Atom.pptx
- Количество слайдов: 6
Atom Dmytro Abdulakh
Presentation structure • History of an atom. • Definition of an atom and modern atomic concept. • References
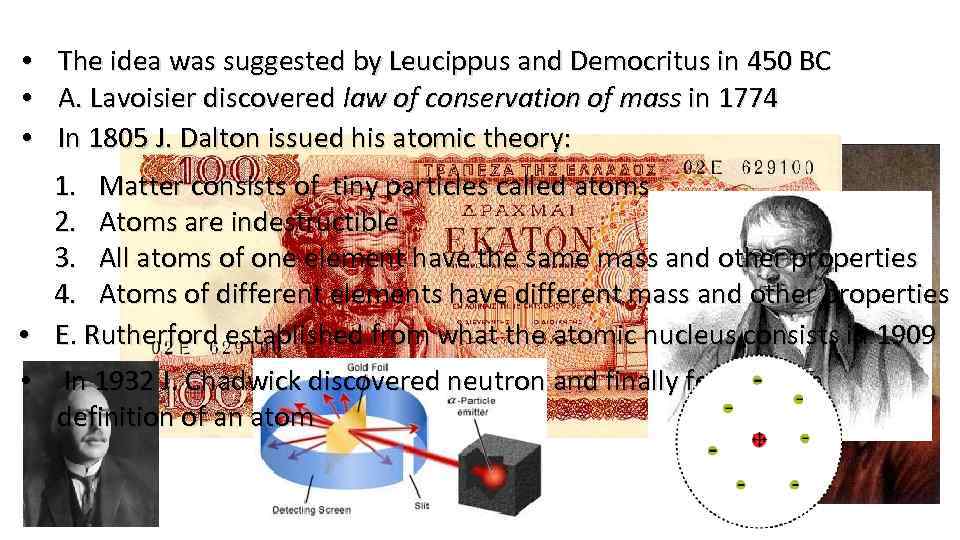
• • • The idea was suggested by Leucippus and Democritus in 450 BC A. Lavoisier discovered law of conservation of mass in 1774 In 1805 J. Dalton issued his atomic theory: 1. Matter consists of tiny particles called atoms 2. Atoms are indestructible 3. All atoms of one element have the same mass and other properties 4. Atoms of different elements have different mass and other properties • E. Rutherford established from what the atomic nucleus consists in 1909 • In 1932 J. Chadwick discovered neutron and finally formed the definition of an atom
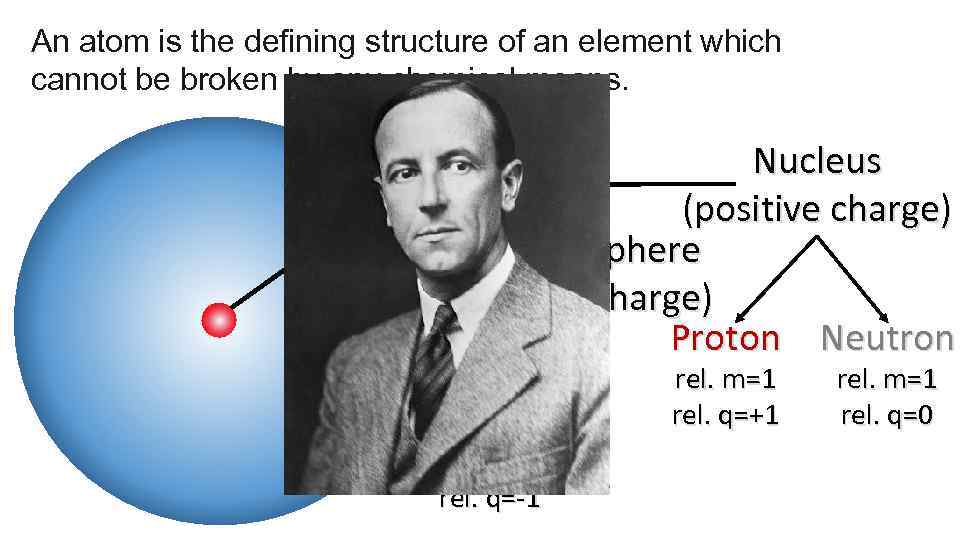
An atom is the defining structure of an element which cannot be broken by any chemical means. Nucleus (positive charge) Electron Sphere (negative charge) Proton Neutron Electron rel. m=0 rel. q=-1 rel. m=1 rel. q=+1 rel. m=1 rel. q=0
References q Blackman, A. , Bottle S. , Schmid S. , Mocerino M. , Wille U. , Brady J. , Senese, F. et al. (2008). Chemistry. Milton: JW & S, pp. 6 -12 q Burrows, A. , Holman, J. , Parsons, A. , Pilling G. , Price, G. (2009). Chemistry 3. Oxford: Oxford UP. , pp. 74 -77 q Wikipedia, the free encyclopedia. (2015). John Dalton [Online]. Available at: https: //en. wikipedia. org/wiki/John_Dalton. [Accessed 2 October 2015] q Wikipedia, the free encyclopedia. (2015). Antoine Lavoisier [Online]. Available at: https: //en. wikipedia. org/wiki/Antoine_Lavoisier. [Accessed: 2 October 2015] q Wikipedia, the free encyclopedia. (2015). Rutherford model [Online]. Available at: https: //en. wikipedia. org/wiki/Rutherford_model. [Accessed: 2 October 2015]

Thank you for your attention!
Atom.pptx