a9e8b2976d39fce1ac413f97b48d3aa4.ppt
- Количество слайдов: 30
 Atmospheric transport and chemistry lecture I. III. IV. V. VII. V/ Introduction Fundamental concepts in atmospheric dynamics: Brewer-Dobson circulation and waves Radiative transfer, heating and vertical transport Stratospheric ozone chemistry The (tropical) tropopause Greenhouse gasses (GHG) and climate Solar (decadal) variability and dynamical coupling
Atmospheric transport and chemistry lecture I. III. IV. V. VII. V/ Introduction Fundamental concepts in atmospheric dynamics: Brewer-Dobson circulation and waves Radiative transfer, heating and vertical transport Stratospheric ozone chemistry The (tropical) tropopause Greenhouse gasses (GHG) and climate Solar (decadal) variability and dynamical coupling
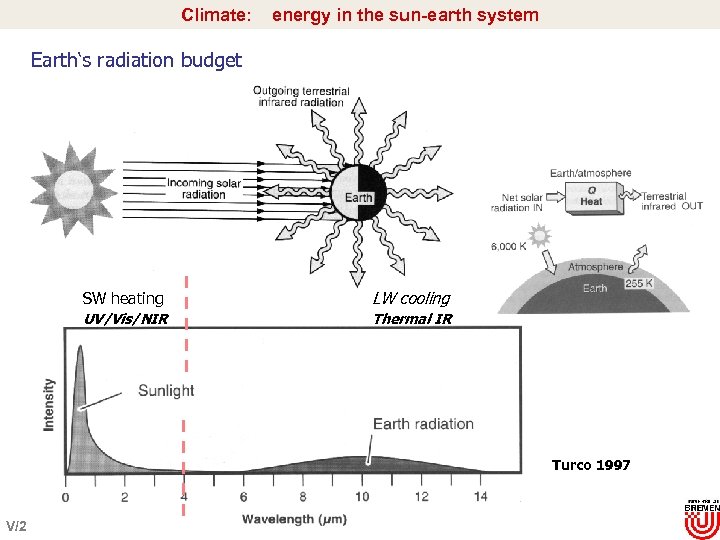 Climate: energy in the sun-earth system Earth‘s radiation budget SW heating LW cooling UV/Vis/NIR Thermal IR Turco 1997 V/2
Climate: energy in the sun-earth system Earth‘s radiation budget SW heating LW cooling UV/Vis/NIR Thermal IR Turco 1997 V/2
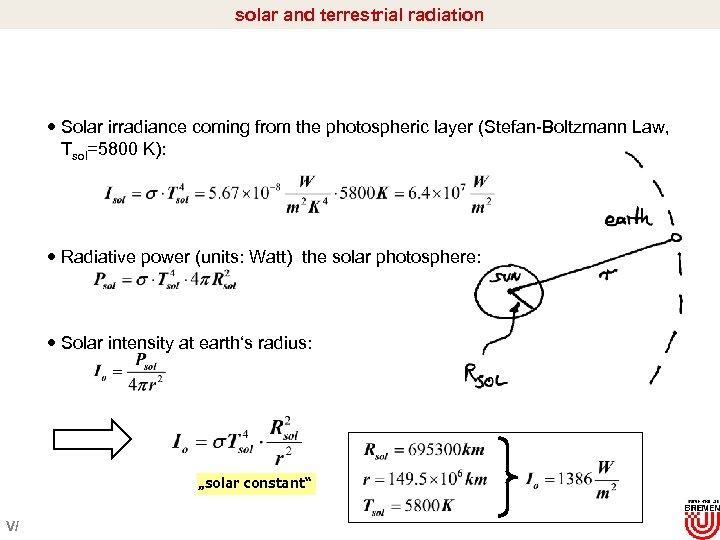 solar and terrestrial radiation Solar irradiance coming from the photospheric layer (Stefan-Boltzmann Law, Tsol=5800 K): Radiative power (units: Watt) the solar photosphere: Solar intensity at earth‘s radius: „solar constant“ V/
solar and terrestrial radiation Solar irradiance coming from the photospheric layer (Stefan-Boltzmann Law, Tsol=5800 K): Radiative power (units: Watt) the solar photosphere: Solar intensity at earth‘s radius: „solar constant“ V/
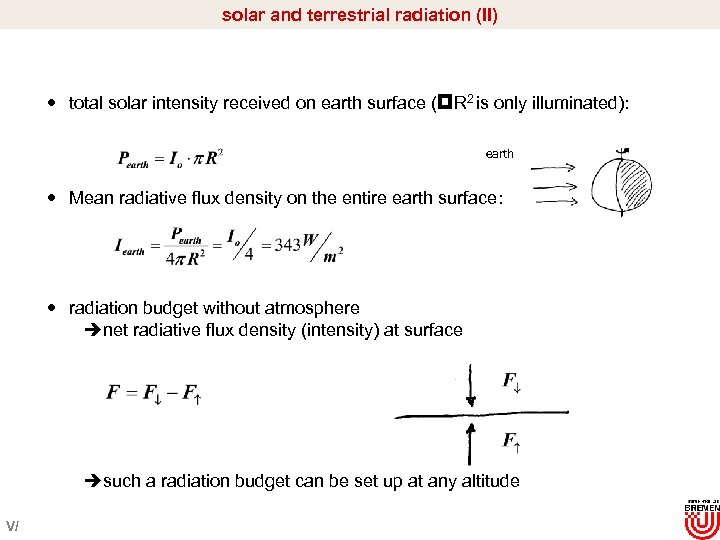 solar and terrestrial radiation (II) total solar intensity received on earth surface ( R 2 is only illuminated): earth Mean radiative flux density on the entire earth surface: radiation budget without atmosphere ènet radiative flux density (intensity) at surface èsuch a radiation budget can be set up at any altitude V/
