7c564246ab4c069adfcb82a58a3595c3.ppt
- Количество слайдов: 27
 Assessment Workshop Copenhagen – January 2011 QIS/QOS: The new PQP quality templates 1| Lynda Paleshnuik | January 2011
Assessment Workshop Copenhagen – January 2011 QIS/QOS: The new PQP quality templates 1| Lynda Paleshnuik | January 2011
 Overview § What is a template § Introduction to the two templates: I: QOS – quality overall summary II: QIS – quality information summary § Uses/tips for QIS § Questions raised 2| Lynda Paleshnuik | January 2011
Overview § What is a template § Introduction to the two templates: I: QOS – quality overall summary II: QIS – quality information summary § Uses/tips for QIS § Questions raised 2| Lynda Paleshnuik | January 2011
 What is a template? Template: a preset format for a document. The dossier template (QOS-PD) = the backbone of the quality review The QIS = the summary of quality information = mini-QOS 3| Lynda Paleshnuik | January 2011
What is a template? Template: a preset format for a document. The dossier template (QOS-PD) = the backbone of the quality review The QIS = the summary of quality information = mini-QOS 3| Lynda Paleshnuik | January 2011
 QOS vs QIS § PQP has moved to ICH CTD format, which is a common format for PDs to be submitted to an agency. § The QOS is part of the ICH CTD structure of a product dossier (PD). As part of the CTD, it has been available since November 2000 (current version Sept 2002). § The QOS-PD is based on the CTD QOS, modified/expanded to be of the most use to WHO. 4| Lynda Paleshnuik | January 2011
QOS vs QIS § PQP has moved to ICH CTD format, which is a common format for PDs to be submitted to an agency. § The QOS is part of the ICH CTD structure of a product dossier (PD). As part of the CTD, it has been available since November 2000 (current version Sept 2002). § The QOS-PD is based on the CTD QOS, modified/expanded to be of the most use to WHO. 4| Lynda Paleshnuik | January 2011
 QOS vs QIS § The QIS is a regional document. It is not part of the CTD. § The QIS is a document with many uses within an agency or PQ. § Note that the QIS has an introductory page. The QOSPD does not, as the QOS instructions are found throughout the quality guideline 5| Lynda Paleshnuik | January 2011
QOS vs QIS § The QIS is a regional document. It is not part of the CTD. § The QIS is a document with many uses within an agency or PQ. § Note that the QIS has an introductory page. The QOSPD does not, as the QOS instructions are found throughout the quality guideline 5| Lynda Paleshnuik | January 2011
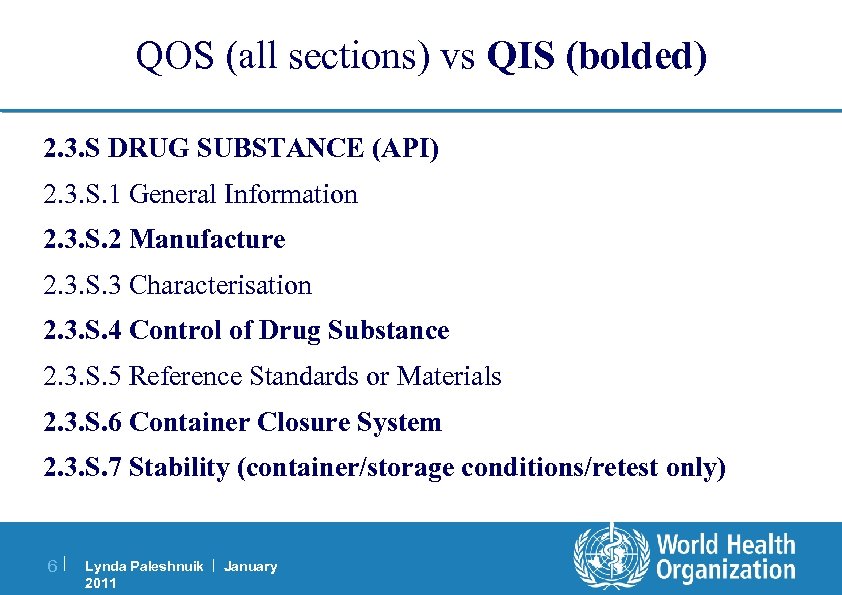 QOS (all sections) vs QIS (bolded) 2. 3. S DRUG SUBSTANCE (API) 2. 3. S. 1 General Information 2. 3. S. 2 Manufacture 2. 3. S. 3 Characterisation 2. 3. S. 4 Control of Drug Substance 2. 3. S. 5 Reference Standards or Materials 2. 3. S. 6 Container Closure System 2. 3. S. 7 Stability (container/storage conditions/retest only) 6| Lynda Paleshnuik | January 2011
QOS (all sections) vs QIS (bolded) 2. 3. S DRUG SUBSTANCE (API) 2. 3. S. 1 General Information 2. 3. S. 2 Manufacture 2. 3. S. 3 Characterisation 2. 3. S. 4 Control of Drug Substance 2. 3. S. 5 Reference Standards or Materials 2. 3. S. 6 Container Closure System 2. 3. S. 7 Stability (container/storage conditions/retest only) 6| Lynda Paleshnuik | January 2011
 QOS (all sections) vs QIS (bolded) 2. 3. P DRUG PRODUCT (FPP) 2. 3. P. 1 Description and Composition of the Drug Product 2. 3. P. 2 Pharmaceutical Development (batch summaries only) 2. 3. P. 3 Manufacture 2. 3. P. 4 Control of Excipients 2. 3. P. 5 Control of Drug Product 2. 3. P. 6 Reference Standards or Materials 2. 3. P. 7 Container Closure System 2. 3. P. 8 Stability (protocols, container/storage/retest) 7| Lynda Paleshnuik | January 2011
QOS (all sections) vs QIS (bolded) 2. 3. P DRUG PRODUCT (FPP) 2. 3. P. 1 Description and Composition of the Drug Product 2. 3. P. 2 Pharmaceutical Development (batch summaries only) 2. 3. P. 3 Manufacture 2. 3. P. 4 Control of Excipients 2. 3. P. 5 Control of Drug Product 2. 3. P. 6 Reference Standards or Materials 2. 3. P. 7 Container Closure System 2. 3. P. 8 Stability (protocols, container/storage/retest) 7| Lynda Paleshnuik | January 2011
