3f32eb94124e3d68ca24fe74fbc5aaa7.ppt
- Количество слайдов: 20

ATLAS TIMI 46 C. Michael Gibson, Jessica L. Mega, Christopher J. Hammett, Vasil Hricak, Pascual Bordes, Adam Witkowski, Valentin Markov, Paul Burton, and Eugene Braunwald for the TIMI 46 Study Group Anti-Xa Therapy to Lower cardiovascular events in addition to Aspirin with or without thienopyridine therapy in Subjects with Acute Coronary Syndrome – Thrombolysis in Myocardial Infarction 46 Trial Funded by a Research Grant from Johnson and Bayer to Brigham & Women’s Hospital. Dr. Gibson has received honoraria & consulting fees from J&J and Bayer
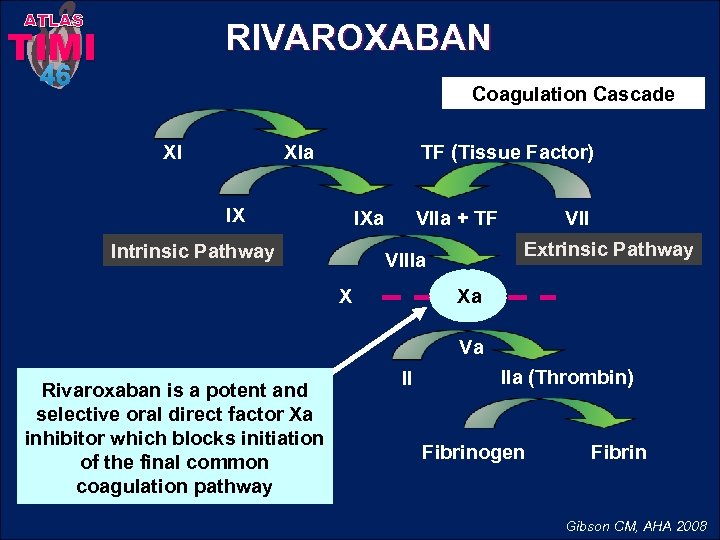
ATLAS RIVAROXABAN TIMI 46 Coagulation Cascade TF (Tissue Factor) XIa XI IX IXa Intrinsic Pathway VIIa + TF Extrinsic Pathway VIIIa X VII Xa Va Rivaroxaban is a potent and selective oral direct factor Xa inhibitor which blocks initiation of the final common coagulation pathway II IIa (Thrombin) Fibrinogen Fibrin Gibson CM, AHA 2008
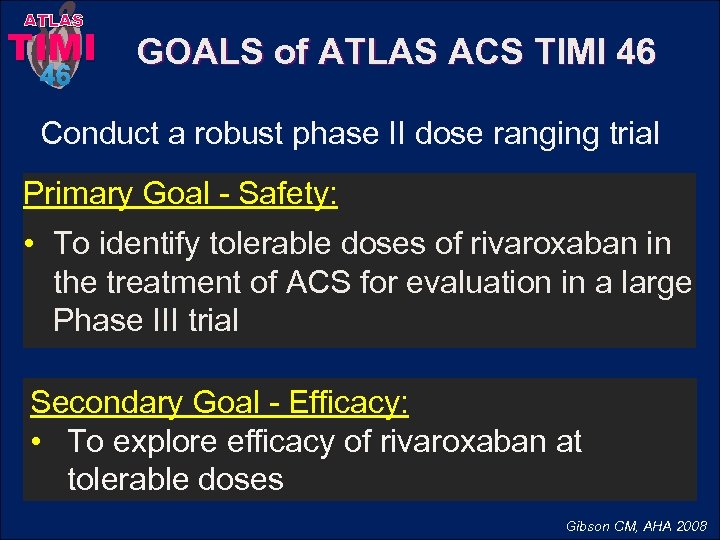
ATLAS TIMI 46 GOALS of ATLAS ACS TIMI 46 Conduct a robust phase II dose ranging trial Primary Goal - Safety: • To identify tolerable doses of rivaroxaban in the treatment of ACS for evaluation in a large Phase III trial Secondary Goal - Efficacy: • To explore efficacy of rivaroxaban at tolerable doses Gibson CM, AHA 2008
