7a832f7447905d5cf150ce7a00e7b84d.ppt
- Количество слайдов: 25
 Assignment: atoms and riddle sheet. Study for the quiz!! Know the nicknames of groups 1, 2, 17, 18; The particles of the atom; General characteristics of metals non-metals and metalloids; the ID card numbers of atomic number and mass and the nomenclature of how we show isotopes
Assignment: atoms and riddle sheet. Study for the quiz!! Know the nicknames of groups 1, 2, 17, 18; The particles of the atom; General characteristics of metals non-metals and metalloids; the ID card numbers of atomic number and mass and the nomenclature of how we show isotopes
 Homework Review
Homework Review
 The true nature of the atom
The true nature of the atom
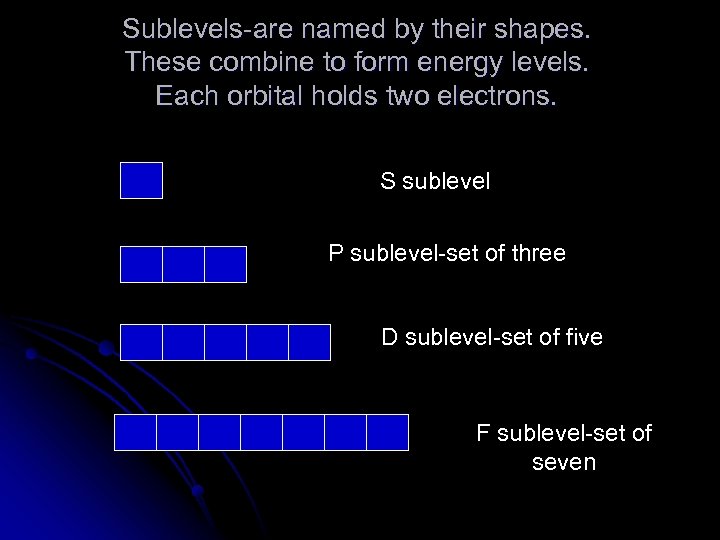 Sublevels-are named by their shapes. These combine to form energy levels. Each orbital holds two electrons. S sublevel P sublevel-set of three D sublevel-set of five F sublevel-set of seven
Sublevels-are named by their shapes. These combine to form energy levels. Each orbital holds two electrons. S sublevel P sublevel-set of three D sublevel-set of five F sublevel-set of seven
 Atomic s-orbitals Atomic p-orbitals Atomic d-orbitals Atomic f-orbitals G- orbitals? ?
Atomic s-orbitals Atomic p-orbitals Atomic d-orbitals Atomic f-orbitals G- orbitals? ?
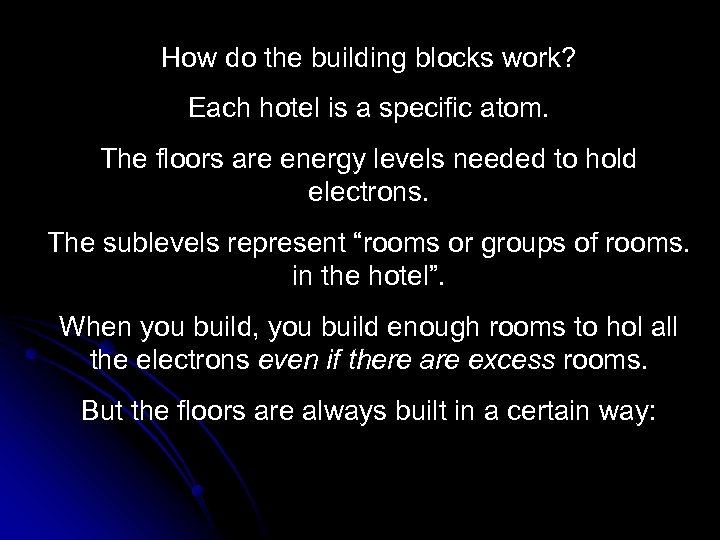 How do the building blocks work? Each hotel is a specific atom. The floors are energy levels needed to hold electrons. The sublevels represent “rooms or groups of rooms. in the hotel”. When you build, you build enough rooms to hol all the electrons even if there are excess rooms. But the floors are always built in a certain way:
How do the building blocks work? Each hotel is a specific atom. The floors are energy levels needed to hold electrons. The sublevels represent “rooms or groups of rooms. in the hotel”. When you build, you build enough rooms to hol all the electrons even if there are excess rooms. But the floors are always built in a certain way:
 Imagine we are going to build a series of hotels, and fill it with guests. Each one is a little bigger than the next. To build each hotel, we need to learn: 1. The building blocks 2. How the building blocks go together. 3. How to fill the rooms. There are rules for each Of course, each hotel is an atom, and our floors are built with rooms called orbitals. But orbitals come in singles, threes, fives and sevens called sublevels. These are the building blocks.
