7e00b427478fdb632e5af17d88a57260.ppt
- Количество слайдов: 32
 ATTENTION The slides in this set which contain the VALUE study logo are unedited slides from the VALUE study / investigators. There additional notes on slides #16, 23 in addition to those from VALUE. The rest of the slides are ALLHAT investigators’ slides and not approved by the VALUE investigators.
ATTENTION The slides in this set which contain the VALUE study logo are unedited slides from the VALUE study / investigators. There additional notes on slides #16, 23 in addition to those from VALUE. The rest of the slides are ALLHAT investigators’ slides and not approved by the VALUE investigators.
 VALUE: Primary Hypothesis In hypertensive patients at high cardiovascular risk, for the same level of blood pressure control, valsartan will be more effective than amlodipine in reducing cardiac morbidity and mortality Julius S et al. Lancet. June 2004; 363.
VALUE: Primary Hypothesis In hypertensive patients at high cardiovascular risk, for the same level of blood pressure control, valsartan will be more effective than amlodipine in reducing cardiac morbidity and mortality Julius S et al. Lancet. June 2004; 363.
 VALUE: Primary Endpoint • Composite cardiac morbidity and mortality – sudden cardiac death – fatal/nonfatal MI – evidence of recent MI on autopsy – emergency thrombolytic/fibrinolytic treatment and/or emergency PTCA/CABG to avoid MI – death during/after PTCA/CABG – new or chronic CHF requiring hospital management – heart failure death Mann J, Julius S. Blood Press. 1998; 7: 176– 183.
VALUE: Primary Endpoint • Composite cardiac morbidity and mortality – sudden cardiac death – fatal/nonfatal MI – evidence of recent MI on autopsy – emergency thrombolytic/fibrinolytic treatment and/or emergency PTCA/CABG to avoid MI – death during/after PTCA/CABG – new or chronic CHF requiring hospital management – heart failure death Mann J, Julius S. Blood Press. 1998; 7: 176– 183.
 VALUE: Secondary Endpoints and Pre-specified Analyses • Secondary Endpoints: – fatal/non-fatal myocardial infarction – fatal/non-fatal stroke – fatal/non-fatal heart failure • Pre-specified Analyses: – all-cause mortality – new-onset diabetes Julius S et al. Lancet. June 2004; 363.
VALUE: Secondary Endpoints and Pre-specified Analyses • Secondary Endpoints: – fatal/non-fatal myocardial infarction – fatal/non-fatal stroke – fatal/non-fatal heart failure • Pre-specified Analyses: – all-cause mortality – new-onset diabetes Julius S et al. Lancet. June 2004; 363.
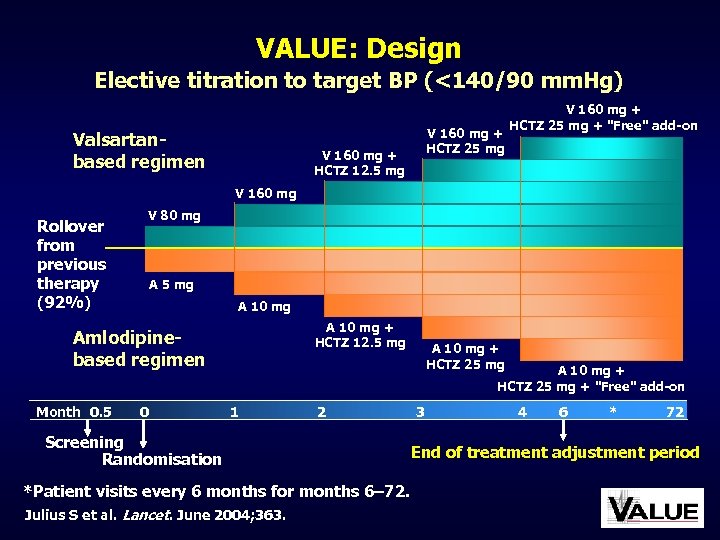 VALUE: Design Elective titration to target BP (<140/90 mm. Hg) Valsartanbased regimen V 160 mg + HCTZ 25 mg V 160 mg + HCTZ 12. 5 mg V 160 mg + HCTZ 25 mg + "Free" add-on V 160 mg Rollover from previous therapy (92%) V 80 mg A 5 mg A 10 mg + HCTZ 12. 5 mg Amlodipinebased regimen Month 0. 5 0 A 10 mg + HCTZ 25 mg + "Free" add-on 1 2 Screening Randomisation *Patient visits every 6 months for months 6– 72. Julius S et al. Lancet. June 2004; 363. 3 4 6 * 72 End of treatment adjustment period
VALUE: Design Elective titration to target BP (<140/90 mm. Hg) Valsartanbased regimen V 160 mg + HCTZ 25 mg V 160 mg + HCTZ 12. 5 mg V 160 mg + HCTZ 25 mg + "Free" add-on V 160 mg Rollover from previous therapy (92%) V 80 mg A 5 mg A 10 mg + HCTZ 12. 5 mg Amlodipinebased regimen Month 0. 5 0 A 10 mg + HCTZ 25 mg + "Free" add-on 1 2 Screening Randomisation *Patient visits every 6 months for months 6– 72. Julius S et al. Lancet. June 2004; 363. 3 4 6 * 72 End of treatment adjustment period
 VALUE: Patient Population • Treated or untreated hypertensive patients – entry criteria for untreated hypertension: 160– 210 mm. Hg systolic, 95– 105 mm. Hg diastolic • Age ≥ 50 years, male or female • High-risk for cardiac events – one or more defined risk factors or diseases Mann J, Julius S. Blood Press. 1998; 7: 176– 183.
VALUE: Patient Population • Treated or untreated hypertensive patients – entry criteria for untreated hypertension: 160– 210 mm. Hg systolic, 95– 105 mm. Hg diastolic • Age ≥ 50 years, male or female • High-risk for cardiac events – one or more defined risk factors or diseases Mann J, Julius S. Blood Press. 1998; 7: 176– 183.