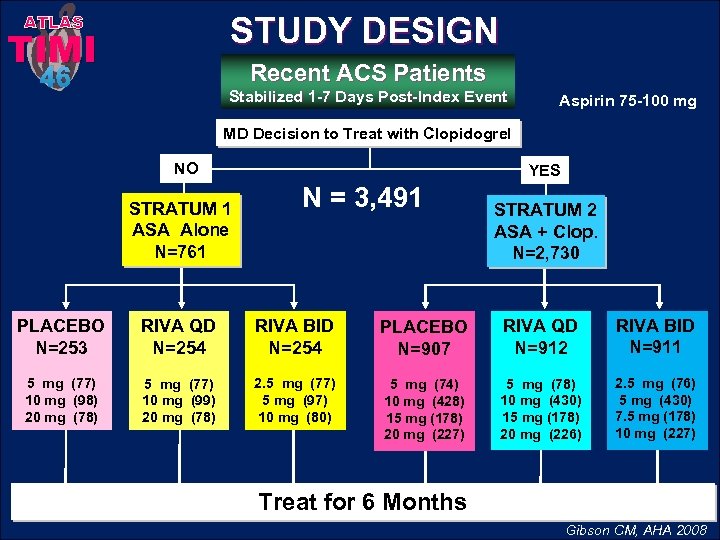
ATLAS STUDY DESIGN 46 Recent ACS Patients TIMI Stabilized 1 -7 Days Post-Index Event Aspirin 75 -100 mg MD Decision to Treat with Clopidogrel NO STRATUM 1 ASA Alone N=761 YES N = 3, 491 STRATUM 2 ASA + Clop. N=2, 730 PLACEBO N=253 RIVA QD N=254 RIVA BID N=254 PLACEBO N=907 RIVA QD N=912 RIVA BID N=911 5 mg (77) 10 mg (98) 20 mg (78) 5 mg (77) 10 mg (99) 20 mg (78) 2. 5 mg (77) 5 mg (97) 10 mg (80) 5 mg (74) 10 mg (428) 15 mg (178) 20 mg (227) 5 mg (78) 10 mg (430) 15 mg (178) 20 mg (226) 2. 5 mg (76) 5 mg (430) 7. 5 mg (178) 10 mg (227) Treat for 6 Months Gibson CM, AHA 2008
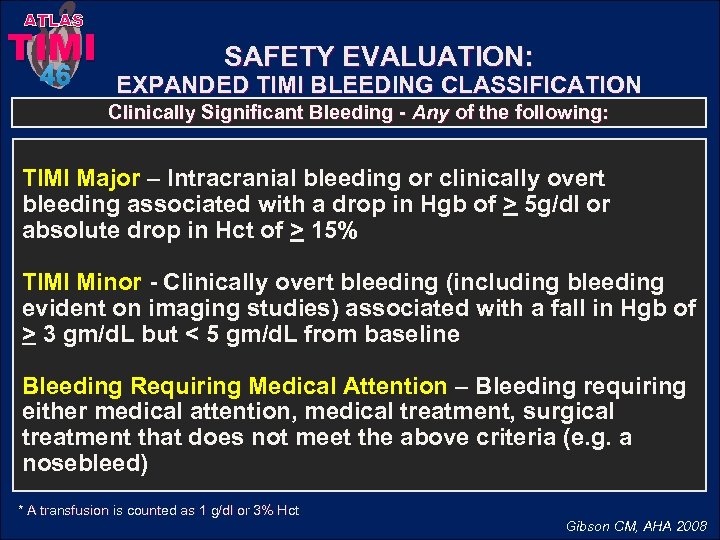
ATLAS TIMI 46 SAFETY EVALUATION: EXPANDED TIMI BLEEDING CLASSIFICATION Clinically Significant Bleeding - Any of the following: TIMI Major – Intracranial bleeding or clinically overt bleeding associated with a drop in Hgb of > 5 g/dl or absolute drop in Hct of > 15% TIMI Minor - Clinically overt bleeding (including bleeding evident on imaging studies) associated with a fall in Hgb of > 3 gm/d. L but < 5 gm/d. L from baseline Bleeding Requiring Medical Attention – Bleeding requiring either medical attention, medical treatment, surgical treatment that does not meet the above criteria (e. g. a nosebleed) * A transfusion is counted as 1 g/dl or 3% Hct Gibson CM, AHA 2008