Imagine we are going to build a series of hotels, and fill it with guests. Each one is a little bigger than the next. To build each hotel, we need to learn: 1. The building blocks 2. How the building blocks go together. 3. How to fill the rooms. There are rules for each Of course, each hotel is an atom, and our floors are built with rooms called orbitals. But orbitals come in singles, threes, fives and sevens called sublevels. These are the building blocks.
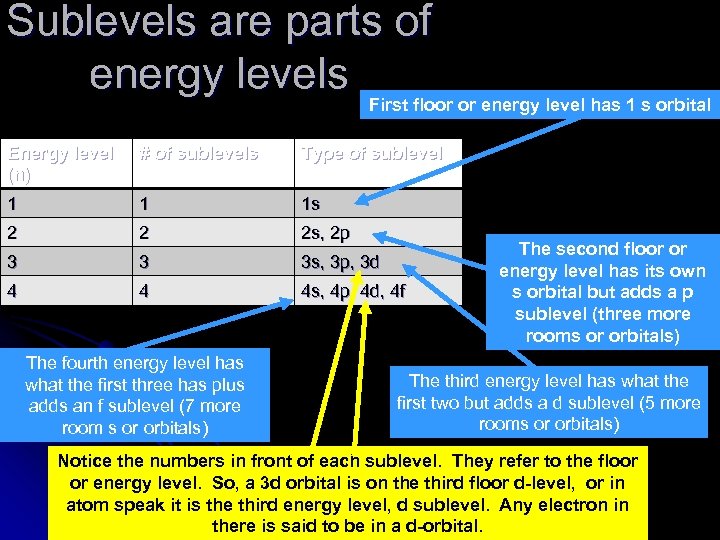 Sublevels are parts of energy levels First floor or energy level has 1 s orbital Energy level (n) # of sublevels Type of sublevel 1 1 1 s 2 2 2 s, 2 p 3 3 3 s, 3 p, 3 d 4 4 4 s, 4 p, 4 d, 4 f The fourth energy level has what the first three has plus adds an f sublevel (7 more room s or orbitals) The second floor or energy level has its own s orbital but adds a p sublevel (three more rooms or orbitals) The third energy level has what the first two but adds a d sublevel (5 more rooms or orbitals) Notice the numbers in front of each sublevel. They refer to the floor or energy level. So, a 3 d orbital is on the third floor d-level, or in atom speak it is the third energy level, d sublevel. Any electron in there is said to be in a d-orbital.
Sublevels are parts of energy levels First floor or energy level has 1 s orbital Energy level (n) # of sublevels Type of sublevel 1 1 1 s 2 2 2 s, 2 p 3 3 3 s, 3 p, 3 d 4 4 4 s, 4 p, 4 d, 4 f The fourth energy level has what the first three has plus adds an f sublevel (7 more room s or orbitals) The second floor or energy level has its own s orbital but adds a p sublevel (three more rooms or orbitals) The third energy level has what the first two but adds a d sublevel (5 more rooms or orbitals) Notice the numbers in front of each sublevel. They refer to the floor or energy level. So, a 3 d orbital is on the third floor d-level, or in atom speak it is the third energy level, d sublevel. Any electron in there is said to be in a d-orbital.
