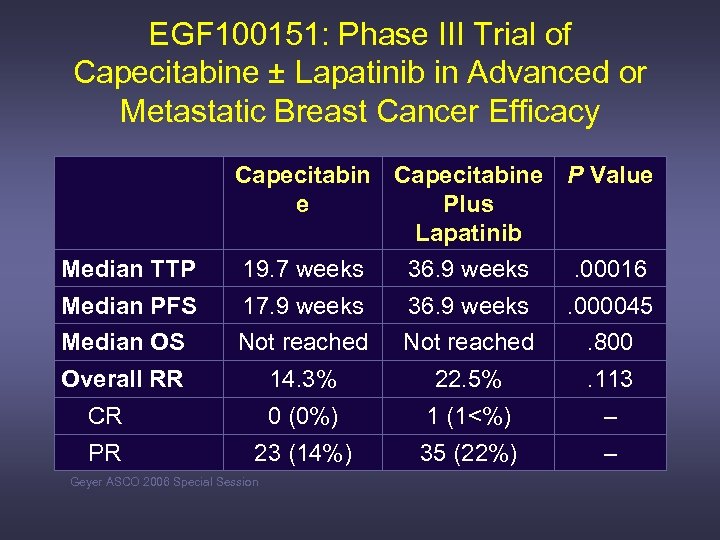 EGF 100151: Phase III Trial of Capecitabine ± Lapatinib in Advanced or Metastatic Breast Cancer Efficacy Median TTP Capecitabine P Value e Plus Lapatinib 19. 7 weeks 36. 9 weeks. 00016 Median PFS 17. 9 weeks 36. 9 weeks . 000045 Median OS Not reached . 800 Overall RR 14. 3% 22. 5% . 113 CR 0 (0%) 1 (1<%) – PR 23 (14%) 35 (22%) – Geyer ASCO 2006 Special Session
EGF 100151: Phase III Trial of Capecitabine ± Lapatinib in Advanced or Metastatic Breast Cancer Efficacy Median TTP Capecitabine P Value e Plus Lapatinib 19. 7 weeks 36. 9 weeks. 00016 Median PFS 17. 9 weeks 36. 9 weeks . 000045 Median OS Not reached . 800 Overall RR 14. 3% 22. 5% . 113 CR 0 (0%) 1 (1<%) – PR 23 (14%) 35 (22%) – Geyer ASCO 2006 Special Session
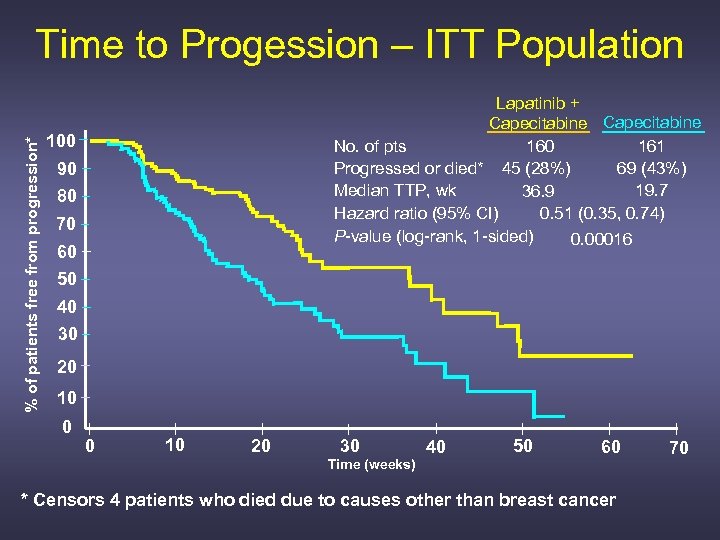 % of patients free from progression* Time to Progession – ITT Population Lapatinib + Capecitabine No. of pts 160 161 Progressed or died* 45 (28%) 69 (43%) Median TTP, wk 19. 7 36. 9 Hazard ratio (95% CI) 0. 51 (0. 35, 0. 74) P-value (log-rank, 1 -sided) 0. 00016 100 90 80 70 60 50 40 30 20 10 0 0 10 20 30 Time (weeks) 40 50 60 * Censors 4 patients who died due to causes other than breast cancer 70
% of patients free from progression* Time to Progession – ITT Population Lapatinib + Capecitabine No. of pts 160 161 Progressed or died* 45 (28%) 69 (43%) Median TTP, wk 19. 7 36. 9 Hazard ratio (95% CI) 0. 51 (0. 35, 0. 74) P-value (log-rank, 1 -sided) 0. 00016 100 90 80 70 60 50 40 30 20 10 0 0 10 20 30 Time (weeks) 40 50 60 * Censors 4 patients who died due to causes other than breast cancer 70