ATLAS TIMI 46 EFFICACY EVALUATION Primary Efficacy Endpoint: Secondary Efficacy Endpoint: • Death • MI • Stroke • Severe Recurrent Ischemia Requiring Revascularization Gibson CM, AHA 2008
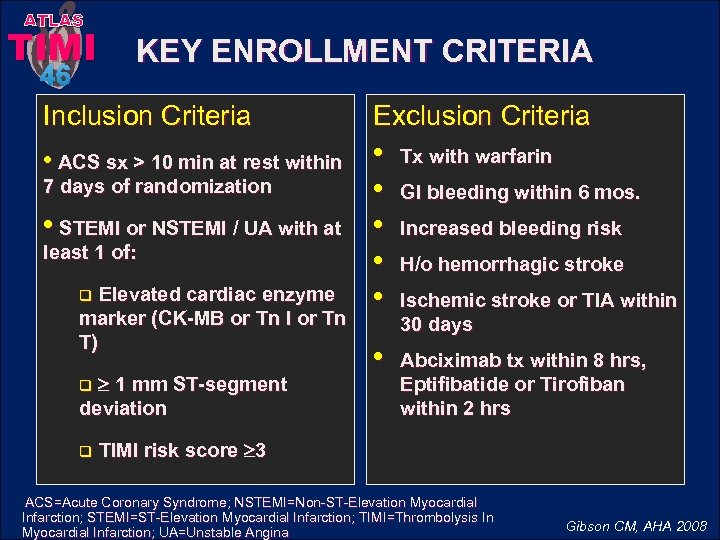
ATLAS TIMI KEY ENROLLMENT CRITERIA 46 Inclusion Criteria • ACS sx > 10 min at rest within 7 days of randomization • STEMI or NSTEMI / UA with at least 1 of: Elevated cardiac enzyme marker (CK-MB or Tn I or Tn T) q 1 mm ST-segment deviation q q Exclusion Criteria • • • Tx with warfarin • Abciximab tx within 8 hrs, Eptifibatide or Tirofiban within 2 hrs GI bleeding within 6 mos. Increased bleeding risk H/o hemorrhagic stroke Ischemic stroke or TIA within 30 days TIMI risk score 3 ACS=Acute Coronary Syndrome; NSTEMI=Non-ST-Elevation Myocardial Infarction; STEMI=ST-Elevation Myocardial Infarction; TIMI=Thrombolysis In Myocardial Infarction; UA=Unstable Angina Gibson CM, AHA 2008

ATLAS TIMI 46 NATIONAL LEAD INVESTIGATORS Poland (502) Slovakia (119) M. Tendera T. Duris Russia (430) Spain (103) M. Ruda A. Betriu United States (401) Israel (99) C. M. Gibson B. Lewis Bulgaria (259) Canada (91) N. Gotcheva P. Theroux Australia (207) United Kingdom (91) P. Aylward R. Wilcox Czech Republic (180) Germany (93) P. Widimsky H. Katus New Zealand (149) Netherlands (89) H. White F. Verheugt Ukraine (161) Belgium (77) Finland (16) F. Van de Werf M. Syvanne Denmark (64) China (4) P. Grande R. Gao A. Parkhomenko Italy (138) D. Ardissino 27 Countries France (49) J. Bassand Korea (47) K. Seung Sweden (47) M. Dellborg Brazil (37) J. Nicolau Hungary (37) E. Ostor Norway (22) D. Atar South Africa (18) A. Dalby 297 Sites Gibson CM, AHA 2008
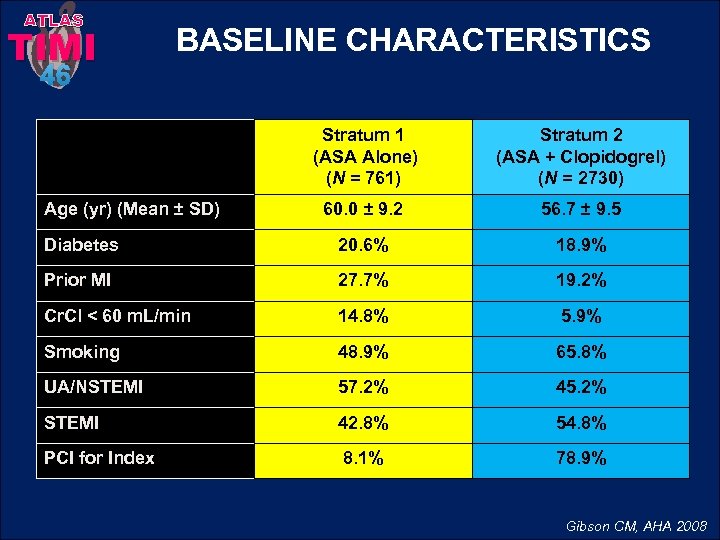
ATLAS TIMI BASELINE CHARACTERISTICS 46 Stratum 1 (ASA Alone) (N = 761) Stratum 2 (ASA + Clopidogrel) (N = 2730) 60. 0 ± 9. 2 56. 7 ± 9. 5 Diabetes 20. 6% 18. 9% Prior MI 27. 7% 19. 2% Cr. Cl < 60 m. L/min 14. 8% 5. 9% Smoking 48. 9% 65. 8% UA/NSTEMI 57. 2% 45. 2% STEMI 42. 8% 54. 8% PCI for Index 8. 1% 78. 9% Age (yr) (Mean ± SD) Gibson CM, AHA 2008
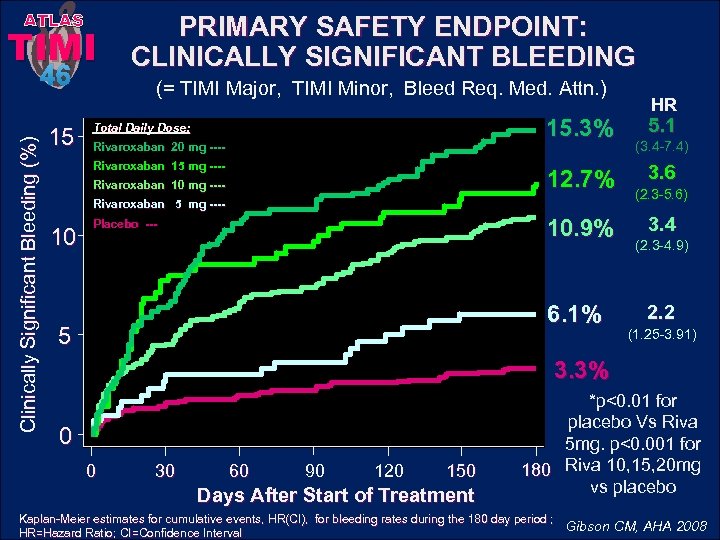
ATLAS TIMI Clinically Significant Bleeding (%) 46 15 PRIMARY SAFETY ENDPOINT: CLINICALLY SIGNIFICANT BLEEDING (= TIMI Major, TIMI Minor, Bleed Req. Med. Attn. ) 15. 3% Total Daily Dose: Rivaroxaban 20 mg ---Rivaroxaban 15 mg ---- 12. 7% Rivaroxaban 10 mg ---Rivaroxaban 5 mg ---- 10 10. 9% Placebo --- 6. 1% 5 HR 5. 1 (3. 4 -7. 4) 3. 6 (2. 3 -5. 6) 3. 4 (2. 3 -4. 9) 2. 2 (1. 25 -3. 91) 3. 3% 0 0 30 60 90 120 150 Days After Start of Treatment *p<0. 01 for placebo Vs Riva 5 mg. p<0. 001 for 180 Riva 10, 15, 20 mg vs placebo Kaplan-Meier estimates for cumulative events, HR(CI), for bleeding rates during the 180 day period ; Gibson CM, AHA 2008 HR=Hazard Ratio; CI=Confidence Interval