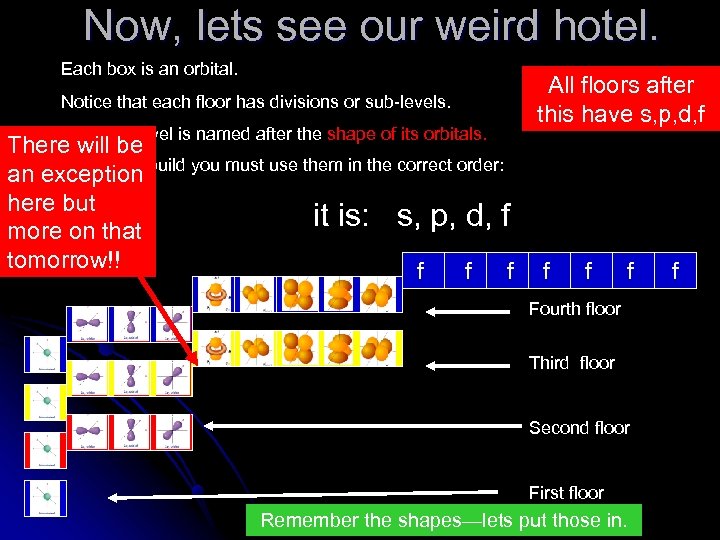 Now, lets see our weird hotel. Each box is an orbital. Notice that each floor has divisions or sub-levels. Each sublevel is named after the shape of its orbitals. There will be In order to an exception build you must use them in the correct order: here but it is: s, p, d, f more on that tomorrow!! f f f All floors after this have s, p, d, f f Fourth floor Third floor Second floor First floor Remember the shapes—lets put those in. f
Now, lets see our weird hotel. Each box is an orbital. Notice that each floor has divisions or sub-levels. Each sublevel is named after the shape of its orbitals. There will be In order to an exception build you must use them in the correct order: here but it is: s, p, d, f more on that tomorrow!! f f f All floors after this have s, p, d, f f Fourth floor Third floor Second floor First floor Remember the shapes—lets put those in. f
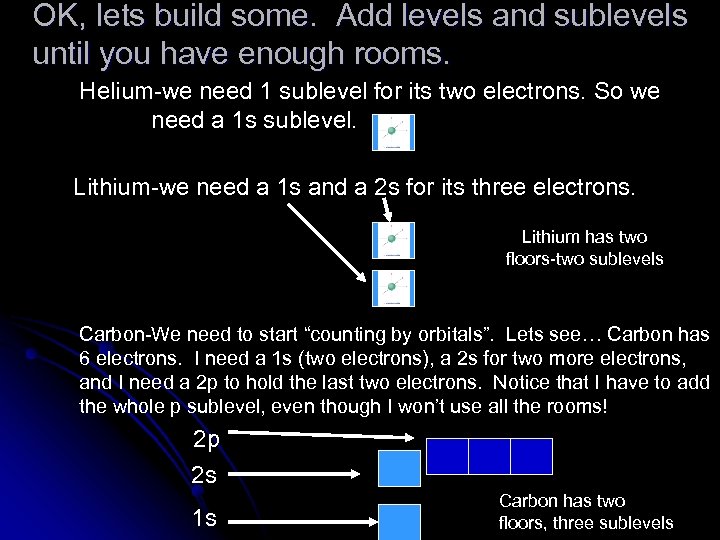 OK, lets build some. Add levels and sublevels until you have enough rooms. Helium-we need 1 sublevel for its two electrons. So we need a 1 s sublevel. Lithium-we need a 1 s and a 2 s for its three electrons. Lithium has two floors-two sublevels Carbon-We need to start “counting by orbitals”. Lets see… Carbon has 6 electrons. I need a 1 s (two electrons), a 2 s for two more electrons, and I need a 2 p to hold the last two electrons. Notice that I have to add the whole p sublevel, even though I won’t use all the rooms! 2 p 2 s 1 s Carbon has two floors, three sublevels