solar and terrestrial radiation (II) total solar intensity received on earth surface ( R 2 is only illuminated): earth Mean radiative flux density on the entire earth surface: radiation budget without atmosphere ènet radiative flux density (intensity) at surface èsuch a radiation budget can be set up at any altitude V/
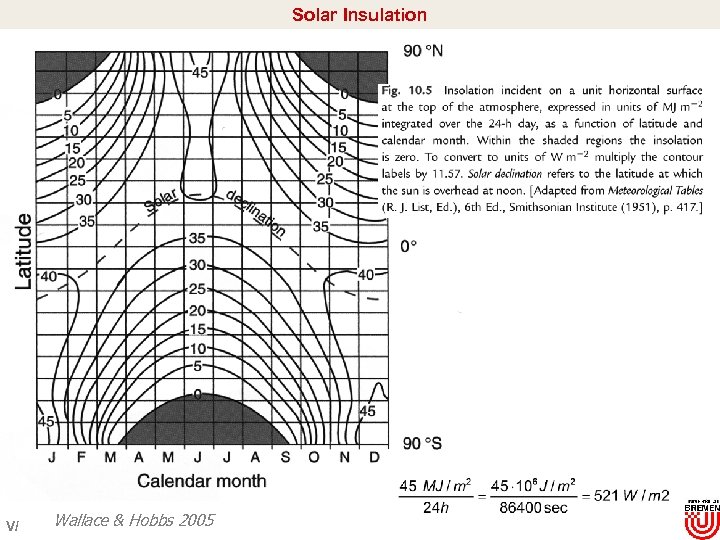 Solar Insulation V/ Wallace & Hobbs 2005
Solar Insulation V/ Wallace & Hobbs 2005
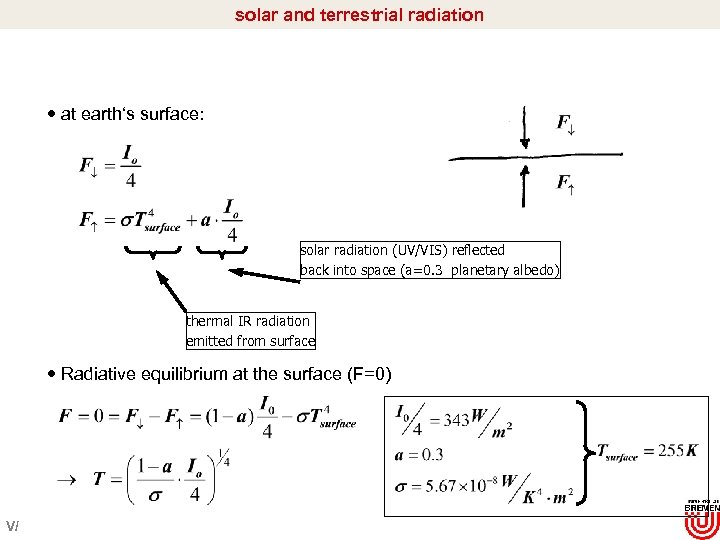 solar and terrestrial radiation at earth‘s surface: solar radiation (UV/VIS) reflected back into space (a=0. 3 planetary albedo) thermal IR radiation emitted from surface Radiative equilibrium at the surface (F=0) V/
solar and terrestrial radiation at earth‘s surface: solar radiation (UV/VIS) reflected back into space (a=0. 3 planetary albedo) thermal IR radiation emitted from surface Radiative equilibrium at the surface (F=0) V/
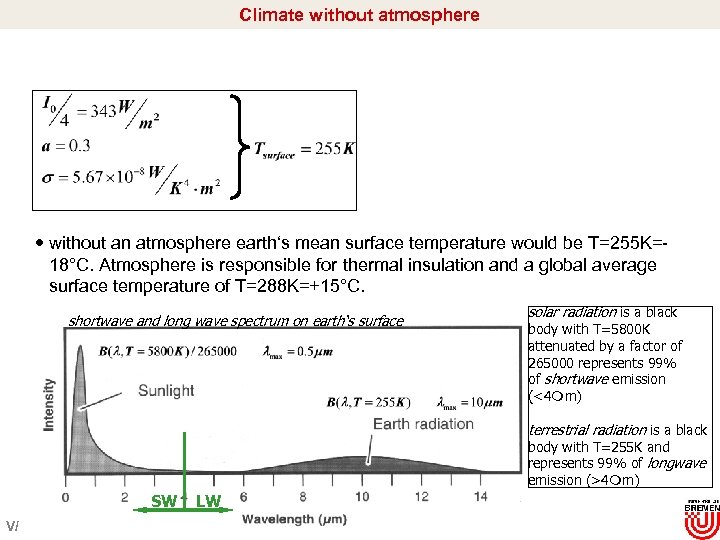 Climate without atmosphere without an atmosphere earth‘s mean surface temperature would be T=255 K=18°C. Atmosphere is responsible for thermal insulation and a global average surface temperature of T=288 K=+15°C. shortwave and long wave spectrum on earth‘s surface solar radiation is a black body with T=5800 K attenuated by a factor of 265000 represents 99% of shortwave emission (<4 m) terrestrial radiation is a black body with T=255 K and represents 99% of longwave emission (>4 m) SW V/ LW
Climate without atmosphere without an atmosphere earth‘s mean surface temperature would be T=255 K=18°C. Atmosphere is responsible for thermal insulation and a global average surface temperature of T=288 K=+15°C. shortwave and long wave spectrum on earth‘s surface solar radiation is a black body with T=5800 K attenuated by a factor of 265000 represents 99% of shortwave emission (<4 m) terrestrial radiation is a black body with T=255 K and represents 99% of longwave emission (>4 m) SW V/ LW