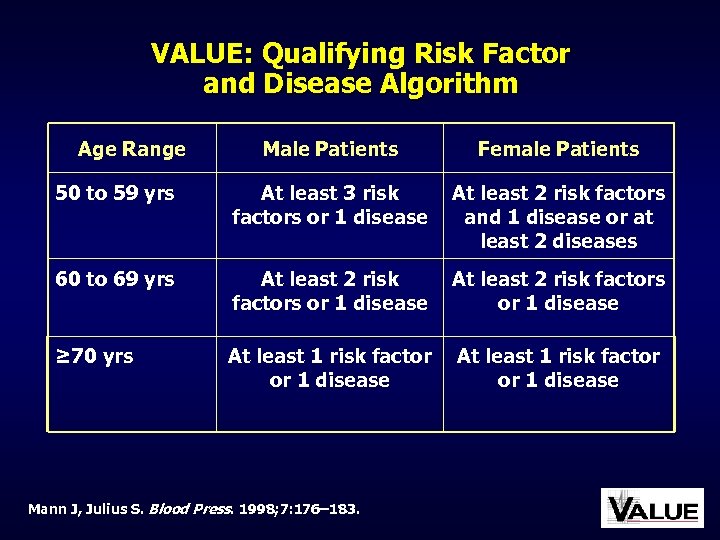 VALUE: Qualifying Risk Factor and Disease Algorithm Age Range Male Patients Female Patients 50 to 59 yrs At least 3 risk factors or 1 disease At least 2 risk factors and 1 disease or at least 2 diseases 60 to 69 yrs At least 2 risk factors or 1 disease ≥ 70 yrs At least 1 risk factor or 1 disease Mann J, Julius S. Blood Press. 1998; 7: 176– 183.
VALUE: Qualifying Risk Factor and Disease Algorithm Age Range Male Patients Female Patients 50 to 59 yrs At least 3 risk factors or 1 disease At least 2 risk factors and 1 disease or at least 2 diseases 60 to 69 yrs At least 2 risk factors or 1 disease ≥ 70 yrs At least 1 risk factor or 1 disease Mann J, Julius S. Blood Press. 1998; 7: 176– 183.
 VALUE: Qualifying Risk Factors and Diseases Risk Factors Diseases • Diabetes mellitus • History of CHD • Cigarette smoking • Peripheral vascular disease • Hypercholesterolemia • Left ventricular hypertrophy (LVH) without strain patterns • Proteinuria • Serum creatinine 150– 265 µmol/L Mann J, Julius S. Blood Press. 1998; 7: 176– 183. • Stroke or transient ischemic attack • LVH with ECG documented strain patterns (ST segment depression)
VALUE: Qualifying Risk Factors and Diseases Risk Factors Diseases • Diabetes mellitus • History of CHD • Cigarette smoking • Peripheral vascular disease • Hypercholesterolemia • Left ventricular hypertrophy (LVH) without strain patterns • Proteinuria • Serum creatinine 150– 265 µmol/L Mann J, Julius S. Blood Press. 1998; 7: 176– 183. • Stroke or transient ischemic attack • LVH with ECG documented strain patterns (ST segment depression)
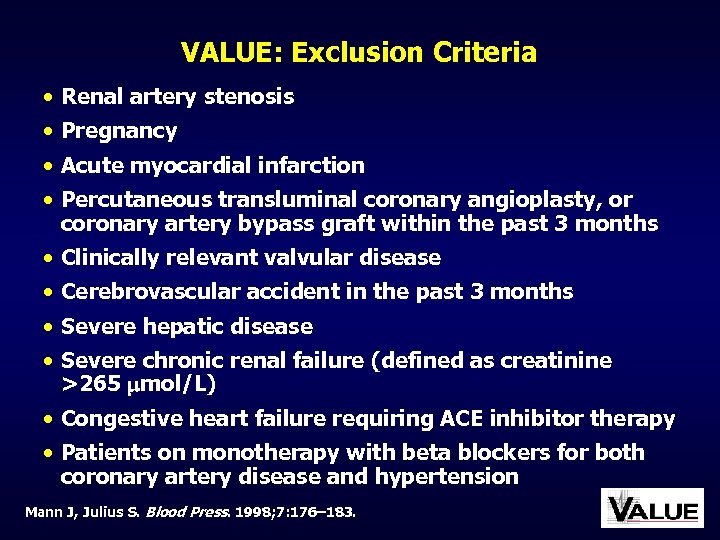 VALUE: Exclusion Criteria • Renal artery stenosis • Pregnancy • Acute myocardial infarction • Percutaneous transluminal coronary angioplasty, or coronary artery bypass graft within the past 3 months • Clinically relevant valvular disease • Cerebrovascular accident in the past 3 months • Severe hepatic disease • Severe chronic renal failure (defined as creatinine >265 mol/L) • Congestive heart failure requiring ACE inhibitor therapy • Patients on monotherapy with beta blockers for both coronary artery disease and hypertension Mann J, Julius S. Blood Press. 1998; 7: 176– 183.
VALUE: Exclusion Criteria • Renal artery stenosis • Pregnancy • Acute myocardial infarction • Percutaneous transluminal coronary angioplasty, or coronary artery bypass graft within the past 3 months • Clinically relevant valvular disease • Cerebrovascular accident in the past 3 months • Severe hepatic disease • Severe chronic renal failure (defined as creatinine >265 mol/L) • Congestive heart failure requiring ACE inhibitor therapy • Patients on monotherapy with beta blockers for both coronary artery disease and hypertension Mann J, Julius S. Blood Press. 1998; 7: 176– 183.
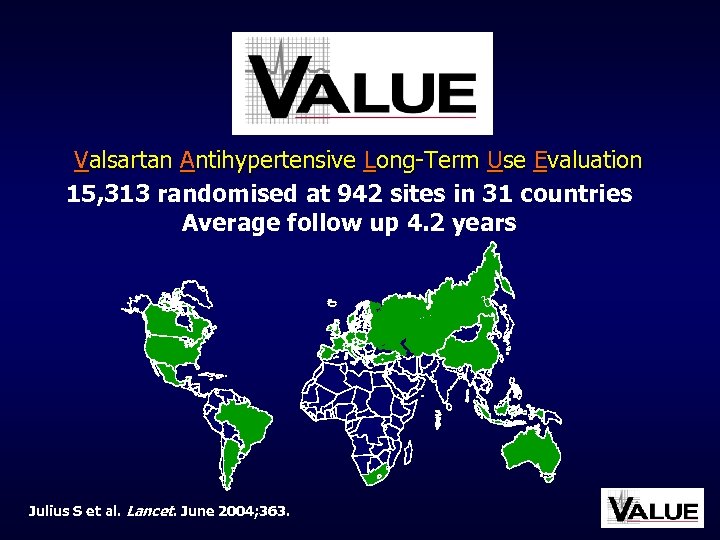 Valsartan Antihypertensive Long-Term Use Evaluation 15, 313 randomised at 942 sites in 31 countries Average follow up 4. 2 years Julius S et al. Lancet. June 2004; 363.