OK, lets build some. Add levels and sublevels until you have enough rooms. Helium-we need 1 sublevel for its two electrons. So we need a 1 s sublevel. Lithium-we need a 1 s and a 2 s for its three electrons. Lithium has two floors-two sublevels Carbon-We need to start “counting by orbitals”. Lets see… Carbon has 6 electrons. I need a 1 s (two electrons), a 2 s for two more electrons, and I need a 2 p to hold the last two electrons. Notice that I have to add the whole p sublevel, even though I won’t use all the rooms! 2 p 2 s 1 s Carbon has two floors, three sublevels
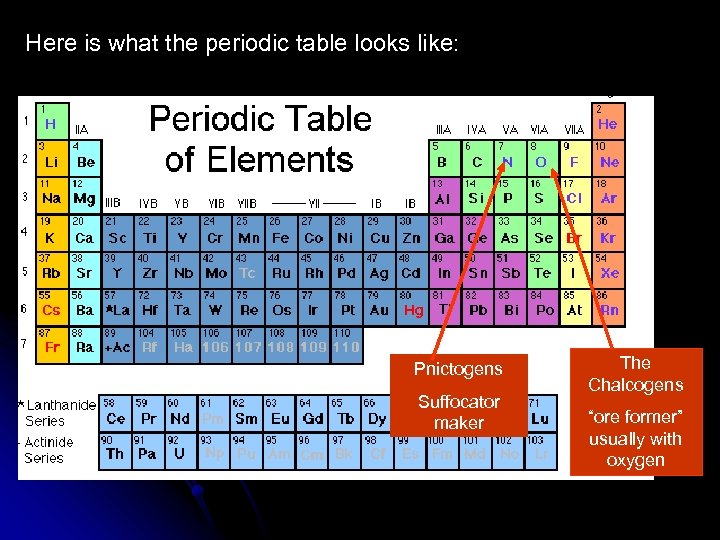 Here is what the periodic table looks like: Pnictogens Suffocator maker The Chalcogens “ore former” usually with oxygen
Here is what the periodic table looks like: Pnictogens Suffocator maker The Chalcogens “ore former” usually with oxygen
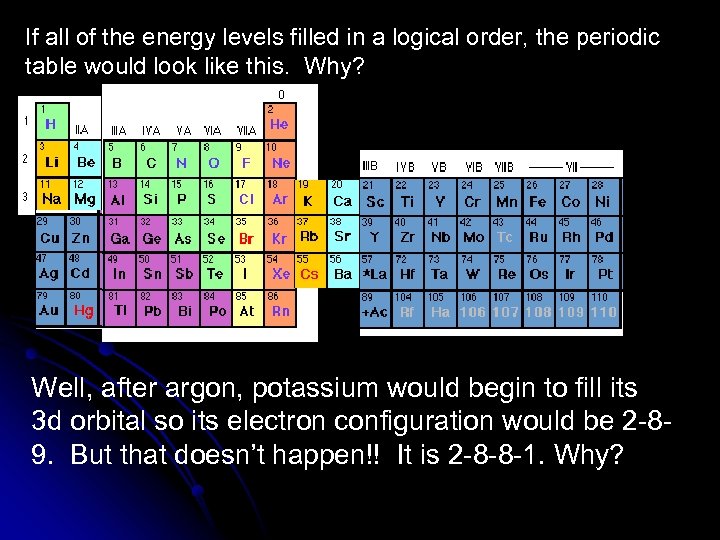 If all of the energy levels filled in a logical order, the periodic table would look like this. Why? Well, after argon, potassium would begin to fill its 3 d orbital so its electron configuration would be 2 -89. But that doesn’t happen!! It is 2 -8 -8 -1. Why?
If all of the energy levels filled in a logical order, the periodic table would look like this. Why? Well, after argon, potassium would begin to fill its 3 d orbital so its electron configuration would be 2 -89. But that doesn’t happen!! It is 2 -8 -8 -1. Why?