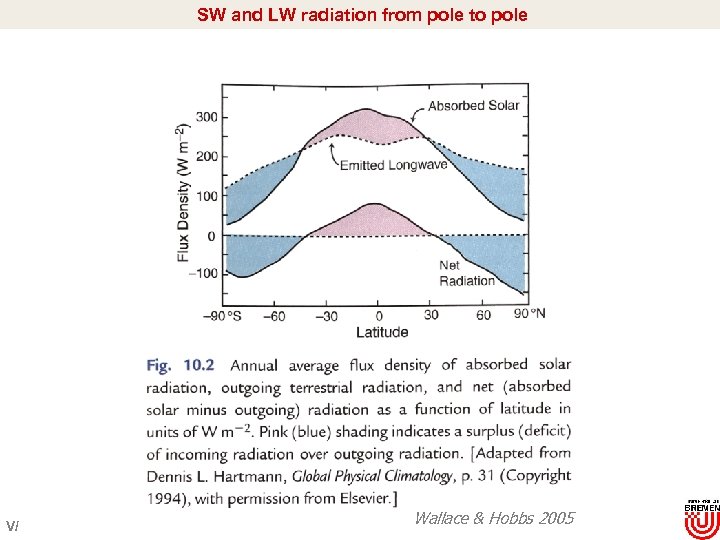 SW and LW radiation from pole to pole V/ Wallace & Hobbs 2005
SW and LW radiation from pole to pole V/ Wallace & Hobbs 2005
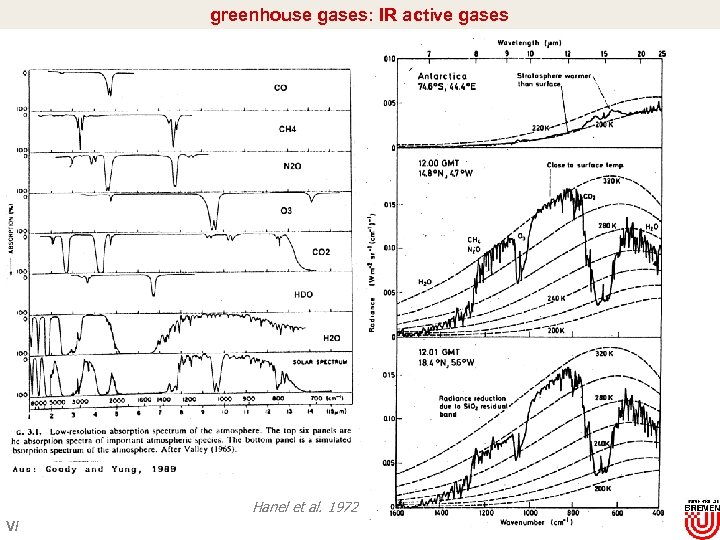 greenhouse gases: IR active gases Hanel et al. 1972 V/
greenhouse gases: IR active gases Hanel et al. 1972 V/
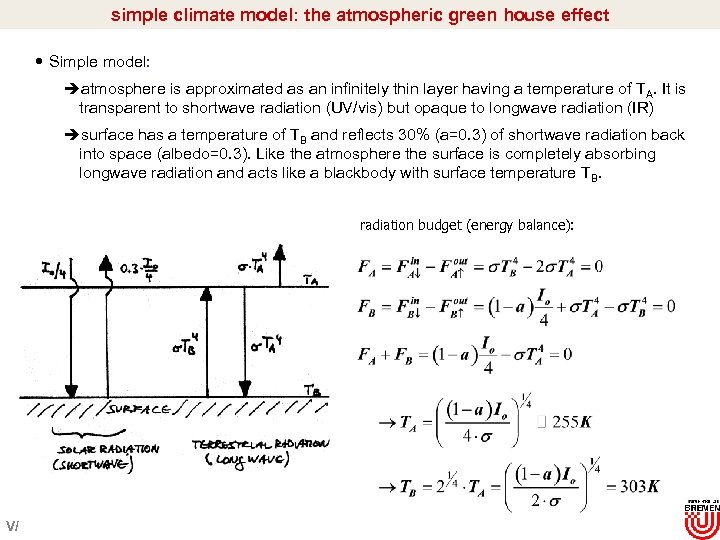 simple climate model: the atmospheric green house effect Simple model: èatmosphere is approximated as an infinitely thin layer having a temperature of TA. It is transparent to shortwave radiation (UV/vis) but opaque to longwave radiation (IR) èsurface has a temperature of TB and reflects 30% (a=0. 3) of shortwave radiation back into space (albedo=0. 3). Like the atmosphere the surface is completely absorbing longwave radiation and acts like a blackbody with surface temperature TB. radiation budget (energy balance): V/
simple climate model: the atmospheric green house effect Simple model: èatmosphere is approximated as an infinitely thin layer having a temperature of TA. It is transparent to shortwave radiation (UV/vis) but opaque to longwave radiation (IR) èsurface has a temperature of TB and reflects 30% (a=0. 3) of shortwave radiation back into space (albedo=0. 3). Like the atmosphere the surface is completely absorbing longwave radiation and acts like a blackbody with surface temperature TB. radiation budget (energy balance): V/