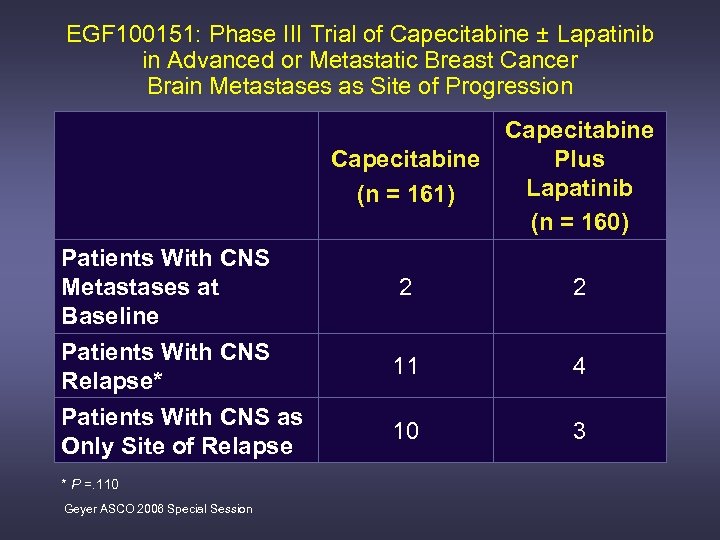 EGF 100151: Phase III Trial of Capecitabine ± Lapatinib in Advanced or Metastatic Breast Cancer Brain Metastases as Site of Progression Capecitabine (n = 161) Patients With CNS Metastases at Baseline Patients With CNS Relapse* Patients With CNS as Only Site of Relapse * P =. 110 Geyer ASCO 2006 Special Session Capecitabine Plus Lapatinib (n = 160) 2 2 11 4 10 3
EGF 100151: Phase III Trial of Capecitabine ± Lapatinib in Advanced or Metastatic Breast Cancer Brain Metastases as Site of Progression Capecitabine (n = 161) Patients With CNS Metastases at Baseline Patients With CNS Relapse* Patients With CNS as Only Site of Relapse * P =. 110 Geyer ASCO 2006 Special Session Capecitabine Plus Lapatinib (n = 160) 2 2 11 4 10 3
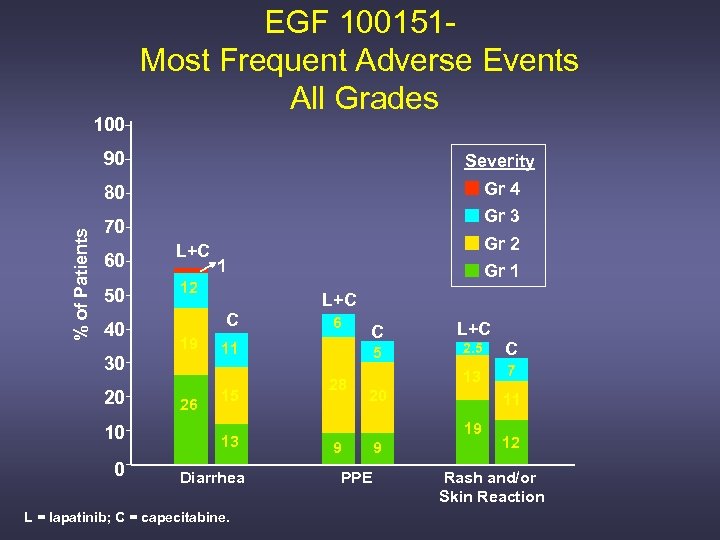 100 EGF 100151 Most Frequent Adverse Events All Grades Severity 80 % of Patients 90 Gr 4 Gr 3 70 60 50 40 L+C 10 0 1 12 C 19 30 20 Gr 2 26 Gr 1 L+C 6 11 15 13 Diarrhea L = lapatinib; C = capecitabine. C 2. 5 C 13 5 28 L+C 7 20 11 19 9 PPE 9 12 Rash and/or Skin Reaction
100 EGF 100151 Most Frequent Adverse Events All Grades Severity 80 % of Patients 90 Gr 4 Gr 3 70 60 50 40 L+C 10 0 1 12 C 19 30 20 Gr 2 26 Gr 1 L+C 6 11 15 13 Diarrhea L = lapatinib; C = capecitabine. C 2. 5 C 13 5 28 L+C 7 20 11 19 9 PPE 9 12 Rash and/or Skin Reaction