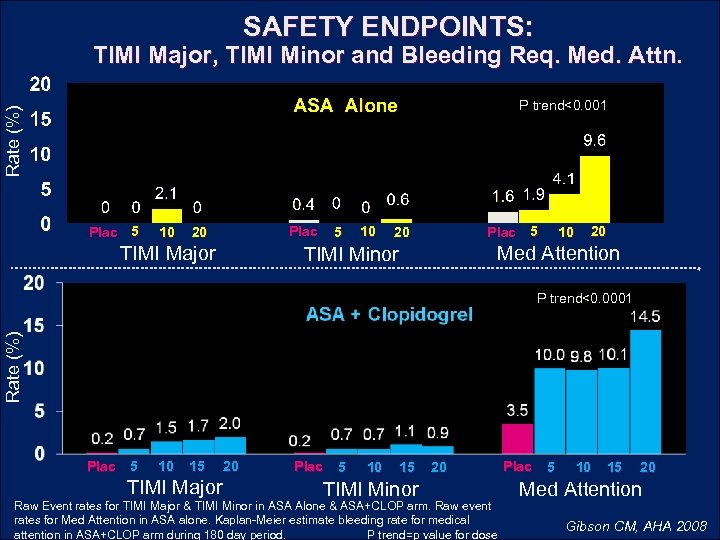
SAFETY ENDPOINTS: TIMI Major, TIMI Minor and Bleeding Req. Med. Attn. Rate (%) P trend<0. 001 Plac 5 10 Plac 20 TIMI Major 5 10 20 Plac 5 10 20 Med Attention TIMI Minor Rate (%) P trend<0. 0001 Plac 5 10 15 TIMI Major 20 Plac 5 10 15 TIMI Minor 20 Raw Event rates for TIMI Major & TIMI Minor in ASA Alone & ASA+CLOP arm. Raw event rates for Med Attention in ASA alone. Kaplan-Meier estimate bleeding rate for medical attention in ASA+CLOP arm during 180 day period. P trend=p value for dose Plac 5 10 15 20 Med Attention Gibson CM, AHA 2008
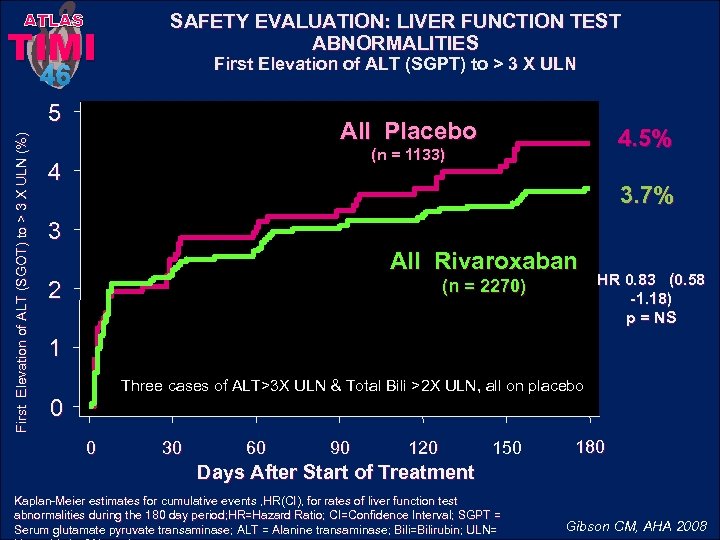
ATLAS TIMI SAFETY EVALUATION: LIVER FUNCTION TEST ABNORMALITIES First Elevation of ALT (SGPT) to > 3 X ULN 46 First Elevation of ALT (SGOT) to > 3 X ULN (%) 5 All Placebo 4. 5% (n = 1133) 4 3. 7% 3 All Rivaroxaban (n = 2270) 2 HR 0. 83 (0. 58 -1. 18) p = NS 1 Three cases of ALT>3 X ULN & Total Bili >2 X ULN, all on placebo 0 0 30 60 90 120 150 180 Days After Start of Treatment Kaplan-Meier estimates for cumulative events , HR(CI), for rates of liver function test abnormalities during the 180 day period; HR=Hazard Ratio; CI=Confidence Interval; SGPT = Serum glutamate pyruvate transaminase; ALT = Alanine transaminase; Bili=Bilirubin; ULN= Gibson CM, AHA 2008