 simple climate model: the green house effect TA=255 K corresponds to the mean temperature at 5. 5 km altitude (~500 h. Pa). This altitude divides the real atmospheric mass in about two halves. TB=303 K=30°C is about 15°C larger than the global mean surface temperature of 288 K. The heating of the atmosphere occurs because of IR absorption of H 2 O, CO 2, CH 4 etc. However, in a real atmosphere: è Some of the IR region is transparent (atmospheric window) è UV/vis region is not completely transparent mainly due to O 3, O 2, and H 2 O absorption è Clouds modify the planetary albedo (a=0. 6 -1. 0) Analogy to a real green house: è glas is 60% transparent to UV/vis radiation but much less transparent to IR è heat-up of the glas house is mainly due to convection (wind protection!). This is the major difference to the real atmosphere V/
simple climate model: the green house effect TA=255 K corresponds to the mean temperature at 5. 5 km altitude (~500 h. Pa). This altitude divides the real atmospheric mass in about two halves. TB=303 K=30°C is about 15°C larger than the global mean surface temperature of 288 K. The heating of the atmosphere occurs because of IR absorption of H 2 O, CO 2, CH 4 etc. However, in a real atmosphere: è Some of the IR region is transparent (atmospheric window) è UV/vis region is not completely transparent mainly due to O 3, O 2, and H 2 O absorption è Clouds modify the planetary albedo (a=0. 6 -1. 0) Analogy to a real green house: è glas is 60% transparent to UV/vis radiation but much less transparent to IR è heat-up of the glas house is mainly due to convection (wind protection!). This is the major difference to the real atmosphere V/
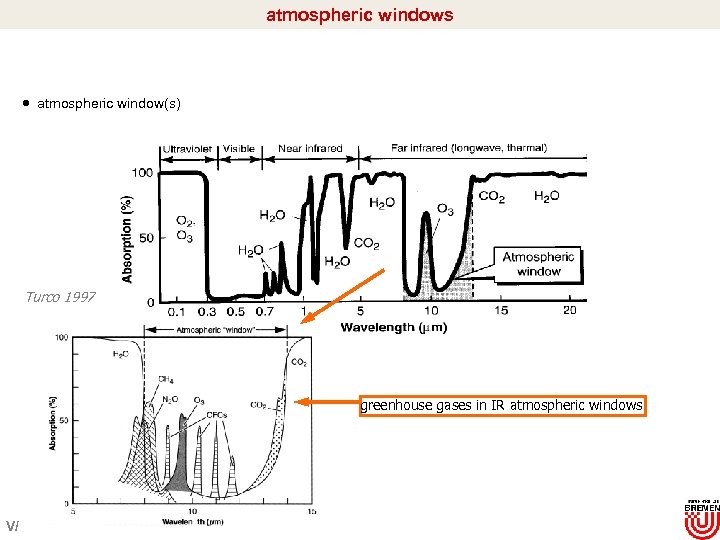 atmospheric windows atmospheric window(s) Turco 1997 greenhouse gases in IR atmospheric windows V/
atmospheric windows atmospheric window(s) Turco 1997 greenhouse gases in IR atmospheric windows V/
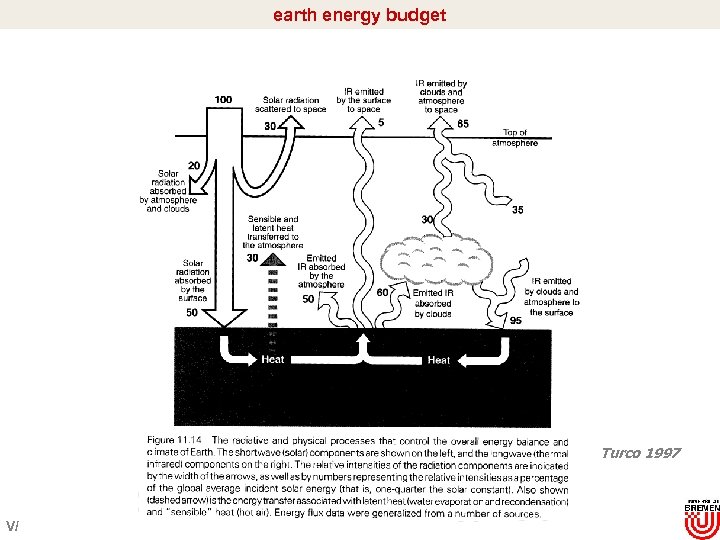 earth energy budget Turco 1997 V/