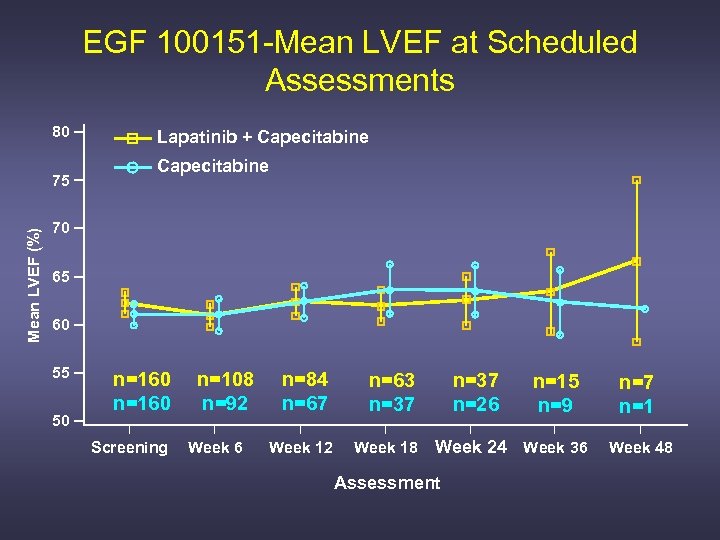 EGF 100151 -Mean LVEF at Scheduled Assessments 80 Mean LVEF (%) 75 Lapatinib + Capecitabine 70 65 60 55 50 n=160 Screening n=108 n=92 Week 6 n=84 n=67 n=63 n=37 Week 12 Week 18 n=37 n=26 n=15 n=9 Week 24 Week 36 Assessment n=7 n=1 Week 48
EGF 100151 -Mean LVEF at Scheduled Assessments 80 Mean LVEF (%) 75 Lapatinib + Capecitabine 70 65 60 55 50 n=160 Screening n=108 n=92 Week 6 n=84 n=67 n=63 n=37 Week 12 Week 18 n=37 n=26 n=15 n=9 Week 24 Week 36 Assessment n=7 n=1 Week 48
 Conclusions Lapatinib with capecitabine is an effective new regimen for advanced HER 2+ breast cancer and should be considered a new standard of care for women meeting eligibility criteria of this trial
Conclusions Lapatinib with capecitabine is an effective new regimen for advanced HER 2+ breast cancer and should be considered a new standard of care for women meeting eligibility criteria of this trial
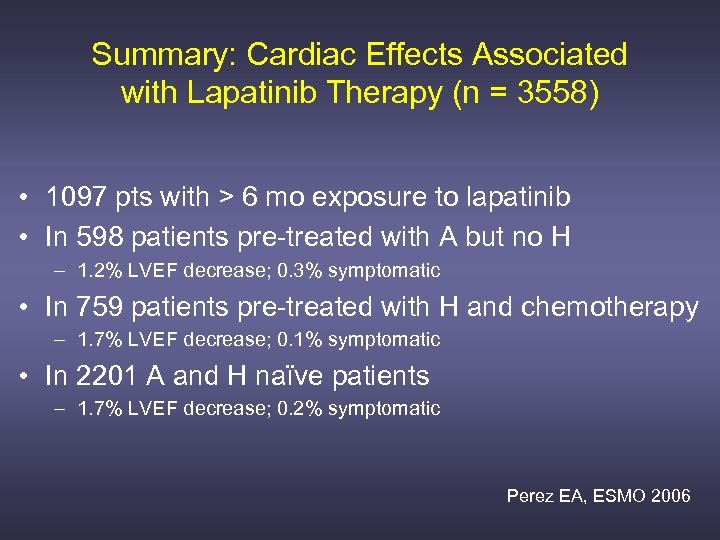 Summary: Cardiac Effects Associated with Lapatinib Therapy (n = 3558) • 1097 pts with > 6 mo exposure to lapatinib • In 598 patients pre-treated with A but no H – 1. 2% LVEF decrease; 0. 3% symptomatic • In 759 patients pre-treated with H and chemotherapy – 1. 7% LVEF decrease; 0. 1% symptomatic • In 2201 A and H naïve patients – 1. 7% LVEF decrease; 0. 2% symptomatic Perez EA, ESMO 2006
Summary: Cardiac Effects Associated with Lapatinib Therapy (n = 3558) • 1097 pts with > 6 mo exposure to lapatinib • In 598 patients pre-treated with A but no H – 1. 2% LVEF decrease; 0. 3% symptomatic • In 759 patients pre-treated with H and chemotherapy – 1. 7% LVEF decrease; 0. 1% symptomatic • In 2201 A and H naïve patients – 1. 7% LVEF decrease; 0. 2% symptomatic Perez EA, ESMO 2006