 Introduction to the QOS-PD template § Filled out by applicant as part of a PD § Becomes the basis of the quality assessment report ► Data summarized in the QOS-PD is checked against the official documents in the PD, e. g. specifications, master production records, executed biobatch records ► Assessors’ remarks/questions/conclusions are added, creating the assessment report. 8| Lynda Paleshnuik | January 2011
Introduction to the QOS-PD template § Filled out by applicant as part of a PD § Becomes the basis of the quality assessment report ► Data summarized in the QOS-PD is checked against the official documents in the PD, e. g. specifications, master production records, executed biobatch records ► Assessors’ remarks/questions/conclusions are added, creating the assessment report. 8| Lynda Paleshnuik | January 2011
 Introduction to the QOS-PD template The QOS begins with a reference to the Q guide (1. 5, 3 and 4) for general/detailed instructions. Introductory information Cross-references Compendial references 9| Lynda Paleshnuik | January 2011
Introduction to the QOS-PD template The QOS begins with a reference to the Q guide (1. 5, 3 and 4) for general/detailed instructions. Introductory information Cross-references Compendial references 9| Lynda Paleshnuik | January 2011
 Introductory Info Cross-referencing Examples: Same API from same API manufacturer(s): if there are no major changes since the cross-referenced dossier(s) – include a list of changes to the API information and associated variations; This may substitute for all API information, ie 3. 2. S. (e. g. very recent PQ and complete variations), or substitute for some API information with supportive information, e. g. current API specs, updated stability, etc 10 | Lynda Paleshnuik | January 2011
Introductory Info Cross-referencing Examples: Same API from same API manufacturer(s): if there are no major changes since the cross-referenced dossier(s) – include a list of changes to the API information and associated variations; This may substitute for all API information, ie 3. 2. S. (e. g. very recent PQ and complete variations), or substitute for some API information with supportive information, e. g. current API specs, updated stability, etc 10 | Lynda Paleshnuik | January 2011
 Introductory Info Cross-referencing § Same API-excipient combination or API-API/excipient combination in another PQ dossier– compatibility data. § Same excipients (same standards) in another PQ dossier – excipient specifications. § Same packaging system - including materials - as another PQ dossier – packaging suitability tests (solid orals), packaging specifications. 11 | Lynda Paleshnuik | January 2011
Introductory Info Cross-referencing § Same API-excipient combination or API-API/excipient combination in another PQ dossier– compatibility data. § Same excipients (same standards) in another PQ dossier – excipient specifications. § Same packaging system - including materials - as another PQ dossier – packaging suitability tests (solid orals), packaging specifications. 11 | Lynda Paleshnuik | January 2011
 Introductory Information Cont’d Compendial references - confirm up-to-date (i. e. “most recent” column) - have available monographs on hand 12 | Lynda Paleshnuik | January 2011
Introductory Information Cont’d Compendial references - confirm up-to-date (i. e. “most recent” column) - have available monographs on hand 12 | Lynda Paleshnuik | January 2011
 Introduction to the QOS-PD template § Review of labelling and samples § API sections begin ► See Q guide section 1. 5 Guidance on format ► Indication of API form – tables give reference to information on which sections to fill out (full details in Q guide) 13 | Lynda Paleshnuik | January 2011
