0bbe34f28fdf16c1330f64d72e11aff1.ppt
- Количество слайдов: 14

Assignment!!! Copy this before class. Lewis dot diagrams—draw Lewis dot diagrams for ions showing them bonded as we did in class. • K+F • Mg + I • Be + S • Na + O • Al + Br Also: page 110 in review book, 26 -30, 32, 33 You can buy review books in Mr. Sciavi’s room

Homework Review In Review book do: pg 105 14 -17; Pg 113 2, 3, 6, 8, 12, 15 Pg 114, 16, 17, 18, 20
Lewis Dot Structures for Atoms and Ions After the lesson you will know: 1. What they are and how the dot diagrams work. 2. How to draw atoms and ions. 3. How to represent ionic bonding when electrons are transferred.

As you know, in chemical bonding, electrons are either transferred or shared. One way to represent bonds is to actually use dots as electrons and the symbol to represent the element. Once you know how to do this, you can use the dots to show the bonding. These diagrams are called Lewis dot diagrams.
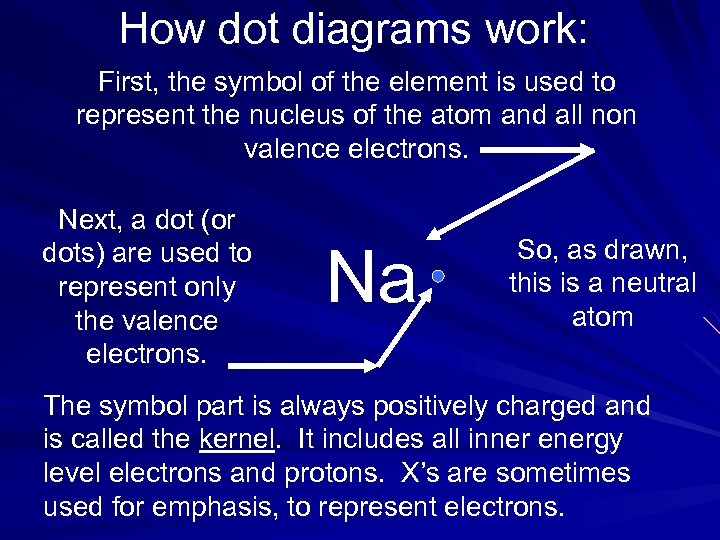
How dot diagrams work: First, the symbol of the element is used to represent the nucleus of the atom and all non valence electrons. Next, a dot (or dots) are used to represent only the valence electrons. Na So, as drawn, this is a neutral atom The symbol part is always positively charged and is called the kernel. It includes all inner energy level electrons and protons. X’s are sometimes used for emphasis, to represent electrons.

Arrangement of the dots: Some students get hung up on this. The convention says to start with the first two on “top” at the twelve o’clock position. However, as you will see some variations are allowed depending on whether you are drawing an atom or a bonded atom. For example: C O Cl Al P 2 -4 2 -6 2 -8 -7 2 -8 -3 2 -8 -5 For any element, check the electron configuration. The last number is/are the valence electrons. Add those around the symbol.

Now try to remember what happens when bonds form. All atoms are noble gas “wannabees”. That means they try to achieve a noble gas configuration. They can only do this by sharing or losing electrons. Let’s take the case of ionic bonding first, where electrons transfer. Usually in Regents you will get a metal with a non metal. The difference in electronegativity will be 1. 7 or greater.
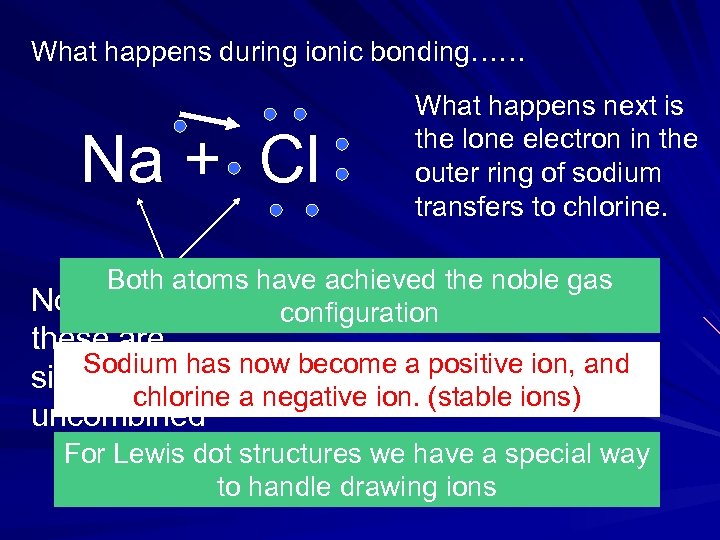
What happens during ionic bonding…… Na + Cl What happens next is the lone electron in the outer ring of sodium transfers to chlorine. Both atoms have achieved the noble gas Notice that configuration these are Sodium has single atoms now become a positive ion, and chlorine a negative ion. (stable ions) uncombined For Lewis dot structures we have a special way to handle drawing ions