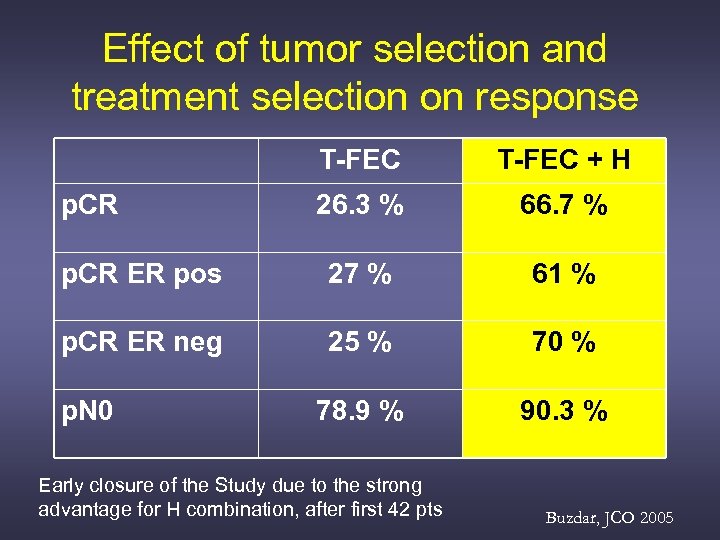 Effect of tumor selection and treatment selection on response T-FEC + H 26. 3 % 66. 7 % p. CR ER pos 27 % 61 % p. CR ER neg 25 % 70 % 78. 9 % 90. 3 % p. CR p. N 0 Early closure of the Study due to the strong advantage for H combination, after first 42 pts Buzdar, JCO 2005
Effect of tumor selection and treatment selection on response T-FEC + H 26. 3 % 66. 7 % p. CR ER pos 27 % 61 % p. CR ER neg 25 % 70 % 78. 9 % 90. 3 % p. CR p. N 0 Early closure of the Study due to the strong advantage for H combination, after first 42 pts Buzdar, JCO 2005
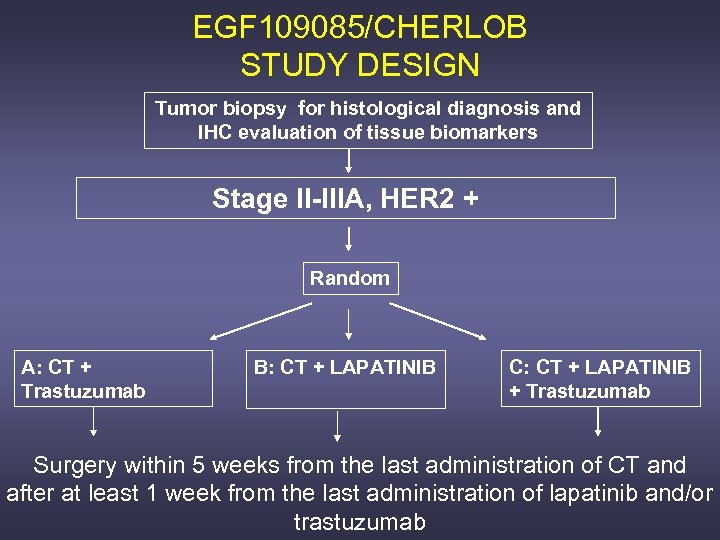 EGF 109085/CHERLOB STUDY DESIGN Tumor biopsy for histological diagnosis and IHC evaluation of tissue biomarkers Stage II-IIIA, HER 2 + Random A: CT + Trastuzumab B: CT + LAPATINIB C: CT + LAPATINIB + Trastuzumab Surgery within 5 weeks from the last administration of CT and after at least 1 week from the last administration of lapatinib and/or trastuzumab
EGF 109085/CHERLOB STUDY DESIGN Tumor biopsy for histological diagnosis and IHC evaluation of tissue biomarkers Stage II-IIIA, HER 2 + Random A: CT + Trastuzumab B: CT + LAPATINIB C: CT + LAPATINIB + Trastuzumab Surgery within 5 weeks from the last administration of CT and after at least 1 week from the last administration of lapatinib and/or trastuzumab