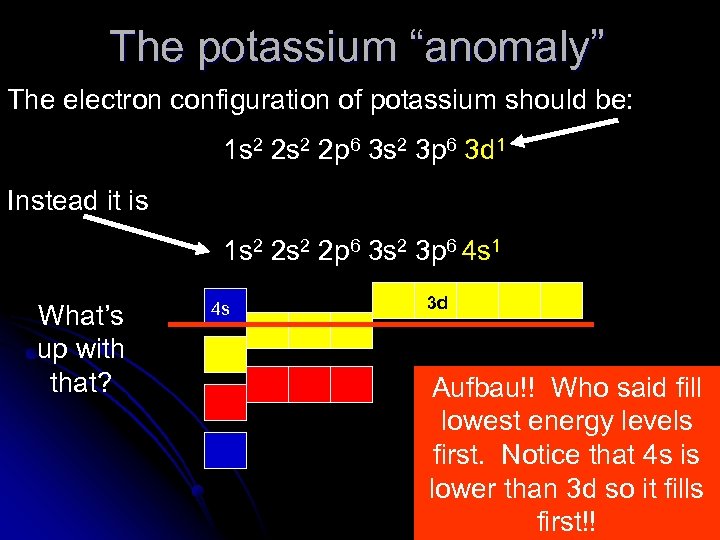 The potassium “anomaly” The electron configuration of potassium should be: 1 s 2 2 p 6 3 s 2 3 p 6 3 d 1 Instead it is 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 What’s up with that? 4 s 3 d Aufbau!! Who said fill lowest energy levels first. Notice that 4 s is lower than 3 d so it fills first!!
The potassium “anomaly” The electron configuration of potassium should be: 1 s 2 2 p 6 3 s 2 3 p 6 3 d 1 Instead it is 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 What’s up with that? 4 s 3 d Aufbau!! Who said fill lowest energy levels first. Notice that 4 s is lower than 3 d so it fills first!!
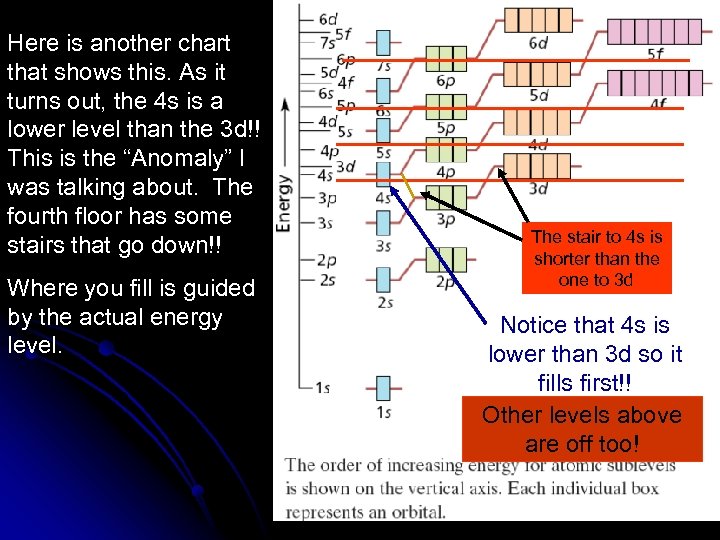 Here is another chart that shows this. As it turns out, the 4 s is a lower level than the 3 d!! This is the “Anomaly” I was talking about. The fourth floor has some stairs that go down!! Where you fill is guided by the actual energy level. The stair to 4 s is shorter than the one to 3 d Notice that 4 s is lower than 3 d so it fills first!! Other levels above are off too!
Here is another chart that shows this. As it turns out, the 4 s is a lower level than the 3 d!! This is the “Anomaly” I was talking about. The fourth floor has some stairs that go down!! Where you fill is guided by the actual energy level. The stair to 4 s is shorter than the one to 3 d Notice that 4 s is lower than 3 d so it fills first!! Other levels above are off too!
 Oh man, I just had this right, now he pulls this on me. Howm I ever gonna git dis straight?
Oh man, I just had this right, now he pulls this on me. Howm I ever gonna git dis straight?