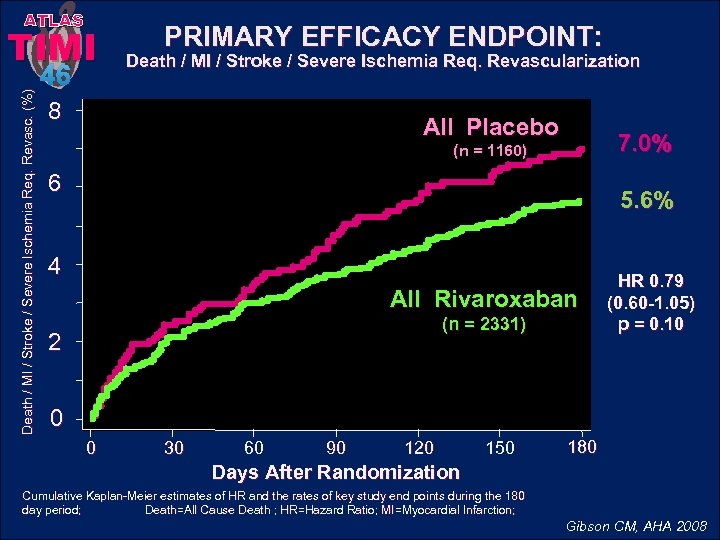
ATLAS Death / MI / Stroke / Severe Ischemia Req. Revasc. (%) TIMI 46 PRIMARY EFFICACY ENDPOINT: Death / MI / Stroke / Severe Ischemia Req. Revascularization 8 All Placebo 7. 0% (n = 1160) 6 5. 6% 4 All Rivaroxaban (n = 2331) 2 HR 0. 79 (0. 60 -1. 05) p = 0. 10 0 0 30 60 90 120 150 180 Days After Randomization Cumulative Kaplan-Meier estimates of HR and the rates of key study end points during the 180 day period; Death=All Cause Death ; HR=Hazard Ratio; MI=Myocardial Infarction; Gibson CM, AHA 2008
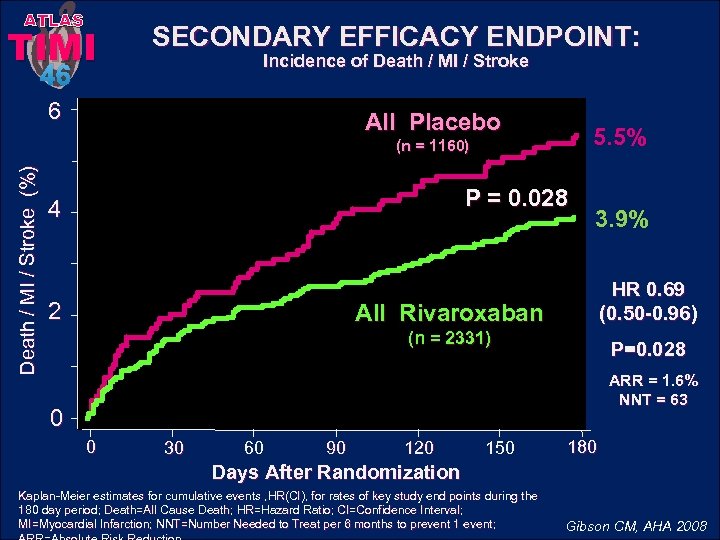
ATLAS TIMI SECONDARY EFFICACY ENDPOINT: Incidence of Death / MI / Stroke 46 6 All Placebo 5. 5% Death / MI / Stroke (%) (n = 1160) P = 0. 028 4 2 3. 9% HR 0. 69 (0. 50 -0. 96) All Rivaroxaban (n = 2331) P=0. 028 ARR = 1. 6% NNT = 63 0 0 30 60 90 120 150 180 Days After Randomization Kaplan-Meier estimates for cumulative events , HR(CI), for rates of key study end points during the 180 day period; Death=All Cause Death; HR=Hazard Ratio; CI=Confidence Interval; MI=Myocardial Infarction; NNT=Number Needed to Treat per 6 months to prevent 1 event; Gibson CM, AHA 2008
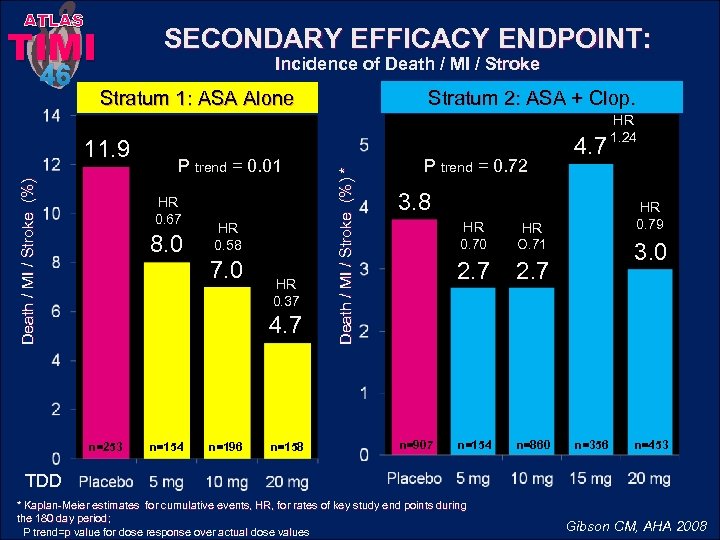