earth energy budget Turco 1997 V/
 climate feedbacks: direct (radiation) and indirect Stratospheric aerosols (major volcanic eruption): èdirect effect: changes in albedo (scattering/cooling) and absorption (soot/warming) èIndirect effect: increases amount of CCN, more cloud can form Role of clouds: èCloud cover changes modify planetary albedo Chemical feedback èOzone depletion contrbutes to stratospheric cooling èWarmer troposphere leads to higher water vapor amounts, modifies clouds èMethane oxydation enhances stratospheric H 2 O (CH 4+OH CH 3+H 2 O), additional IR cooling èChemical response to temperature changes ècirculation changes (transport & chemistry) V/ Turco 1997
climate feedbacks: direct (radiation) and indirect Stratospheric aerosols (major volcanic eruption): èdirect effect: changes in albedo (scattering/cooling) and absorption (soot/warming) èIndirect effect: increases amount of CCN, more cloud can form Role of clouds: èCloud cover changes modify planetary albedo Chemical feedback èOzone depletion contrbutes to stratospheric cooling èWarmer troposphere leads to higher water vapor amounts, modifies clouds èMethane oxydation enhances stratospheric H 2 O (CH 4+OH CH 3+H 2 O), additional IR cooling èChemical response to temperature changes ècirculation changes (transport & chemistry) V/ Turco 1997
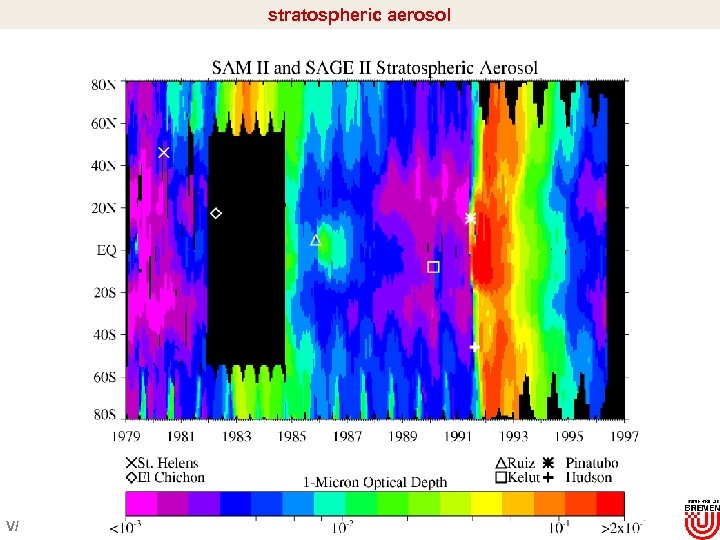 stratospheric aerosol V/
stratospheric aerosol V/
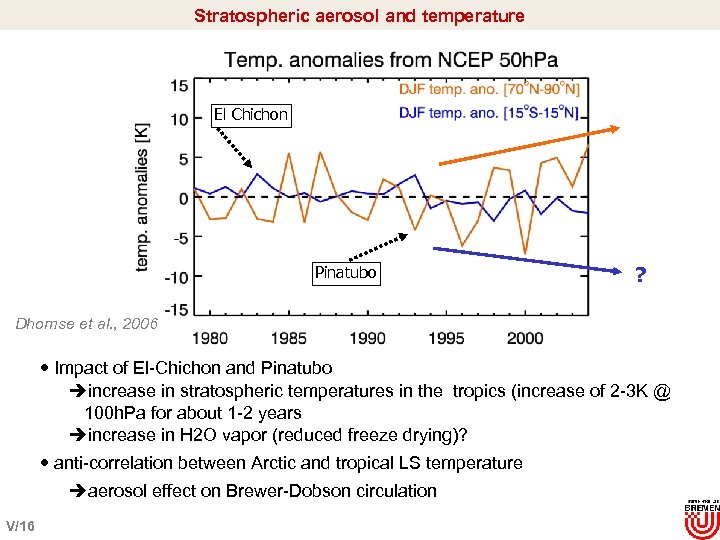 Stratospheric aerosol and temperature El Chichon Pinatubo ? Dhomse et al. , 2006 Impact of El-Chichon and Pinatubo èincrease in stratospheric temperatures in the tropics (increase of 2 -3 K @ 100 h. Pa for about 1 -2 years èincrease in H 2 O vapor (reduced freeze drying)? anti-correlation between Arctic and tropical LS temperature èaerosol effect on Brewer-Dobson circulation V/16