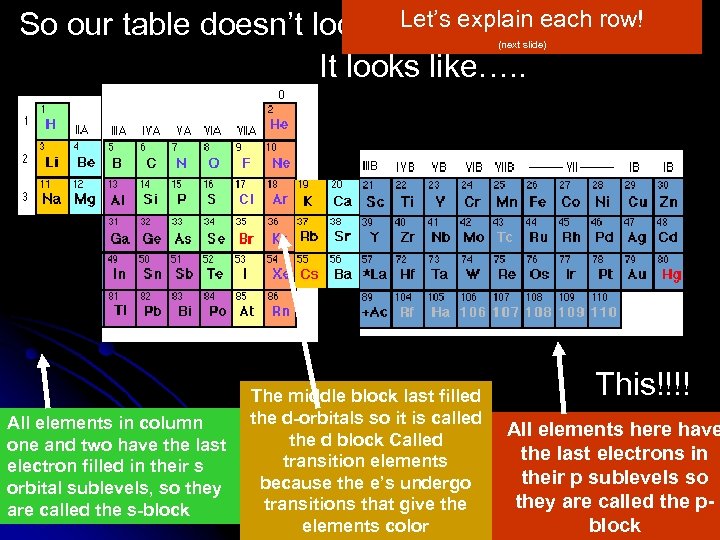 Let’s explain So our table doesn’t look like this…. each row! It looks like…. . (next slide) All elements in column one and two have the last electron filled in their s orbital sublevels, so they are called the s-block The middle block last filled the d-orbitals so it is called the d block Called transition elements because the e’s undergo transitions that give the elements color This!!!! All elements here have the last electrons in their p sublevels so they are called the pblock
Let’s explain So our table doesn’t look like this…. each row! It looks like…. . (next slide) All elements in column one and two have the last electron filled in their s orbital sublevels, so they are called the s-block The middle block last filled the d-orbitals so it is called the d block Called transition elements because the e’s undergo transitions that give the elements color This!!!! All elements here have the last electrons in their p sublevels so they are called the pblock
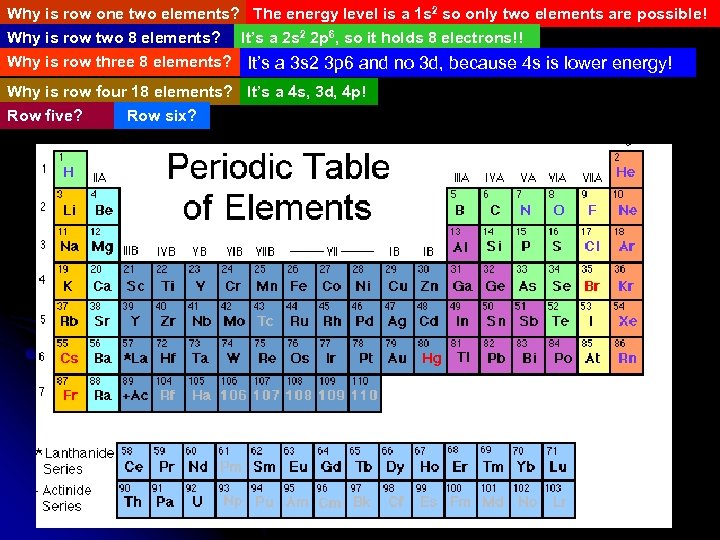 Why is row one two elements? The energy level is a 1 s 2 so only two elements are possible! Why is row two 8 elements? It’s a 2 s 2 2 p 6, so it holds 8 electrons!! Why is row three 8 elements? It’s a 3 s 2 3 p 6 and no 3 d, because 4 s is lower energy! Why is row four 18 elements? It’s a 4 s, 3 d, 4 p! Row five? Row six?
Why is row one two elements? The energy level is a 1 s 2 so only two elements are possible! Why is row two 8 elements? It’s a 2 s 2 2 p 6, so it holds 8 electrons!! Why is row three 8 elements? It’s a 3 s 2 3 p 6 and no 3 d, because 4 s is lower energy! Why is row four 18 elements? It’s a 4 s, 3 d, 4 p! Row five? Row six?
 A Cool Periodic Table Something cool—David’s Whizzy Periodic Table
A Cool Periodic Table Something cool—David’s Whizzy Periodic Table









