c0663b967b1a57a4a2a68cfe48d5189d.ppt
- Количество слайдов: 1
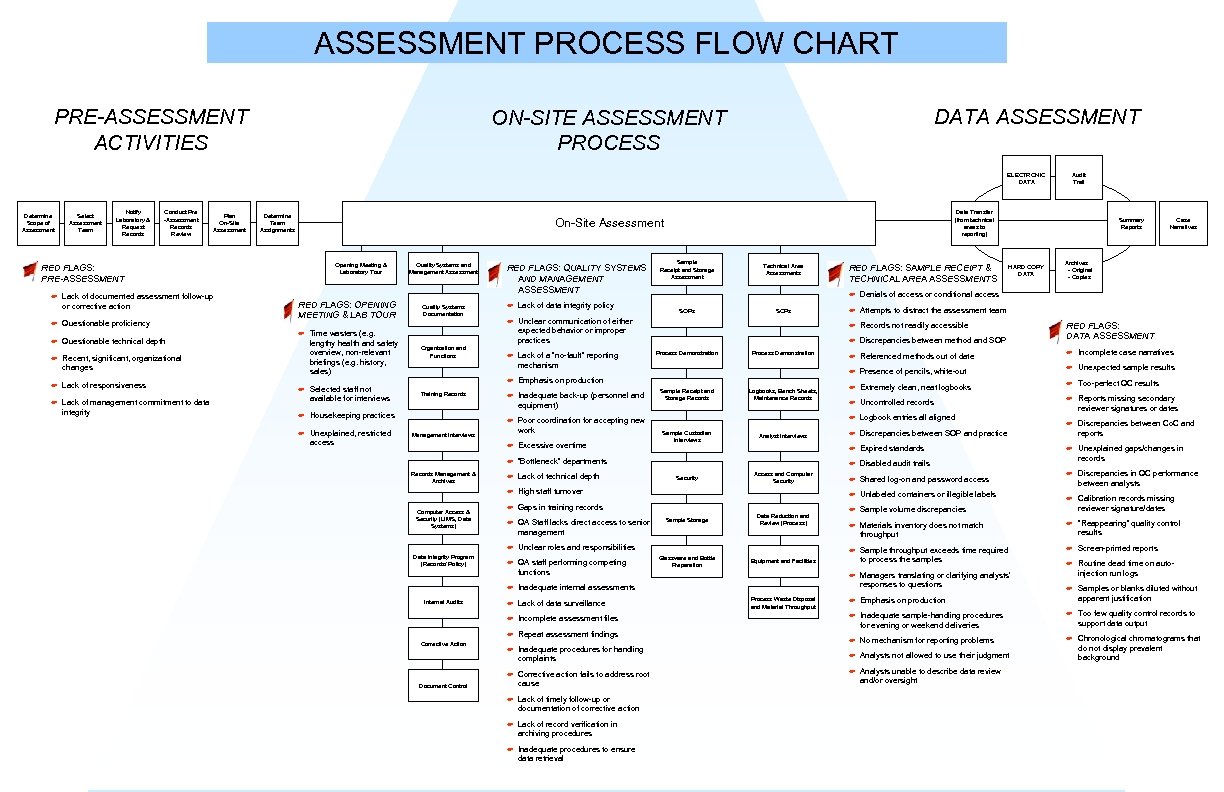 ASSESSMENT PROCESS FLOW CHART PRE-ASSESSMENT ACTIVITIES DATA ASSESSMENT ON-SITE ASSESSMENT PROCESS ELECTRONIC DATA Determine Scope of Assessment Select Assessment Team Notify Laboratory & Request Records Conduct Pre -Assessment Records Review Plan On-Site Assessment Determine Team Assignments F Lack of documented assessment follow-up or corrective action F Questionable proficiency F Questionable technical depth F Opening Meeting & Laboratory Tour RED FLAGS: OPENING MEETING & LAB TOUR Recent, significant, organizational changes F F Lack of responsiveness Lack of management commitment to data integrity Data Transfer (from technical areas to reporting) On-Site Assessment RED FLAGS: PRE-ASSESSMENT F F Time wasters (e. g. lengthy health and safety overview, non-relevant briefings (e. g. history, sales) Selected staff not available for interviews F Unexplained, restricted access RED FLAGS: QUALITY SYSTEMS AND MANAGEMENT ASSESSMENT Organization and Functions F Lack of data integrity policy F Quality Systems Documentation F Lack of a “no-fault” reporting mechanism Technical Area Assessments F Inadequate back-up (personnel and equipment) Poor coordination for accepting new work Management Interviews RED FLAGS: SAMPLE RECEIPT & TECHNICAL AREA ASSESSMENTS Summary Reports HARD COPY DATA Case Narratives Archives - Original - Copies F Analyst Interviews F Referenced methods out of date F Incomplete case narratives Presence of pencils, white-out F Unexpected sample results F Extremely clean, neat logbooks F Too-perfect QC results F Uncontrolled records F Reports missing secondary reviewer signatures or dates Logbook entries all aligned F F Discrepancies between SOP and practice Discrepancies between Co. C and reports Expired standards F F Sample Custodian Interviews Logbooks, Bench Sheets, Maintenance Records Discrepancies between method and SOP F Sample Receipt and Storage Records not readily accessible F Process Demonstration Attempts to distract the assessment team F Process Demonstration F F SOPs Denials of access or conditional access F SOPs Emphasis on production F Training Records Sample Receipt and Storage Assessment Unclear communication of either expected behavior or improper practices F Housekeeping practices F Quality Systems and Management Assessment Audit Trail Disabled audit trails Unexplained gaps/changes in records F Shared log-on and password access F Discrepancies in QC performance between analysts F Calibration records missing reviewer signature/dates RED FLAGS: DATA ASSESSMENT F Data Integrity Program (Records/ Policy) F Lack of technical depth High staff turnover F Unlabeled containers or illegible labels F Gaps in training records F Sample volume discrepancies F QA Staff lacks direct access to senior management F Materials inventory does not match throughput F “Reappearing” quality control results F Computer Access & Security (LIMS, Data Systems) “Bottleneck” departments F Records Management & Archives Excessive overtime F Unclear roles and responsibilities F Sample throughput exceeds time required to process the samples F Screen-printed reports F F Managers translating or clarifying analysts’ responses to questions Routine dead time on autoinjection run logs F F Emphasis on production Samples or blanks diluted without apparent justification F Inadequate sample-handling procedures for evening or weekend deliveries F Too few quality control records to support data output F No mechanism for reporting problems F F Analysts not allowed to use their judgment Chronological chromatograms that do not display prevalent background F Analysts unable to describe data review and/or oversight F F QA staff performing competing functions F Lack of data surveillance Incomplete assessment files F Repeat assessment findings F Inadequate procedures for handling complaints F Corrective Action Corrective action fails to address root cause F Lack of timely follow-up or documentation of corrective action F Lack of record verification in archiving procedures F Inadequate procedures to ensure data retrieval Document Control Sample Storage Data Reduction and Review (Process) Glassware and Bottle Preparation Equipment and Facilities Inadequate internal assessments F Internal Audits Security Access and Computer Security Process Waste Disposal and Material Throughput