Stratospheric aerosol and temperature El Chichon Pinatubo ? Dhomse et al. , 2006 Impact of El-Chichon and Pinatubo èincrease in stratospheric temperatures in the tropics (increase of 2 -3 K @ 100 h. Pa for about 1 -2 years èincrease in H 2 O vapor (reduced freeze drying)? anti-correlation between Arctic and tropical LS temperature èaerosol effect on Brewer-Dobson circulation V/16
![Trends in greenhouse gases (surface): CO 2 Note today: [CO 2] 382 ppmv [CH Trends in greenhouse gases (surface): CO 2 Note today: [CO 2] 382 ppmv [CH](https://present5.com/presentation/a9e8b2976d39fce1ac413f97b48d3aa4/image-17.jpg) Trends in greenhouse gases (surface): CO 2 Note today: [CO 2] 382 ppmv [CH 4] 1800 ppbv Mouna Loa Hawaii Ahrens 1999 V/
Trends in greenhouse gases (surface): CO 2 Note today: [CO 2] 382 ppmv [CH 4] 1800 ppbv Mouna Loa Hawaii Ahrens 1999 V/
![Trends in greenhouse gases (surface) Note today: [CO 2] 370 ppmv [CH 4] 1800 Trends in greenhouse gases (surface) Note today: [CO 2] 370 ppmv [CH 4] 1800](https://present5.com/presentation/a9e8b2976d39fce1ac413f97b48d3aa4/image-18.jpg) Trends in greenhouse gases (surface) Note today: [CO 2] 370 ppmv [CH 4] 1800 ppbv IPCC 2001 V/
Trends in greenhouse gases (surface) Note today: [CO 2] 370 ppmv [CH 4] 1800 ppbv IPCC 2001 V/
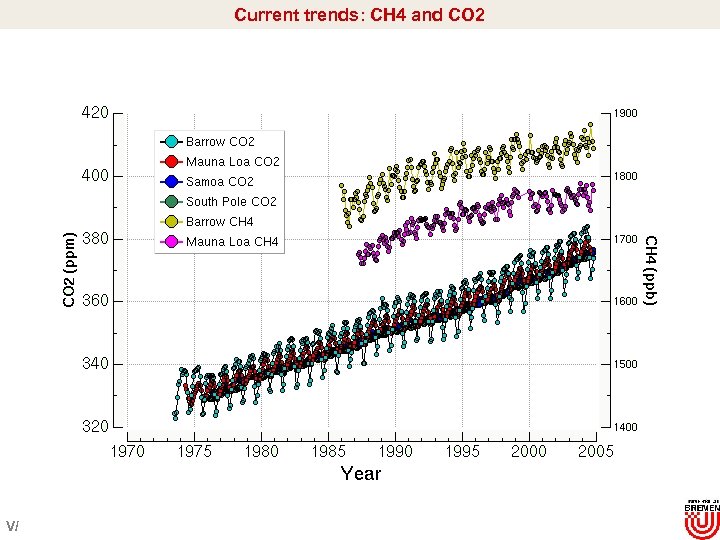 Current trends: CH 4 and CO 2 V/
Current trends: CH 4 and CO 2 V/
![GHG in the past fromice cores Note today: [CO 2] 370 ppmv [CH 4] GHG in the past fromice cores Note today: [CO 2] 370 ppmv [CH 4]](https://present5.com/presentation/a9e8b2976d39fce1ac413f97b48d3aa4/image-20.jpg) GHG in the past fromice cores Note today: [CO 2] 370 ppmv [CH 4] 1700 ppbv 0 ky V/ 150 ky Age in kyears
GHG in the past fromice cores Note today: [CO 2] 370 ppmv [CH 4] 1700 ppbv 0 ky V/ 150 ky Age in kyears