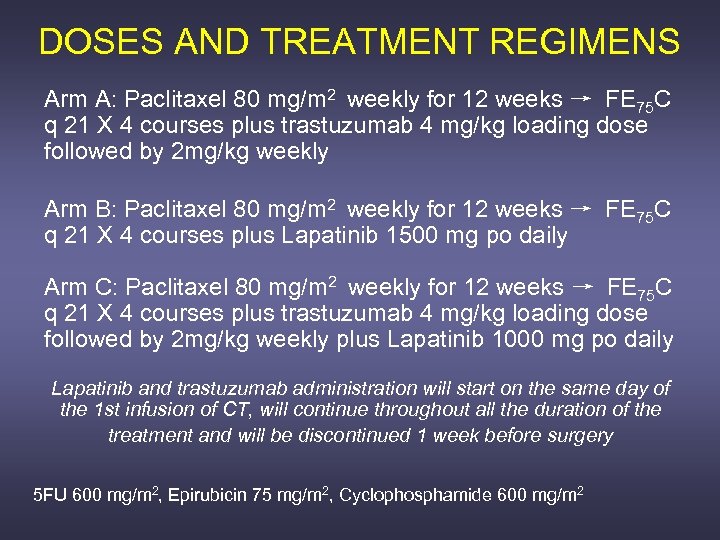 DOSES AND TREATMENT REGIMENS Arm A: Paclitaxel 80 mg/m 2 weekly for 12 weeks → FE 75 C q 21 X 4 courses plus trastuzumab 4 mg/kg loading dose followed by 2 mg/kg weekly Arm B: Paclitaxel 80 mg/m 2 weekly for 12 weeks → FE 75 C q 21 X 4 courses plus Lapatinib 1500 mg po daily Arm C: Paclitaxel 80 mg/m 2 weekly for 12 weeks → FE 75 C q 21 X 4 courses plus trastuzumab 4 mg/kg loading dose followed by 2 mg/kg weekly plus Lapatinib 1000 mg po daily Lapatinib and trastuzumab administration will start on the same day of the 1 st infusion of CT, will continue throughout all the duration of the treatment and will be discontinued 1 week before surgery 5 FU 600 mg/m 2, Epirubicin 75 mg/m 2, Cyclophosphamide 600 mg/m 2
DOSES AND TREATMENT REGIMENS Arm A: Paclitaxel 80 mg/m 2 weekly for 12 weeks → FE 75 C q 21 X 4 courses plus trastuzumab 4 mg/kg loading dose followed by 2 mg/kg weekly Arm B: Paclitaxel 80 mg/m 2 weekly for 12 weeks → FE 75 C q 21 X 4 courses plus Lapatinib 1500 mg po daily Arm C: Paclitaxel 80 mg/m 2 weekly for 12 weeks → FE 75 C q 21 X 4 courses plus trastuzumab 4 mg/kg loading dose followed by 2 mg/kg weekly plus Lapatinib 1000 mg po daily Lapatinib and trastuzumab administration will start on the same day of the 1 st infusion of CT, will continue throughout all the duration of the treatment and will be discontinued 1 week before surgery 5 FU 600 mg/m 2, Epirubicin 75 mg/m 2, Cyclophosphamide 600 mg/m 2
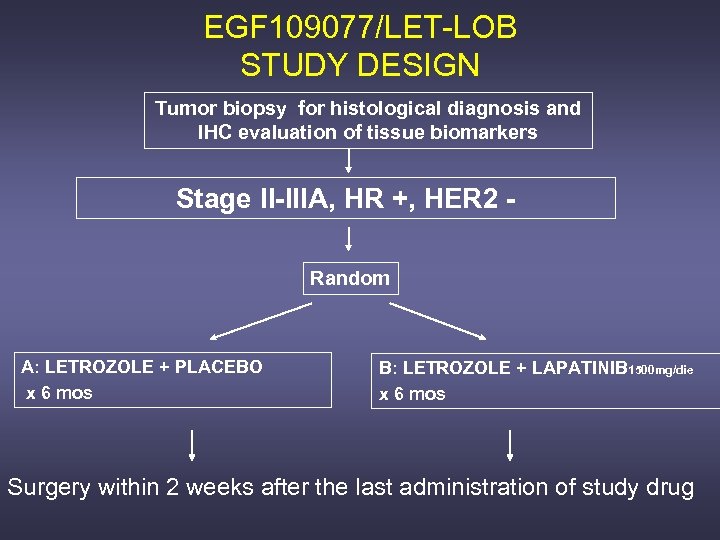 EGF 109077/LET-LOB STUDY DESIGN Tumor biopsy for histological diagnosis and IHC evaluation of tissue biomarkers Stage II-IIIA, HR +, HER 2 Random A: LETROZOLE + PLACEBO x 6 mos B: LETROZOLE + LAPATINIB 1500 mg/die x 6 mos Surgery within 2 weeks after the last administration of study drug