ATLAS TIMI Incidence of Death / MI / Stroke Stratum 2: ASA + Clop. Stratum 1: ASA Alone Death / MI / Stroke (%) 11. 9 P trend = 0. 01 HR 0. 67 8. 0 HR 0. 58 7. 0 HR 0. 37 4. 7 n=253 n=154 n=196 n=158 Death / MI / Stroke (%) * 46 SECONDARY EFFICACY ENDPOINT: P trend = 0. 72 4. 7 3. 8 HR 0. 70 n=907 2. 7 n=154 n=860 HR 0. 79 HR O. 71 2. 7 HR 1. 24 3. 0 n=356 n=453 TDD * Kaplan-Meier estimates for cumulative events, HR, for rates of key study end points during the 180 day period; P trend=p value for dose response over actual dose values Gibson CM, AHA 2008
ATLAS TIMI SUMMARY- SAFETY 46 • There was increased bleeding associated with higher doses of rivaroxaban. • Most bleeding was bleeding requiring medical attention, rather than TIMI major or TIMI minor bleeding • No evidence of drug induced liver injury Gibson CM, AHA 2008
ATLAS TIMI 46 SUMMARY-EFFICACY 1 o Endpoint: 21% RRR (HR 0. 79, p=0. 10) in death, MI, stroke, or severe recurrent ischemia requiring revascularization 2 o Endpoint: 31% RRR in the risk of death, MI, or stroke (HR 0. 69, p=0. 028) Gibson CM, AHA 2008
ATLAS TIMI 46 SELECTION OF DOSES FOR PHASE III Based upon: 1. Efficacy at lower doses of rivaroxaban 2. Graded increase in bleeding at higher doses of rivaroxaban 3. A trend for BID doses of rivaroxaban to be safer and more efficacious than QD dosing of rivaroxaban in ACS • Two low doses, 2. 5 mg BID and 5 mg BID, have been selected for the Phase III trial Gibson CM, AHA 2008