Valsartan Antihypertensive Long-Term Use Evaluation 15, 313 randomised at 942 sites in 31 countries Average follow up 4. 2 years Julius S et al. Lancet. June 2004; 363.
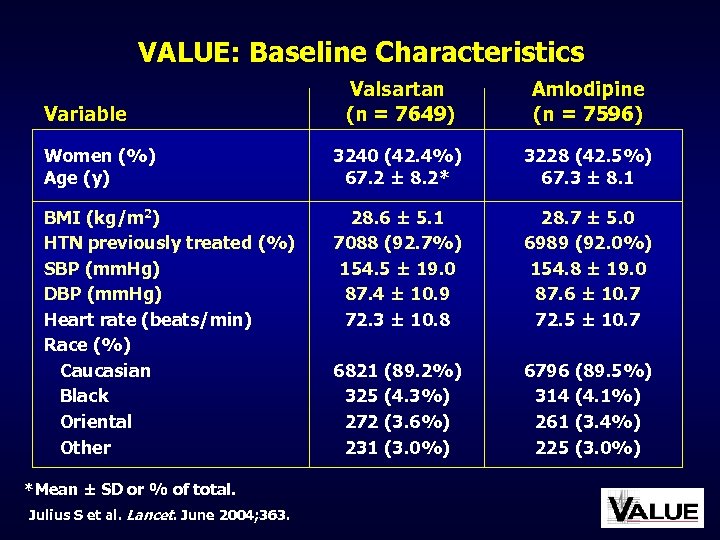 VALUE: Baseline Characteristics Valsartan (n = 7649) Amlodipine (n = 7596) Women (%) Age (y) 3240 (42. 4%) 67. 2 ± 8. 2* 3228 (42. 5%) 67. 3 ± 8. 1 BMI (kg/m 2) HTN previously treated (%) SBP (mm. Hg) DBP (mm. Hg) Heart rate (beats/min) Race (%) Caucasian Black Oriental Other 28. 6 ± 5. 1 7088 (92. 7%) 154. 5 ± 19. 0 87. 4 ± 10. 9 72. 3 ± 10. 8 28. 7 ± 5. 0 6989 (92. 0%) 154. 8 ± 19. 0 87. 6 ± 10. 7 72. 5 ± 10. 7 6821 (89. 2%) 325 (4. 3%) 272 (3. 6%) 231 (3. 0%) 6796 (89. 5%) 314 (4. 1%) 261 (3. 4%) 225 (3. 0%) Variable *Mean ± SD or % of total. Julius S et al. Lancet. June 2004; 363.
VALUE: Baseline Characteristics Valsartan (n = 7649) Amlodipine (n = 7596) Women (%) Age (y) 3240 (42. 4%) 67. 2 ± 8. 2* 3228 (42. 5%) 67. 3 ± 8. 1 BMI (kg/m 2) HTN previously treated (%) SBP (mm. Hg) DBP (mm. Hg) Heart rate (beats/min) Race (%) Caucasian Black Oriental Other 28. 6 ± 5. 1 7088 (92. 7%) 154. 5 ± 19. 0 87. 4 ± 10. 9 72. 3 ± 10. 8 28. 7 ± 5. 0 6989 (92. 0%) 154. 8 ± 19. 0 87. 6 ± 10. 7 72. 5 ± 10. 7 6821 (89. 2%) 325 (4. 3%) 272 (3. 6%) 231 (3. 0%) 6796 (89. 5%) 314 (4. 1%) 261 (3. 4%) 225 (3. 0%) Variable *Mean ± SD or % of total. Julius S et al. Lancet. June 2004; 363.
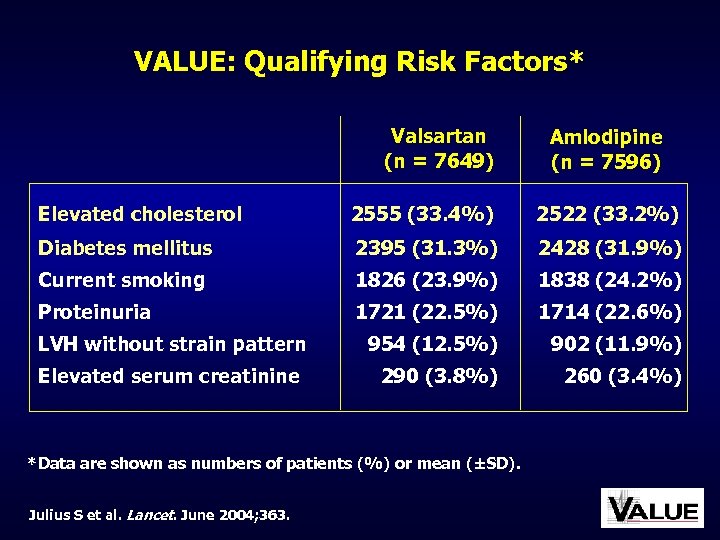 VALUE: Qualifying Risk Factors* Valsartan (n = 7649) Amlodipine (n = 7596) Elevated cholesterol 2555 (33. 4%) 2522 (33. 2%) Diabetes mellitus 2395 (31. 3%) 2428 (31. 9%) Current smoking 1826 (23. 9%) 1838 (24. 2%) Proteinuria 1721 (22. 5%) 1714 (22. 6%) 954 (12. 5%) 902 (11. 9%) 290 (3. 8%) 260 (3. 4%) LVH without strain pattern Elevated serum creatinine *Data are shown as numbers of patients (%) or mean (±SD). Julius S et al. Lancet. June 2004; 363.