EGF 109077/LET-LOB STUDY DESIGN Tumor biopsy for histological diagnosis and IHC evaluation of tissue biomarkers Stage II-IIIA, HR +, HER 2 Random A: LETROZOLE + PLACEBO x 6 mos B: LETROZOLE + LAPATINIB 1500 mg/die x 6 mos Surgery within 2 weeks after the last administration of study drug
 BIOMARKER EVALUATION The following biomarkers will be evaluated, to define the inhibition of the down-stream pathways of EGFR-family: - EGFR, HER 2 - p. TEN, p. AKT, p. MAPK - Apoptosis with TUNEL Test - Ki 67 These parameters will be evaluated by IHC, on paraffinembedded specimens obtained from diagnostic core biopsy of the primary lesion and from surgical specimens. Fresh tumor tissue from core-biopsy must be snap frozen, to perform a microarray analysis of the gene expression profile before treatment and to evaluate its correlation with response.
BIOMARKER EVALUATION The following biomarkers will be evaluated, to define the inhibition of the down-stream pathways of EGFR-family: - EGFR, HER 2 - p. TEN, p. AKT, p. MAPK - Apoptosis with TUNEL Test - Ki 67 These parameters will be evaluated by IHC, on paraffinembedded specimens obtained from diagnostic core biopsy of the primary lesion and from surgical specimens. Fresh tumor tissue from core-biopsy must be snap frozen, to perform a microarray analysis of the gene expression profile before treatment and to evaluate its correlation with response.