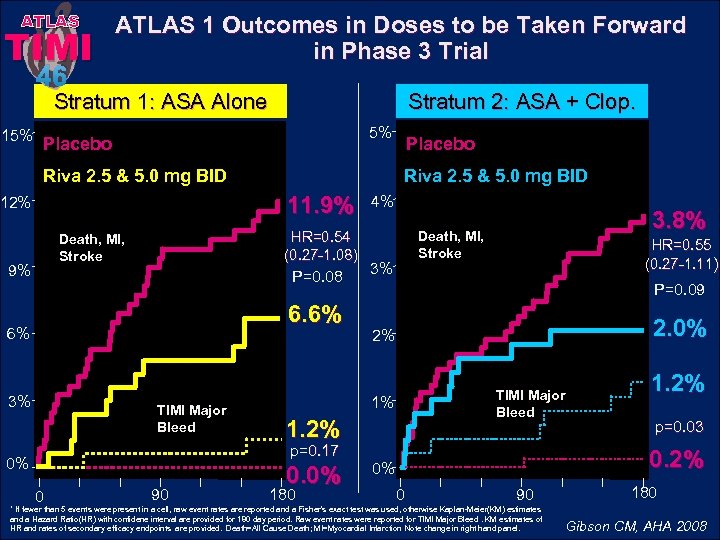
ATLAS TIMI 46 ATLAS 1 Outcomes in Doses to be Taken Forward in Phase 3 Trial Stratum 1: ASA Alone 15% Stratum 2: ASA + Clop. 5% Placebo Riva 2. 5 & 5. 0 mg BID 11. 9% 12% HR=0. 54 (0. 27 -1. 08) Death, MI, Stroke 9% P=0. 08 6. 6% 6% 3% TIMI Major Bleed 0 90 4% 3% 180 HR=0. 55 (0. 27 -1. 11) P=0. 09 2. 0% 2% TIMI Major Bleed 1. 2% 0. 0% 3. 8% Death, MI, Stroke 1% p=0. 17 0% Placebo 1. 2% p=0. 03 0. 2% 0% 0 90 * If fewer than 5 events were present in a cell, raw event rates are reported and a Fisher’s exact test was used, otherwise Kaplan-Meier(KM) estimates and a Hazard Ratio(HR) with confidene interval are provided for 180 day period. Raw event rates were reported for TIMI Major Bleed. KM estimates of HR and rates of secondary efficacy endpoints are provided. Death=All Cause Death; MI=Myocardial Infarction Note change in right hand panel. 180 Gibson CM, AHA 2008
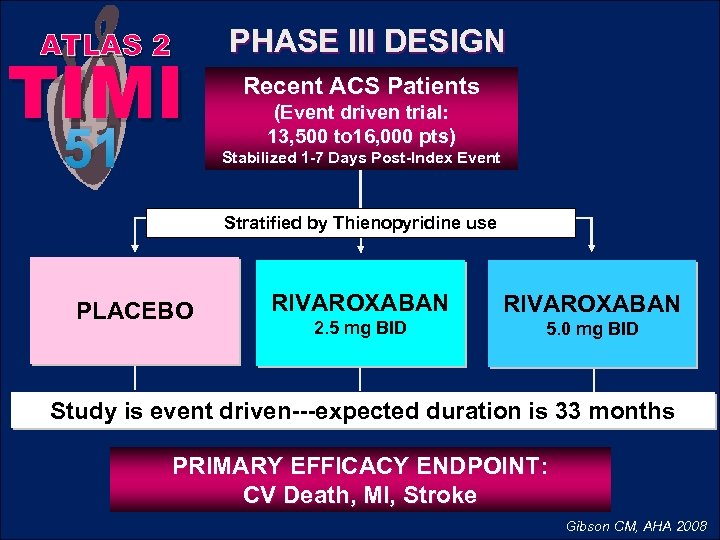
ATLAS 2 TIMI 51 PHASE III DESIGN Recent ACS Patients (Event driven trial: 13, 500 to 16, 000 pts) Stabilized 1 -7 Days Post-Index Event Stratified by Thienopyridine use PLACEBO RIVAROXABAN 2. 5 mg BID 5. 0 mg BID Study is event driven---expected duration is 33 months PRIMARY EFFICACY ENDPOINT: CV Death, MI, Stroke Gibson CM, AHA 2008
3f32eb94124e3d68ca24fe74fbc5aaa7.ppt