Introduction to the QOS-PD template § Review of labelling and samples § API sections begin ► See Q guide section 1. 5 Guidance on format ► Indication of API form – tables give reference to information on which sections to fill out (full details in Q guide) 13 | Lynda Paleshnuik | January 2011
 Submission of API Data Three means of providing API data for an FPP dossier: § CEP: certificate of suitability (EDQM) § APIMF: API master file procedure § All data provided in the dossier 14 | Lynda Paleshnuik | January 2011
Submission of API Data Three means of providing API data for an FPP dossier: § CEP: certificate of suitability (EDQM) § APIMF: API master file procedure § All data provided in the dossier 14 | Lynda Paleshnuik | January 2011
![Submission of API Data - CEP WHEN? Whenever a CEP is available [EDQM Databases]. Submission of API Data - CEP WHEN? Whenever a CEP is available [EDQM Databases].](https://present5.com/presentation/7c564246ab4c069adfcb82a58a3595c3/image-15.jpg) Submission of API Data - CEP WHEN? Whenever a CEP is available [EDQM Databases]. [Encouraged but not required] HOW? Provide the CEP (with all annexes) in the dossier, with the presence highlighted. The access box of the CEP is to be filled out by the CEP holder on behalf of the FPP manufacturer or applicant to PQP. 15 | Lynda Paleshnuik | January 2011
Submission of API Data - CEP WHEN? Whenever a CEP is available [EDQM Databases]. [Encouraged but not required] HOW? Provide the CEP (with all annexes) in the dossier, with the presence highlighted. The access box of the CEP is to be filled out by the CEP holder on behalf of the FPP manufacturer or applicant to PQP. 15 | Lynda Paleshnuik | January 2011
 EDQM Databases 16 | Lynda Paleshnuik | January 2011
EDQM Databases 16 | Lynda Paleshnuik | January 2011
 Introduction to the QOS-PD template QOS-PD is divided (according to CTD structure) into: 2. 3. S Drug substance (or API), and 2. 3. P Drug product (or FPP) 17 | Lynda Paleshnuik | January 2011
Introduction to the QOS-PD template QOS-PD is divided (according to CTD structure) into: 2. 3. S Drug substance (or API), and 2. 3. P Drug product (or FPP) 17 | Lynda Paleshnuik | January 2011
 Introduction to the QOS-PD template Some expansions compared to CTD QOS: Addition of tables (solubility, specifications, etc) Stability protocol sections expanded to include the various stability categories: commitment for primary batches, production batches, ongoing (annual) batches. Batch summaries in Section P. 2. 2. 1 Formulation Development (p. 14 -15 of 26). 18 | Lynda Paleshnuik | January 2011
Introduction to the QOS-PD template Some expansions compared to CTD QOS: Addition of tables (solubility, specifications, etc) Stability protocol sections expanded to include the various stability categories: commitment for primary batches, production batches, ongoing (annual) batches. Batch summaries in Section P. 2. 2. 1 Formulation Development (p. 14 -15 of 26). 18 | Lynda Paleshnuik | January 2011
 Introduction to the QOS-PD template § Regional information 2. 3. R includes where to provide the executed biobatch and proposed master production records § Additional standard templates for summarizing chromatographic methods and their validation. 19 | Lynda Paleshnuik | January 2011
Introduction to the QOS-PD template § Regional information 2. 3. R includes where to provide the executed biobatch and proposed master production records § Additional standard templates for summarizing chromatographic methods and their validation. 19 | Lynda Paleshnuik | January 2011
 Introduction to the QIS template § Filled out by applicant as part of a PD § The QIS is mainly cut-and-pasted information from the QOS-PD, therefore much of it does not need to be separately assessed. § Includes agreed commitments made by the applicant. The final QIS represents the PQ’d/approved quality data in the PD. 20 | Lynda Paleshnuik | January 2011
Introduction to the QIS template § Filled out by applicant as part of a PD § The QIS is mainly cut-and-pasted information from the QOS-PD, therefore much of it does not need to be separately assessed. § Includes agreed commitments made by the applicant. The final QIS represents the PQ’d/approved quality data in the PD. 20 | Lynda Paleshnuik | January 2011