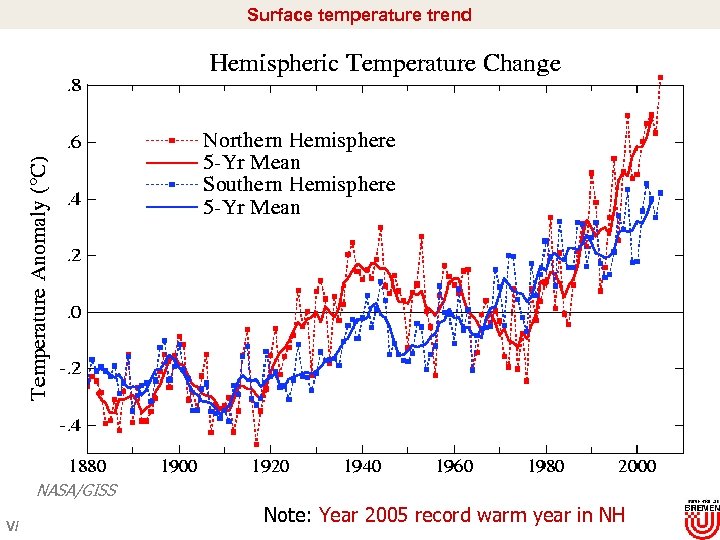 Surface temperature trend NASA/GISS V/ Note: Year 2005 record warm year in NH
Surface temperature trend NASA/GISS V/ Note: Year 2005 record warm year in NH
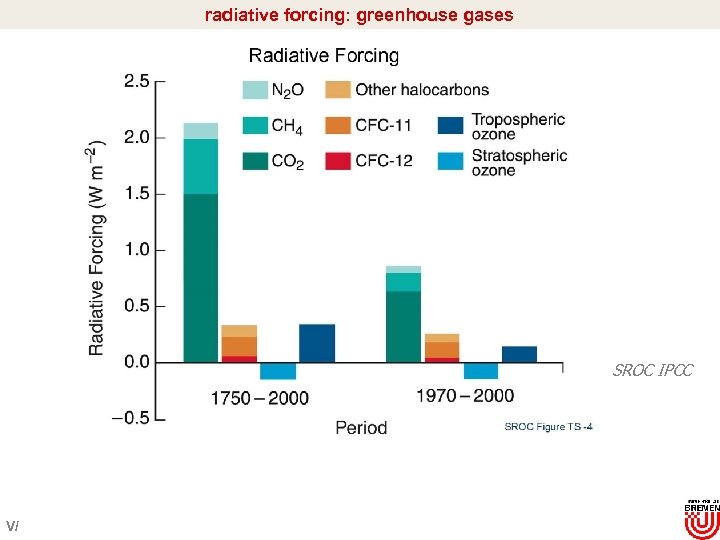 radiative forcing: greenhouse gases SROC IPCC V/
radiative forcing: greenhouse gases SROC IPCC V/
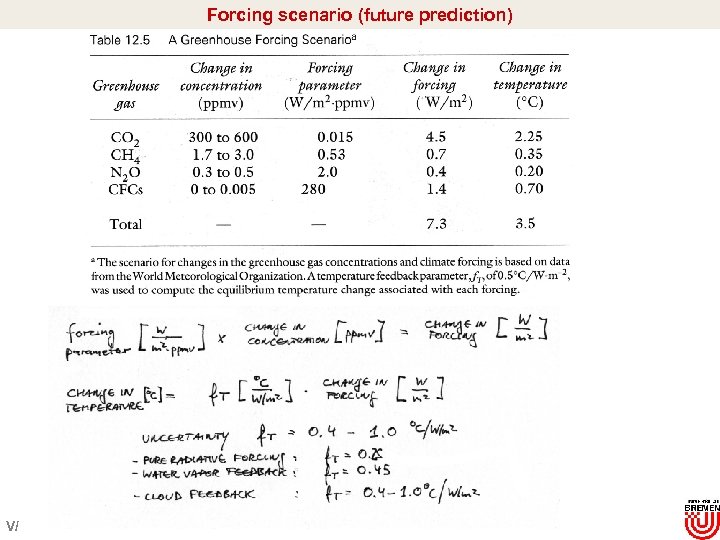 Forcing scenario (future prediction) Turco 1997 V/
Forcing scenario (future prediction) Turco 1997 V/
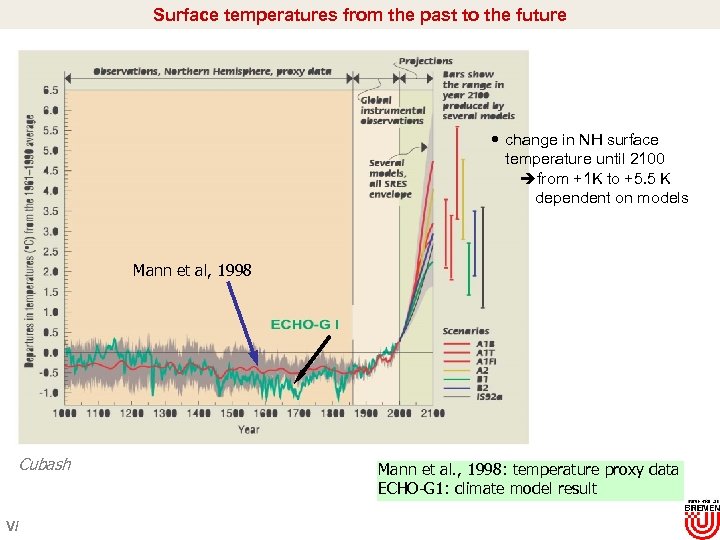 Surface temperatures from the past to the future change in NH surface temperature until 2100 èfrom +1 K to +5. 5 K dependent on models Mann et al, 1998 Cubash V/ Mann et al. , 1998: temperature proxy data ECHO-G 1: climate model result
Surface temperatures from the past to the future change in NH surface temperature until 2100 èfrom +1 K to +5. 5 K dependent on models Mann et al, 1998 Cubash V/ Mann et al. , 1998: temperature proxy data ECHO-G 1: climate model result
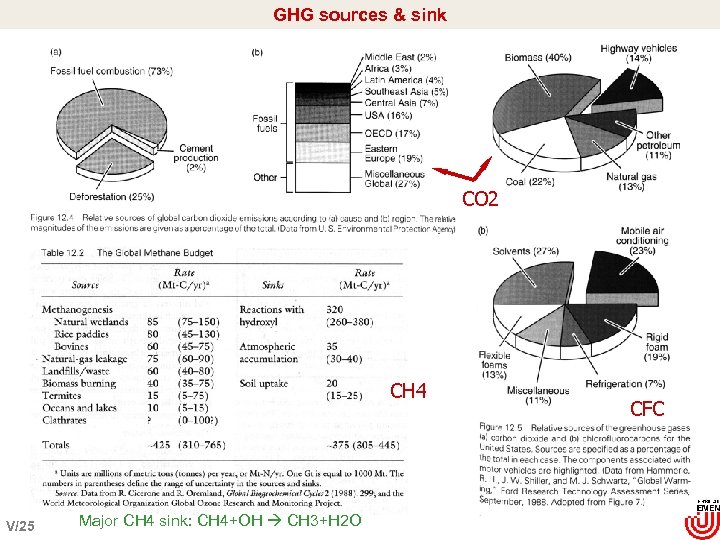 GHG sources & sink CO 2 CH 4 V/25 Major CH 4 sink: CH 4+OH CH 3+H 2 O CFC
GHG sources & sink CO 2 CH 4 V/25 Major CH 4 sink: CH 4+OH CH 3+H 2 O CFC
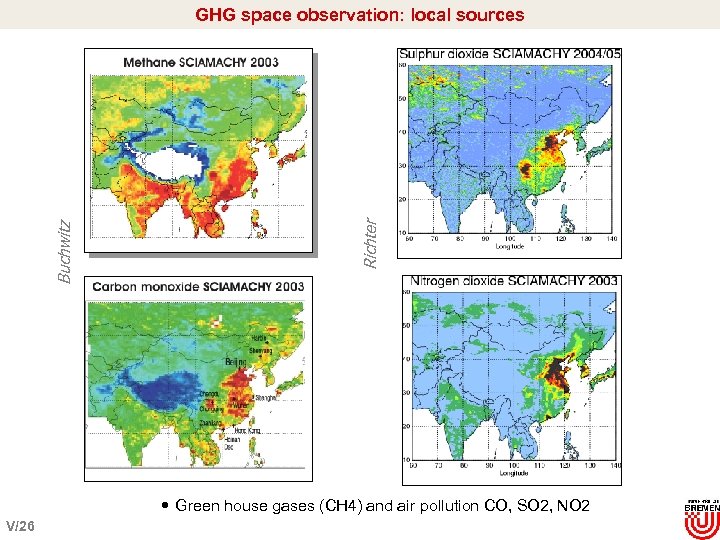 Richter Buchwitz GHG space observation: local sources Green house gases (CH 4) and air pollution CO, SO 2, NO 2 V/26
Richter Buchwitz GHG space observation: local sources Green house gases (CH 4) and air pollution CO, SO 2, NO 2 V/26
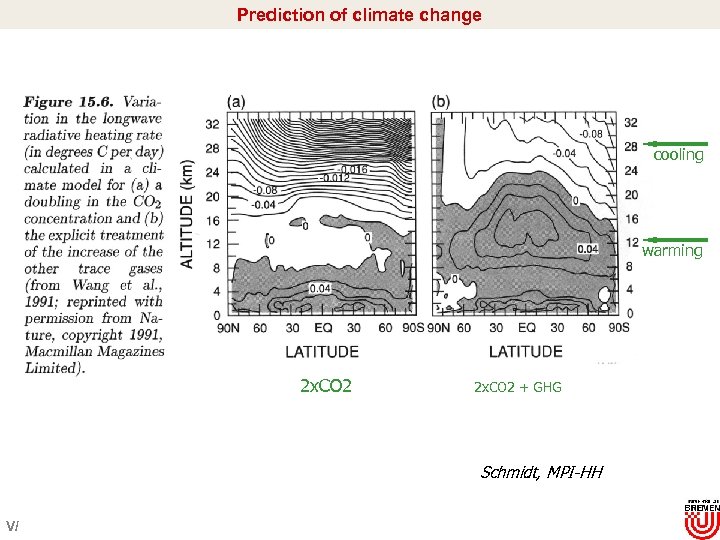 Prediction of climate change cooling warming 2 x. CO 2 + GHG Schmidt, MPI-HH V/
Prediction of climate change cooling warming 2 x. CO 2 + GHG Schmidt, MPI-HH V/