VALUE: Qualifying Risk Factors* Valsartan (n = 7649) Amlodipine (n = 7596) Elevated cholesterol 2555 (33. 4%) 2522 (33. 2%) Diabetes mellitus 2395 (31. 3%) 2428 (31. 9%) Current smoking 1826 (23. 9%) 1838 (24. 2%) Proteinuria 1721 (22. 5%) 1714 (22. 6%) 954 (12. 5%) 902 (11. 9%) 290 (3. 8%) 260 (3. 4%) LVH without strain pattern Elevated serum creatinine *Data are shown as numbers of patients (%) or mean (±SD). Julius S et al. Lancet. June 2004; 363.
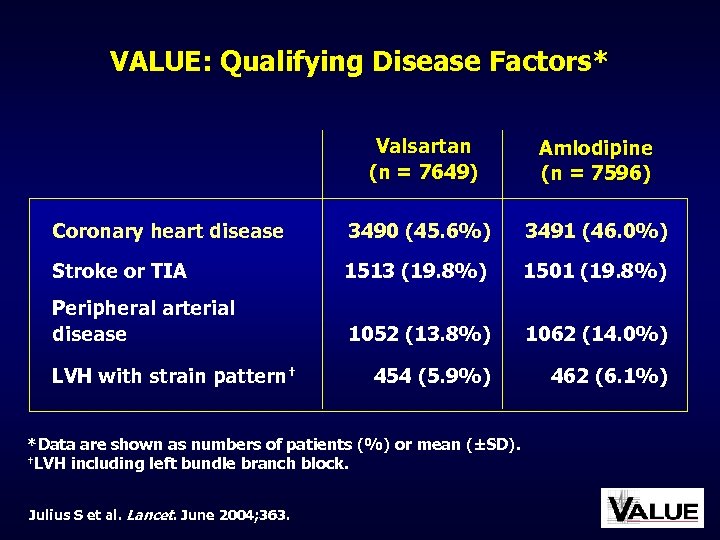 VALUE: Qualifying Disease Factors* Valsartan (n = 7649) Amlodipine (n = 7596) Coronary heart disease 3490 (45. 6%) 3491 (46. 0%) Stroke or TIA 1513 (19. 8%) 1501 (19. 8%) Peripheral arterial disease 1052 (13. 8%) 1062 (14. 0%) 454 (5. 9%) 462 (6. 1%) LVH with strain pattern† *Data are shown as numbers of patients (%) or mean (±SD). †LVH including left bundle branch block. Julius S et al. Lancet. June 2004; 363.
VALUE: Qualifying Disease Factors* Valsartan (n = 7649) Amlodipine (n = 7596) Coronary heart disease 3490 (45. 6%) 3491 (46. 0%) Stroke or TIA 1513 (19. 8%) 1501 (19. 8%) Peripheral arterial disease 1052 (13. 8%) 1062 (14. 0%) 454 (5. 9%) 462 (6. 1%) LVH with strain pattern† *Data are shown as numbers of patients (%) or mean (±SD). †LVH including left bundle branch block. Julius S et al. Lancet. June 2004; 363.
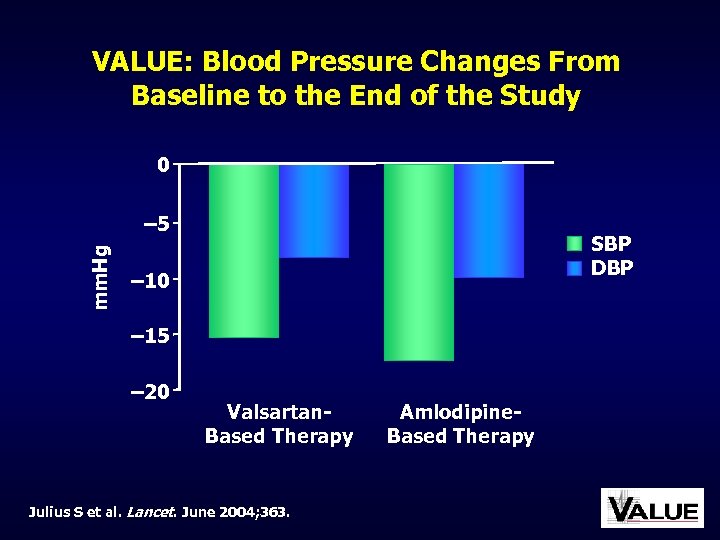 VALUE: Blood Pressure Changes From Baseline to the End of the Study 0 mm. Hg – 5 SBP DBP – 10 – 15 – 20 Valsartan. Based Therapy Julius S et al. Lancet. June 2004; 363. Amlodipine. Based Therapy
VALUE: Blood Pressure Changes From Baseline to the End of the Study 0 mm. Hg – 5 SBP DBP – 10 – 15 – 20 Valsartan. Based Therapy Julius S et al. Lancet. June 2004; 363. Amlodipine. Based Therapy
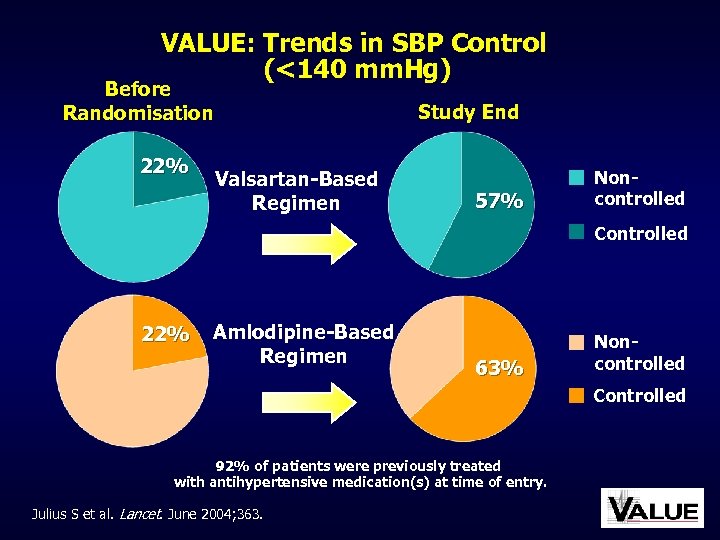 VALUE: Trends in SBP Control (<140 mm. Hg) Before Randomisation 22% Study End Valsartan-Based Regimen 57% Noncontrolled Controlled 22% Amlodipine-Based Regimen 63% Noncontrolled Controlled 92% of patients were previously treated with antihypertensive medication(s) at time of entry. Julius S et al. Lancet. June 2004; 363.