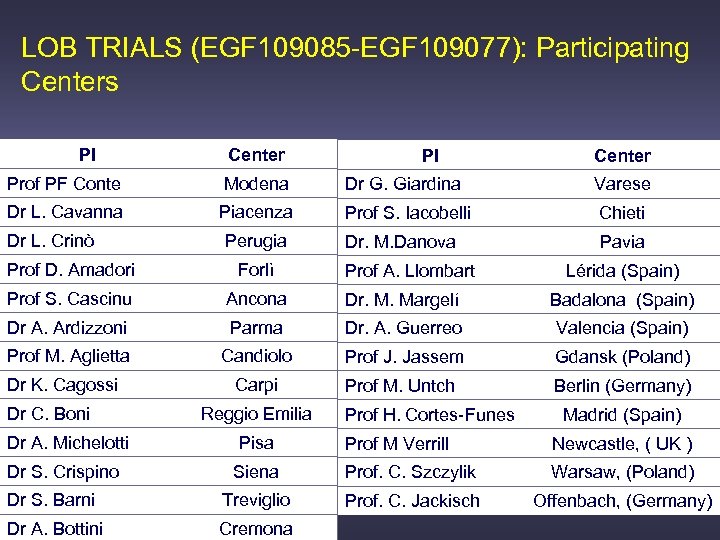 LOB TRIALS (EGF 109085 -EGF 109077): Participating Centers PI Center Prof PF Conte Modena Dr G. Giardina Varese Dr L. Cavanna Piacenza Prof S. Iacobelli Chieti Dr. M. Danova Pavia Dr L. Crinò Perugia Prof D. Amadori Forlì Prof S. Cascinu Ancona Dr. M. Margelí Badalona (Spain) Dr A. Ardizzoni Parma Dr. A. Guerreo Valencia (Spain) Prof M. Aglietta Candiolo Prof J. Jassem Gdansk (Poland) Carpi Prof M. Untch Berlin (Germany) Dr K. Cagossi Dr C. Boni Reggio Emilia Dr A. Michelotti Pisa Dr S. Crispino Prof A. Llombart Prof H. Cortes-Funes Lérida (Spain) Madrid (Spain) Prof M Verrill Newcastle, ( UK ) Siena Prof. C. Szczylik Warsaw, (Poland) Dr S. Barni Treviglio Prof. C. Jackisch Offenbach, (Germany) Dr A. Bottini Cremona
LOB TRIALS (EGF 109085 -EGF 109077): Participating Centers PI Center Prof PF Conte Modena Dr G. Giardina Varese Dr L. Cavanna Piacenza Prof S. Iacobelli Chieti Dr. M. Danova Pavia Dr L. Crinò Perugia Prof D. Amadori Forlì Prof S. Cascinu Ancona Dr. M. Margelí Badalona (Spain) Dr A. Ardizzoni Parma Dr. A. Guerreo Valencia (Spain) Prof M. Aglietta Candiolo Prof J. Jassem Gdansk (Poland) Carpi Prof M. Untch Berlin (Germany) Dr K. Cagossi Dr C. Boni Reggio Emilia Dr A. Michelotti Pisa Dr S. Crispino Prof A. Llombart Prof H. Cortes-Funes Lérida (Spain) Madrid (Spain) Prof M Verrill Newcastle, ( UK ) Siena Prof. C. Szczylik Warsaw, (Poland) Dr S. Barni Treviglio Prof. C. Jackisch Offenbach, (Germany) Dr A. Bottini Cremona
 Molecular subtypes of breast cancer and clinical management • Her 2+ represents a distinct molecular subtype • Her 2+ tumors have a unique clinical behaviour (shorter DFI, more visceral and CNS metastases) • Her 2 + tumors exhibit a peculiar pattern of sensitivity to chemo and hormonal therapy • Her 2 targeting agents have dramatically changed the course of this disease and represent now the foundation of treatment in early and advanced disease
Molecular subtypes of breast cancer and clinical management • Her 2+ represents a distinct molecular subtype • Her 2+ tumors have a unique clinical behaviour (shorter DFI, more visceral and CNS metastases) • Her 2 + tumors exhibit a peculiar pattern of sensitivity to chemo and hormonal therapy • Her 2 targeting agents have dramatically changed the course of this disease and represent now the foundation of treatment in early and advanced disease