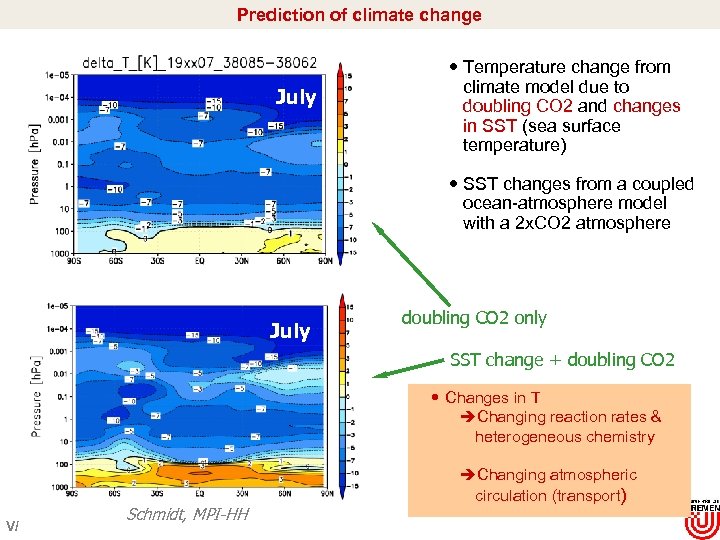 Prediction of climate change July Temperature change from climate model due to doubling CO 2 and changes in SST (sea surface temperature) SST changes from a coupled ocean-atmosphere model with a 2 x. CO 2 atmosphere July doubling CO 2 only SST change + doubling CO 2 Changes in T èChanging reaction rates & heterogeneous chemistry V/ Schmidt, MPI-HH èChanging atmospheric circulation (transport)
Prediction of climate change July Temperature change from climate model due to doubling CO 2 and changes in SST (sea surface temperature) SST changes from a coupled ocean-atmosphere model with a 2 x. CO 2 atmosphere July doubling CO 2 only SST change + doubling CO 2 Changes in T èChanging reaction rates & heterogeneous chemistry V/ Schmidt, MPI-HH èChanging atmospheric circulation (transport)
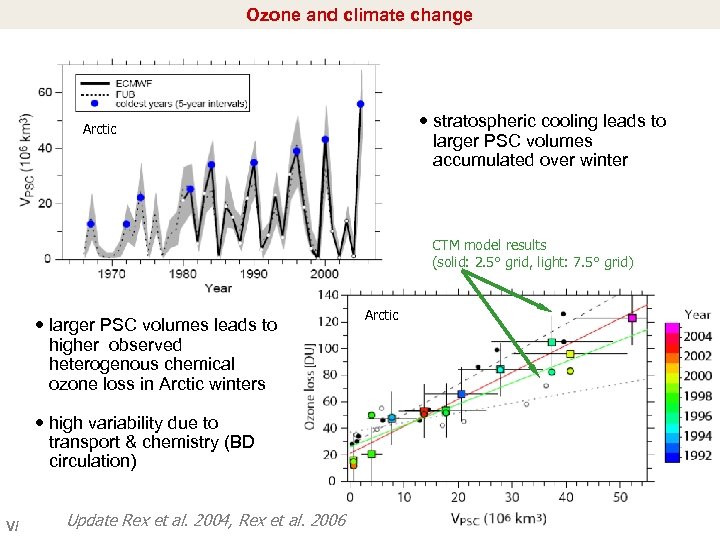 Ozone and climate change stratospheric cooling leads to larger PSC volumes accumulated over winter Arctic CTM model results (solid: 2. 5° grid, light: 7. 5° grid) larger PSC volumes leads to higher observed heterogenous chemical ozone loss in Arctic winters high variability due to transport & chemistry (BD circulation) V/ Update Rex et al. 2004, Rex et al. 2006 Arctic
Ozone and climate change stratospheric cooling leads to larger PSC volumes accumulated over winter Arctic CTM model results (solid: 2. 5° grid, light: 7. 5° grid) larger PSC volumes leads to higher observed heterogenous chemical ozone loss in Arctic winters high variability due to transport & chemistry (BD circulation) V/ Update Rex et al. 2004, Rex et al. 2006 Arctic
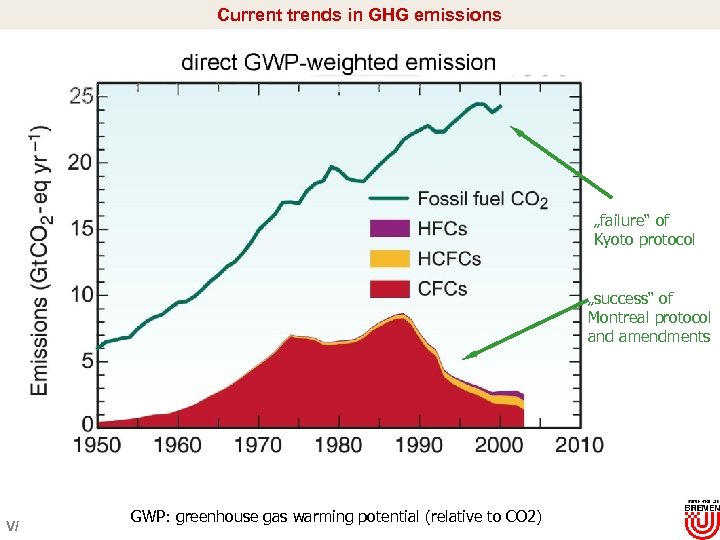 Current trends in GHG emissions „failure“ of Kyoto protocol „success“ of Montreal protocol and amendments V/ GWP: greenhouse gas warming potential (relative to CO 2)
Current trends in GHG emissions „failure“ of Kyoto protocol „success“ of Montreal protocol and amendments V/ GWP: greenhouse gas warming potential (relative to CO 2)