VALUE: Trends in SBP Control (<140 mm. Hg) Before Randomisation 22% Study End Valsartan-Based Regimen 57% Noncontrolled Controlled 22% Amlodipine-Based Regimen 63% Noncontrolled Controlled 92% of patients were previously treated with antihypertensive medication(s) at time of entry. Julius S et al. Lancet. June 2004; 363.
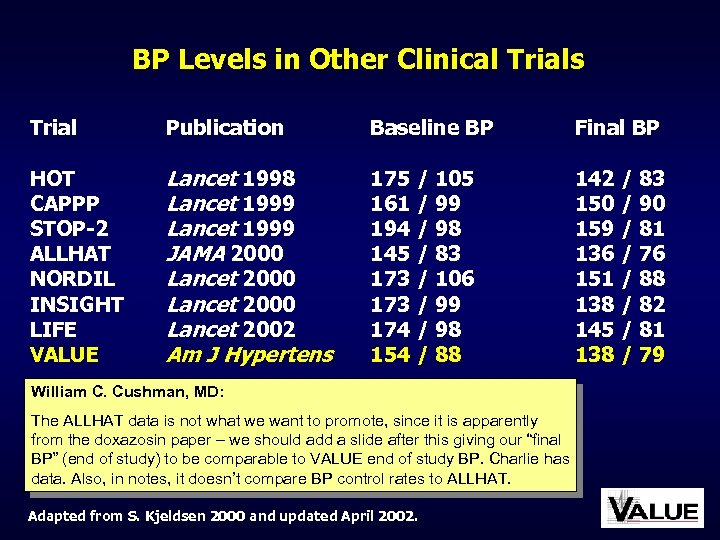 BP Levels in Other Clinical Trials Trial Publication Baseline BP Final BP HOT CAPPP STOP-2 ALLHAT NORDIL INSIGHT LIFE VALUE Lancet 1998 Lancet 1999 JAMA 2000 Lancet 2002 Am J Hypertens 175 / 105 161 / 99 194 / 98 145 / 83 173 / 106 173 / 99 174 / 98 154 / 88 142 / 83 150 / 90 159 / 81 136 / 76 151 / 88 138 / 82 145 / 81 138 / 79 William C. Cushman, MD: The ALLHAT data is not what we want to promote, since it is apparently from the doxazosin paper – we should add a slide after this giving our “final BP” (end of study) to be comparable to VALUE end of study BP. Charlie has data. Also, in notes, it doesn’t compare BP control rates to ALLHAT. Adapted from S. Kjeldsen 2000 and updated April 2002.
BP Levels in Other Clinical Trials Trial Publication Baseline BP Final BP HOT CAPPP STOP-2 ALLHAT NORDIL INSIGHT LIFE VALUE Lancet 1998 Lancet 1999 JAMA 2000 Lancet 2002 Am J Hypertens 175 / 105 161 / 99 194 / 98 145 / 83 173 / 106 173 / 99 174 / 98 154 / 88 142 / 83 150 / 90 159 / 81 136 / 76 151 / 88 138 / 82 145 / 81 138 / 79 William C. Cushman, MD: The ALLHAT data is not what we want to promote, since it is apparently from the doxazosin paper – we should add a slide after this giving our “final BP” (end of study) to be comparable to VALUE end of study BP. Charlie has data. Also, in notes, it doesn’t compare BP control rates to ALLHAT. Adapted from S. Kjeldsen 2000 and updated April 2002.
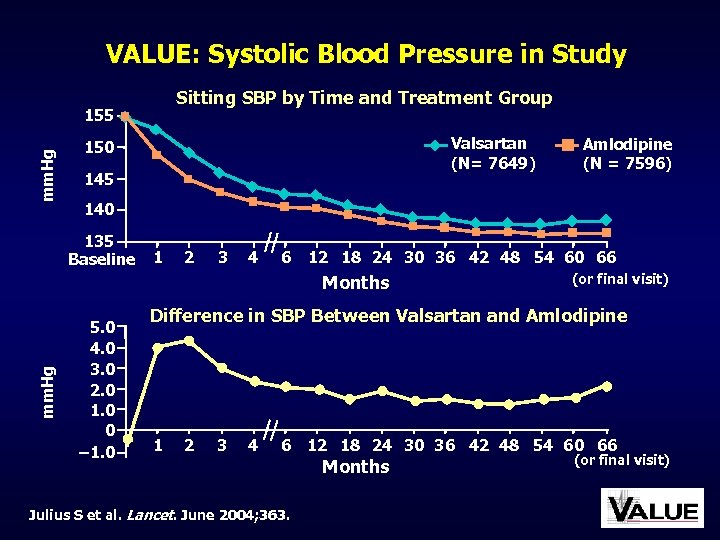 VALUE: Systolic Blood Pressure in Study Sitting SBP by Time and Treatment Group mm. Hg 155 Valsartan (N= 7649) 150 145 140 135 Baseline 1 2 3 4 6 12 18 24 30 36 42 48 54 60 66 Months mm. Hg Amlodipine (N = 7596) 5. 0 4. 0 3. 0 2. 0 1. 0 0 – 1. 0 (or final visit) Difference in SBP Between Valsartan and Amlodipine 1 2 3 4 6 Julius S et al. Lancet. June 2004; 363. 12 18 24 30 36 42 48 54 60 66 Months (or final visit)
VALUE: Systolic Blood Pressure in Study Sitting SBP by Time and Treatment Group mm. Hg 155 Valsartan (N= 7649) 150 145 140 135 Baseline 1 2 3 4 6 12 18 24 30 36 42 48 54 60 66 Months mm. Hg Amlodipine (N = 7596) 5. 0 4. 0 3. 0 2. 0 1. 0 0 – 1. 0 (or final visit) Difference in SBP Between Valsartan and Amlodipine 1 2 3 4 6 Julius S et al. Lancet. June 2004; 363. 12 18 24 30 36 42 48 54 60 66 Months (or final visit)
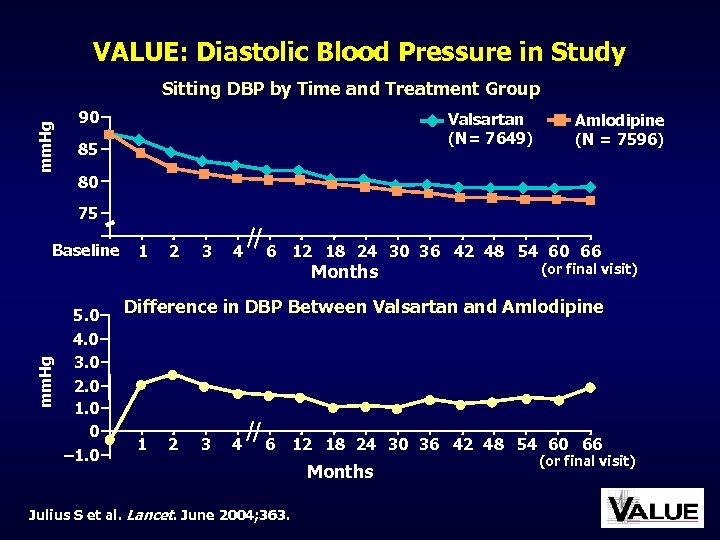 VALUE: Diastolic Blood Pressure in Study mm. Hg Sitting DBP by Time and Treatment Group 90 Valsartan (N= 7649) 85 Amlodipine (N = 7596) 80 75 Baseline 1 2 3 4 6 12 18 24 30 36 42 48 54 60 66 mm. Hg Months 5. 0 4. 0 3. 0 2. 0 1. 0 0 – 1. 0 (or final visit) Difference in DBP Between Valsartan and Amlodipine 1 2 3 4 6 Julius S et al. Lancet. June 2004; 363. 12 18 24 30 36 42 48 54 60 66 Months (or final visit)