 Introduction to the QIS template § As the quality report is created from the QOS-PD, whenever an inconsistency is found in a section which also is included in the QIS, this triggers a comment to the applicant to correct/revise the QIS. 21 | Lynda Paleshnuik | January 2011
Introduction to the QIS template § As the quality report is created from the QOS-PD, whenever an inconsistency is found in a section which also is included in the QIS, this triggers a comment to the applicant to correct/revise the QIS. 21 | Lynda Paleshnuik | January 2011
 Introduction to the QIS template For each Question & Answer round to the applicant, there should be a compiled question regarding the QIS as follows: You are requested to submit a (revised) QIS in electronic format, incorporating all of the changes requested in the preceding comments. In addition,
Introduction to the QIS template For each Question & Answer round to the applicant, there should be a compiled question regarding the QIS as follows: You are requested to submit a (revised) QIS in electronic format, incorporating all of the changes requested in the preceding comments. In addition,
 Uses of the QIS template § Summarizes the key quality data § For variations, the approved/PQ’d data is easily found for comparison § Assessing a similar product with no official standard (no compendial monograph available). Can easily check what was approved/PQ’d for: § Specifications § Retest, shelf-life, storage conditions, etc. 23 | Lynda Paleshnuik | January 2011
Uses of the QIS template § Summarizes the key quality data § For variations, the approved/PQ’d data is easily found for comparison § Assessing a similar product with no official standard (no compendial monograph available). Can easily check what was approved/PQ’d for: § Specifications § Retest, shelf-life, storage conditions, etc. 23 | Lynda Paleshnuik | January 2011
 Uses of the QIS template § Less common manufacturing technique (e. g. direct compression, coated beads, oral powder/granules): can search and easily find the details associated with other similarly manufactured products § Simplifies requalification assessment § Tool for inspectors (sites, process validation protocol codes, commitments) 24 | Lynda Paleshnuik | January 2011
Uses of the QIS template § Less common manufacturing technique (e. g. direct compression, coated beads, oral powder/granules): can search and easily find the details associated with other similarly manufactured products § Simplifies requalification assessment § Tool for inspectors (sites, process validation protocol codes, commitments) 24 | Lynda Paleshnuik | January 2011
 Tips - QIS § Only the most recent (final) QIS should be saved to the report database (or most recent in clearly marked folder). § As the QIS should be revised and submitted with each round of questions, can request confirmation that no unsolicited changes made (saves reviewing the entire QIS). 25 | Lynda Paleshnuik | January 2011
Tips - QIS § Only the most recent (final) QIS should be saved to the report database (or most recent in clearly marked folder). § As the QIS should be revised and submitted with each round of questions, can request confirmation that no unsolicited changes made (saves reviewing the entire QIS). 25 | Lynda Paleshnuik | January 2011
 Implementation of the QOS/QIS PDs in CTD format including QOS and QIS can be submitted now but are optional. For all PDs submitted after 1 March 2011, the QOS and QIS are mandatory. 26 | Lynda Paleshnuik | January 2011
Implementation of the QOS/QIS PDs in CTD format including QOS and QIS can be submitted now but are optional. For all PDs submitted after 1 March 2011, the QOS and QIS are mandatory. 26 | Lynda Paleshnuik | January 2011
 ? Questions? 27 | Lynda Paleshnuik | January 2011
? Questions? 27 | Lynda Paleshnuik | January 2011