ASSESSMENT PROCESS FLOW CHART PRE-ASSESSMENT ACTIVITIES DATA ASSESSMENT ON-SITE ASSESSMENT PROCESS ELECTRONIC DATA Determine Scope of Assessment Select Assessment Team Notify Laboratory & Request Records Conduct Pre -Assessment Records Review Plan On-Site Assessment Determine Team Assignments F Lack of documented assessment follow-up or corrective action F Questionable proficiency F Questionable technical depth F Opening Meeting & Laboratory Tour RED FLAGS: OPENING MEETING & LAB TOUR Recent, significant, organizational changes F F Lack of responsiveness Lack of management commitment to data integrity Data Transfer (from technical areas to reporting) On-Site Assessment RED FLAGS: PRE-ASSESSMENT F F Time wasters (e. g. lengthy health and safety overview, non-relevant briefings (e. g. history, sales) Selected staff not available for interviews F Unexplained, restricted access RED FLAGS: QUALITY SYSTEMS AND MANAGEMENT ASSESSMENT Organization and Functions F Lack of data integrity policy F Quality Systems Documentation F Lack of a “no-fault” reporting mechanism Technical Area Assessments F Inadequate back-up (personnel and equipment) Poor coordination for accepting new work Management Interviews RED FLAGS: SAMPLE RECEIPT & TECHNICAL AREA ASSESSMENTS Summary Reports HARD COPY DATA Case Narratives Archives - Original - Copies F Analyst Interviews F Referenced methods out of date F Incomplete case narratives Presence of pencils, white-out F Unexpected sample results F Extremely clean, neat logbooks F Too-perfect QC results F Uncontrolled records F Reports missing secondary reviewer signatures or dates Logbook entries all aligned F F Discrepancies between SOP and practice Discrepancies between Co. C and reports Expired standards F F Sample Custodian Interviews Logbooks, Bench Sheets, Maintenance Records Discrepancies between method and SOP F Sample Receipt and Storage Records not readily accessible F Process Demonstration Attempts to distract the assessment team F Process Demonstration F F SOPs Denials of access or conditional access F SOPs Emphasis on production F Training Records Sample Receipt and Storage Assessment Unclear communication of either expected behavior or improper practices F Housekeeping practices F Quality Systems and Management Assessment Audit Trail Disabled audit trails Unexplained gaps/changes in records F Shared log-on and password access F Discrepancies in QC performance between analysts F Calibration records missing reviewer signature/dates RED FLAGS: DATA ASSESSMENT F Data Integrity Program (Records/ Policy) F Lack of technical depth High staff turnover F Unlabeled containers or illegible labels F Gaps in training records F Sample volume discrepancies F QA Staff lacks direct access to senior management F Materials inventory does not match throughput F “Reappearing” quality control results F Computer Access & Security (LIMS, Data Systems) “Bottleneck” departments F Records Management & Archives Excessive overtime F Unclear roles and responsibilities F Sample throughput exceeds time required to process the samples F Screen-printed reports F F Managers translating or clarifying analysts’ responses to questions Routine dead time on autoinjection run logs F F Emphasis on production Samples or blanks diluted without apparent justification F Inadequate sample-handling procedures for evening or weekend deliveries F Too few quality control records to support data output F No mechanism for reporting problems F F Analysts not allowed to use their judgment Chronological chromatograms that do not display prevalent background F Analysts unable to describe data review and/or oversight F F QA staff performing competing functions F Lack of data surveillance Incomplete assessment files F Repeat assessment findings F Inadequate procedures for handling complaints F Corrective Action Corrective action fails to address root cause F Lack of timely follow-up or documentation of corrective action F Lack of record verification in archiving procedures F Inadequate procedures to ensure data retrieval Document Control Sample Storage Data Reduction and Review (Process) Glassware and Bottle Preparation Equipment and Facilities Inadequate internal assessments F Internal Audits Security Access and Computer Security Process Waste Disposal and Material Throughput