VALUE: Diastolic Blood Pressure in Study mm. Hg Sitting DBP by Time and Treatment Group 90 Valsartan (N= 7649) 85 Amlodipine (N = 7596) 80 75 Baseline 1 2 3 4 6 12 18 24 30 36 42 48 54 60 66 mm. Hg Months 5. 0 4. 0 3. 0 2. 0 1. 0 0 – 1. 0 (or final visit) Difference in DBP Between Valsartan and Amlodipine 1 2 3 4 6 Julius S et al. Lancet. June 2004; 363. 12 18 24 30 36 42 48 54 60 66 Months (or final visit)
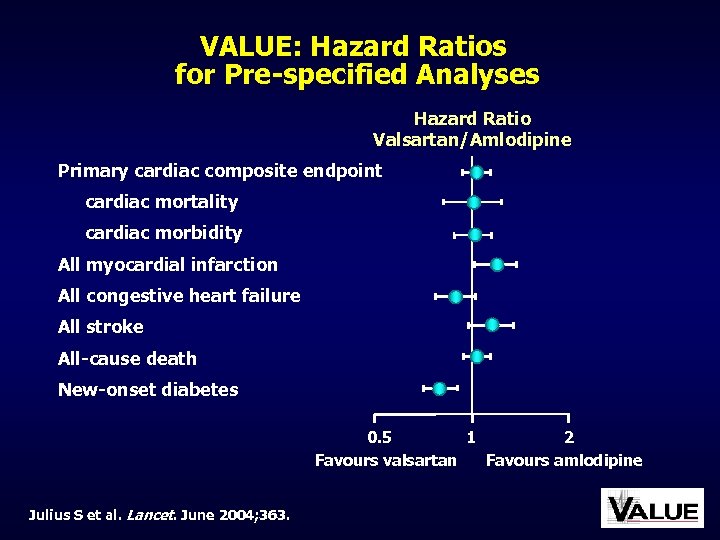 VALUE: Hazard Ratios for Pre-specified Analyses Hazard Ratio Valsartan/Amlodipine Primary cardiac composite endpoint cardiac mortality cardiac morbidity All myocardial infarction All congestive heart failure All stroke All-cause death New-onset diabetes 0. 5 1 2 Favours valsartan Favours amlodipine Julius S et al. Lancet. June 2004; 363.
VALUE: Hazard Ratios for Pre-specified Analyses Hazard Ratio Valsartan/Amlodipine Primary cardiac composite endpoint cardiac mortality cardiac morbidity All myocardial infarction All congestive heart failure All stroke All-cause death New-onset diabetes 0. 5 1 2 Favours valsartan Favours amlodipine Julius S et al. Lancet. June 2004; 363.
 VALUE: Main Results Good BP control was achieved with both treatment regimens, but BP decrease in the amlodipine group was more pronounced, particularly early in the trial Despite BP differences, the primary composite cardiac endpoint in both groups was not different Julius S et al. Lancet. June 2004; 363.
VALUE: Main Results Good BP control was achieved with both treatment regimens, but BP decrease in the amlodipine group was more pronounced, particularly early in the trial Despite BP differences, the primary composite cardiac endpoint in both groups was not different Julius S et al. Lancet. June 2004; 363.
 VALUE: Other Results • Incidence of stroke was lower, but not significantly, in the amlodipine group • Incidence of non-fatal MI was significantly lower in the amlodipine group • There was a positive trend in favour of valsartan for less heart failure but this did not reach significance • There was a highly significant lower rate of new-onset diabetes in the valsartan group Julius S et al. Lancet. June 2004; 363.
VALUE: Other Results • Incidence of stroke was lower, but not significantly, in the amlodipine group • Incidence of non-fatal MI was significantly lower in the amlodipine group • There was a positive trend in favour of valsartan for less heart failure but this did not reach significance • There was a highly significant lower rate of new-onset diabetes in the valsartan group Julius S et al. Lancet. June 2004; 363.
 VALUE: Interpretations • The observed difference in stroke rates appears to be strongly related to differences in achieved BPs • The benefits of valsartan in heart failure prevention emerged later in the study when BP differences were smaller, indicating that there is a potential beneficial effect of valsartan beyond BP control Julius S et al. Lancet. June 2004; 363.