Let’s take the two cases separately First the plus ion…. In these, the outer ring has vanished and we are back to the kernel. To show it is an ion, we use brackets, and indicate the charge by a plus or minus sign. [ Na] + Remember: a kernel with no electrons is positive!!
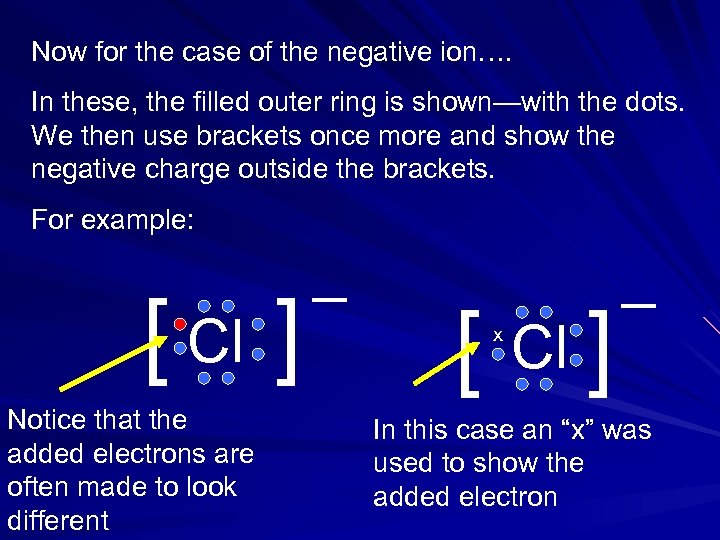
Now for the case of the negative ion…. In these, the filled outer ring is shown—with the dots. We then use brackets once more and show the negative charge outside the brackets. For example: [ Cl ] Notice that the added electrons are often made to look different _ [ x Cl ] _ In this case an “x” was used to show the added electron
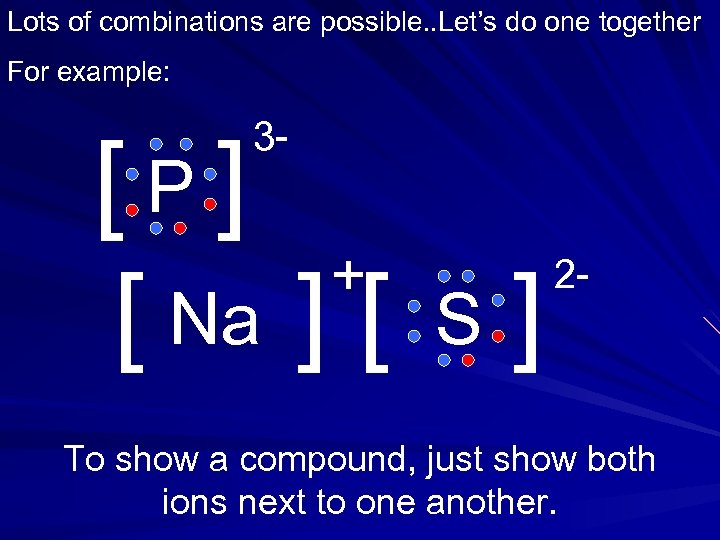
Lots of combinations are possible. . Let’s do one together For example: 3 - [P] + [ Na ] [ S ] 2 - To show a compound, just show both ions next to one another.

Now you try a couple, and I’ll see how you do. Show the lewis dot structures for: Magnesium Nitrogen Draw the lewis structures for magnesium bonded to oxygen

End

This requires some explanation: Rules for determining bond types: 1. If there is no difference—the bond is non-polar note that in this course they will normally give you an example with no difference. (. 5) 2. If there is a difference between 0 and 1. 7, the bond is said to be “polar”. 3. If the difference is greater than 1. 7, the bond is ionic. What does polar mean? What’s the difference between polar and non-polar? Let’s find out!!
0bbe34f28fdf16c1330f64d72e11aff1.ppt