VALUE: Interpretations • The observed difference in stroke rates appears to be strongly related to differences in achieved BPs • The benefits of valsartan in heart failure prevention emerged later in the study when BP differences were smaller, indicating that there is a potential beneficial effect of valsartan beyond BP control Julius S et al. Lancet. June 2004; 363.
 VALUE: Analysis of Results Based on Serial Median Matching Rationale: Differences in achieved BP levels in VALUE precluded valid comparisons of drug effects on outcomes. Therefore, a statistical technique that adjusts for BP differences was applied post hoc to create treatment cohorts with closely similar characteristics Weber MA et al. Lancet. 2004; 363: 2047– 49.
VALUE: Analysis of Results Based on Serial Median Matching Rationale: Differences in achieved BP levels in VALUE precluded valid comparisons of drug effects on outcomes. Therefore, a statistical technique that adjusts for BP differences was applied post hoc to create treatment cohorts with closely similar characteristics Weber MA et al. Lancet. 2004; 363: 2047– 49.
 VALUE: Analysis of Results Based on Serial Median Matching Description: The novel computerised procedure of Serial Median Matching was applied at 6 months, following the treatment adjustments intended to achieve BP control. The programme selected the most median patient (by achieved systolic BP) in the valsartan group; this patient was paired with one from the amlodipine group ( 2 mm. Hg) and was matched also for age, sex and the presence or absence of prior coronary disease, stroke and diabetes. The newly created patient pair was moved to a new database, and the procedure repeated serially until all possible patient pairs were matched. Weber MA et al. Lancet. 2004; 363: 2047– 49.
VALUE: Analysis of Results Based on Serial Median Matching Description: The novel computerised procedure of Serial Median Matching was applied at 6 months, following the treatment adjustments intended to achieve BP control. The programme selected the most median patient (by achieved systolic BP) in the valsartan group; this patient was paired with one from the amlodipine group ( 2 mm. Hg) and was matched also for age, sex and the presence or absence of prior coronary disease, stroke and diabetes. The newly created patient pair was moved to a new database, and the procedure repeated serially until all possible patient pairs were matched. Weber MA et al. Lancet. 2004; 363: 2047– 49.
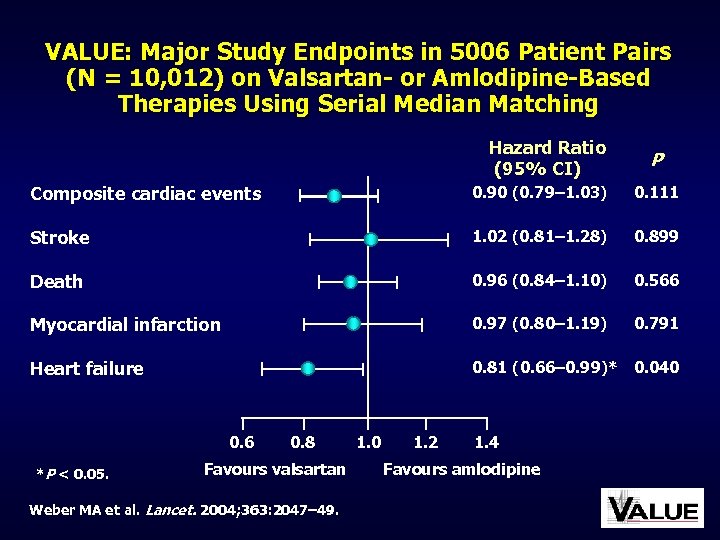 VALUE: Major Study Endpoints in 5006 Patient Pairs (N = 10, 012) on Valsartan- or Amlodipine-Based Therapies Using Serial Median Matching Hazard Ratio (95% CI) P Composite cardiac events 0. 90 (0. 79– 1. 03) 0. 111 Stroke 1. 02 (0. 81– 1. 28) 0. 899 Death 0. 96 (0. 84– 1. 10) 0. 566 Myocardial infarction 0. 97 (0. 80– 1. 19) 0. 791 Heart failure 0. 81 (0. 66– 0. 99)* 0. 040 0. 6 *P < 0. 05. 0. 8 Favours valsartan Weber MA et al. Lancet. 2004; 363: 2047– 49. 1. 0 1. 2 1. 4 Favours amlodipine
VALUE: Major Study Endpoints in 5006 Patient Pairs (N = 10, 012) on Valsartan- or Amlodipine-Based Therapies Using Serial Median Matching Hazard Ratio (95% CI) P Composite cardiac events 0. 90 (0. 79– 1. 03) 0. 111 Stroke 1. 02 (0. 81– 1. 28) 0. 899 Death 0. 96 (0. 84– 1. 10) 0. 566 Myocardial infarction 0. 97 (0. 80– 1. 19) 0. 791 Heart failure 0. 81 (0. 66– 0. 99)* 0. 040 0. 6 *P < 0. 05. 0. 8 Favours valsartan Weber MA et al. Lancet. 2004; 363: 2047– 49. 1. 0 1. 2 1. 4 Favours amlodipine
 VALUE: Analysis of Results Based on Serial Median Matching Conclusions: Serial median matching created valsartanamlodipine patient pairs matched exactly for systolic BP and demographic and clinical characteristics excluding the high and low extremes of achieved BPs. It allowed us to address the original study hypothesis, and demonstrated that for the same achieved BPs, valsartan intermediate dose had effects similar to amlodipine on most CV endpoints, and was more effective in reducing heart failure hospitalisations. Weber MA et al. Lancet. 2004; 363: 2047– 49.
VALUE: Analysis of Results Based on Serial Median Matching Conclusions: Serial median matching created valsartanamlodipine patient pairs matched exactly for systolic BP and demographic and clinical characteristics excluding the high and low extremes of achieved BPs. It allowed us to address the original study hypothesis, and demonstrated that for the same achieved BPs, valsartan intermediate dose had effects similar to amlodipine on most CV endpoints, and was more effective in reducing heart failure hospitalisations. Weber MA et al. Lancet. 2004; 363: 2047– 49.
 VALUE: Analyses of Results Based on BP Control Overall Conclusions: Blood pressure control, and rapidity of response, are critical for reducing events in high-risk hypertension The significant between-group differences in heart failure and diabetes suggest that valsartan may offer benefits beyond BP control Weber MA et al. Lancet. 2004; 363: 2047– 49.
VALUE: Analyses of Results Based on BP Control Overall Conclusions: Blood pressure control, and rapidity of response, are critical for reducing events in high-risk hypertension The significant between-group differences in heart failure and diabetes suggest that valsartan may offer benefits beyond BP control Weber MA et al. Lancet. 2004; 363: 2047– 49.
 VALUE Summary • ARB (valsartan) not more effective than CCB (amlodipine) in decreasing cardiac mortality and morbidity – CCB →lower SBP than ARB (4 mm Hg at 1 -2 months, 2 mm Hg at 6 months) – CCB →significantly lower incidence of MI, higher incidence of new-onset diabetes • Serial median matching adjusted for BP Δ – MI incidence became equivalent for CCB & ARB – Lower rate of HF with ARB Julius S, Kjeldsen SE, Weber M, et al. Lancet 2004; 363: 2022 -31
VALUE Summary • ARB (valsartan) not more effective than CCB (amlodipine) in decreasing cardiac mortality and morbidity – CCB →lower SBP than ARB (4 mm Hg at 1 -2 months, 2 mm Hg at 6 months) – CCB →significantly lower incidence of MI, higher incidence of new-onset diabetes • Serial median matching adjusted for BP Δ – MI incidence became equivalent for CCB & ARB – Lower rate of HF with ARB Julius S, Kjeldsen SE, Weber M, et al. Lancet 2004; 363: 2022 -31
 Comments on VALUE Analyses • Differing odds ratios over time – Cox regressions assume constant hazard ratios, but were used in analyses anyway • Rationale for BP adjustment method? – Discards thousands of observations. – Alternatives: Cox model with BP as timedependent covariate or beginning at 6 months
Comments on VALUE Analyses • Differing odds ratios over time – Cox regressions assume constant hazard ratios, but were used in analyses anyway • Rationale for BP adjustment method? – Discards thousands of observations. – Alternatives: Cox model with BP as timedependent covariate or beginning at 6 months
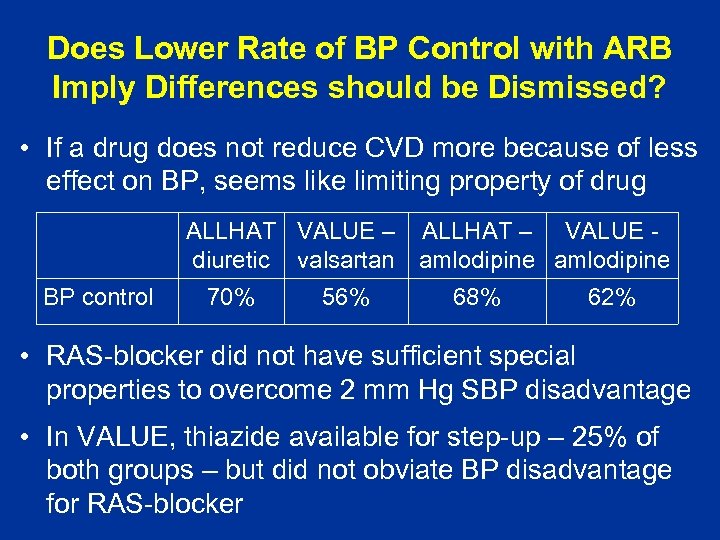 Does Lower Rate of BP Control with ARB Imply Differences should be Dismissed? • If a drug does not reduce CVD more because of less effect on BP, seems like limiting property of drug ALLHAT VALUE – ALLHAT – VALUE diuretic valsartan amlodipine BP control 70% 56% 68% 62% • RAS-blocker did not have sufficient special properties to overcome 2 mm Hg SBP disadvantage • In VALUE, thiazide available for step-up – 25% of both groups – but did not obviate BP disadvantage for RAS-blocker
Does Lower Rate of BP Control with ARB Imply Differences should be Dismissed? • If a drug does not reduce CVD more because of less effect on BP, seems like limiting property of drug ALLHAT VALUE – ALLHAT – VALUE diuretic valsartan amlodipine BP control 70% 56% 68% 62% • RAS-blocker did not have sufficient special properties to overcome 2 mm Hg SBP disadvantage • In VALUE, thiazide available for step-up – 25% of both groups – but did not obviate BP disadvantage for RAS-blocker
 Diabetes Incidence If CCBs raise incidence of new-onset diabetes but lower incidence of MI compared with ARBs, why would an ARB be preferred?
Diabetes Incidence If CCBs raise incidence of new-onset diabetes but lower incidence of MI compared with ARBs, why would an ARB be preferred?
 ARBs & β-blockers may prevent MI less than thiazide-like diuretics, CCBs, ARBs 1. In meta-analysis 1, β-blockers did not reduce coronary events as well as low-dose diuretics 2. No differences in LIFE trial between ARB (losartan) & β-blocker (atenolol)2 3. No differences in ALLHAT between chlorthalidone, lisinopril, and amlodipine for primary outcome 4. Taken together, this suggests ARBs and βblockers may reduce CHD less than thiazidetype diuretics, CCBs, or ACEIs. 1 Psaty BM, Smith ML, Siscovick DS, et al. JAMA 1997; 277: 739 -745. 2 Dahlof B, Devereux RB, Kjeldsen SE, et al. Lancet 2002; 359: 995 -1003.
ARBs & β-blockers may prevent MI less than thiazide-like diuretics, CCBs, ARBs 1. In meta-analysis 1, β-blockers did not reduce coronary events as well as low-dose diuretics 2. No differences in LIFE trial between ARB (losartan) & β-blocker (atenolol)2 3. No differences in ALLHAT between chlorthalidone, lisinopril, and amlodipine for primary outcome 4. Taken together, this suggests ARBs and βblockers may reduce CHD less than thiazidetype diuretics, CCBs, or ACEIs. 1 Psaty BM, Smith ML, Siscovick DS, et al. JAMA 1997; 277: 739 -745. 2 Dahlof B, Devereux RB, Kjeldsen SE, et al. Lancet 2002; 359: 995 -1003.


