e40c67b489bcb4893e189fb66ad17f73.ppt
- Количество слайдов: 36
 ASSESSMENT OF DIAGNOSTIC METHODS AND STANDARD DIAGNOSTIC PROCEDURES Prof. Dr. Ivaylo Chenchev, Ph. D, DVSc National Diagnostic and Research Veterinary Medical Institute, Bulgaria
ASSESSMENT OF DIAGNOSTIC METHODS AND STANDARD DIAGNOSTIC PROCEDURES Prof. Dr. Ivaylo Chenchev, Ph. D, DVSc National Diagnostic and Research Veterinary Medical Institute, Bulgaria
 DIAGNOSTIC METHODS AND STANDARD DIAGNOSTIC PROCEDURES OFFICIAL DOCUMENT FOR LABORATORY METHODS IS REGULATION (EC) No 882/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on official controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare rules
DIAGNOSTIC METHODS AND STANDARD DIAGNOSTIC PROCEDURES OFFICIAL DOCUMENT FOR LABORATORY METHODS IS REGULATION (EC) No 882/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on official controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare rules
 DIAGNOSTIC METHODS AND STANDARD DIAGNOSTIC PROCEDURES OFFICIAL DOCUMENT FOR MOVMENT OF EQUINES IN EU IS REGULATIONS • COMMISSION REGULATION (EU) No 595/2010 of 2 July 2010 amending Annexes VIII, X and XI to Regulation (EC) No 1774/2002 of the European Parliament and of the Council laying down health rules concerning animal by-products not intended for human consumption
DIAGNOSTIC METHODS AND STANDARD DIAGNOSTIC PROCEDURES OFFICIAL DOCUMENT FOR MOVMENT OF EQUINES IN EU IS REGULATIONS • COMMISSION REGULATION (EU) No 595/2010 of 2 July 2010 amending Annexes VIII, X and XI to Regulation (EC) No 1774/2002 of the European Parliament and of the Council laying down health rules concerning animal by-products not intended for human consumption
 DIAGNOSTIC METHODS AND STANDARD DIAGNOSTIC PROCEDURES • • African Horse Sickness (AHS) Equine Infectious anemia (EIA) Equine Viral Arteritis (EVA, EAV) Equine Herpes type 1 (EHV 1) Equine Herpes type 4 (EHV 4) Equine Influenza virus (EIV) West Nile Fever (WNF) Other Encephalomyelitis (EEE, JEE. VEE)
DIAGNOSTIC METHODS AND STANDARD DIAGNOSTIC PROCEDURES • • African Horse Sickness (AHS) Equine Infectious anemia (EIA) Equine Viral Arteritis (EVA, EAV) Equine Herpes type 1 (EHV 1) Equine Herpes type 4 (EHV 4) Equine Influenza virus (EIV) West Nile Fever (WNF) Other Encephalomyelitis (EEE, JEE. VEE)
 African Horse Sickness Mortality • Horses: Mortality 50 -95% • • Pulmonary form – up to 95% Cardiac form – 50% or higher Mixed form – 70 -80% Horsesickness fever - typically recover • Other Equidae – Mules: 50% – European or Asian donkeys: 5 -10% – None in African donkeys and zebras
African Horse Sickness Mortality • Horses: Mortality 50 -95% • • Pulmonary form – up to 95% Cardiac form – 50% or higher Mixed form – 70 -80% Horsesickness fever - typically recover • Other Equidae – Mules: 50% – European or Asian donkeys: 5 -10% – None in African donkeys and zebras
 African Horse Sickness • Refrigerate but do not freeze the following specimens and send them to the laboratory: • Blood, preferably with heparin as an anticoagulant (other anticoagulants can be used), or blood in an equal volume of OCG Pieces of spleen, mediastinal and mesenteric lymph nodes, lung, and liver At least 5 m. L of serum from acute and convalescent animals • In addition, send the following: • Blood smears (at least six) fixed in absolute methanol • Tissues in 10 -percent formalin, from spleen, liver, lung, kidney, heart, lymph nodes, and brain
African Horse Sickness • Refrigerate but do not freeze the following specimens and send them to the laboratory: • Blood, preferably with heparin as an anticoagulant (other anticoagulants can be used), or blood in an equal volume of OCG Pieces of spleen, mediastinal and mesenteric lymph nodes, lung, and liver At least 5 m. L of serum from acute and convalescent animals • In addition, send the following: • Blood smears (at least six) fixed in absolute methanol • Tissues in 10 -percent formalin, from spleen, liver, lung, kidney, heart, lymph nodes, and brain
 African Horse Sickness Laboratory Diagnosis: Virus Isolation • Confirmation of an initial case of AHS in an area normally free of the disease requires isolation and identification of the virus. • AHSV can be isolated from heparinized blood, spleen, lymph node, or lung collected at necropsy using cell culture (BHK 21 or Vero cells) • Intracerebral inoculation of mice that are 2 to 3 days old • Intravenous inoculation of embryonated eggs at day 10 to 12. • The incubation period in mice can be 4 to 20 days; then the mice die. • Viral isolates are identified by group-specific tests such as complement fixation • Enzyme-linked immunosorbent assay (ELISA) • Immunofluorescence • Determination of the serotype is done by plaque reduction or plaque inhibition using known antisera. • Real Time PCR
African Horse Sickness Laboratory Diagnosis: Virus Isolation • Confirmation of an initial case of AHS in an area normally free of the disease requires isolation and identification of the virus. • AHSV can be isolated from heparinized blood, spleen, lymph node, or lung collected at necropsy using cell culture (BHK 21 or Vero cells) • Intracerebral inoculation of mice that are 2 to 3 days old • Intravenous inoculation of embryonated eggs at day 10 to 12. • The incubation period in mice can be 4 to 20 days; then the mice die. • Viral isolates are identified by group-specific tests such as complement fixation • Enzyme-linked immunosorbent assay (ELISA) • Immunofluorescence • Determination of the serotype is done by plaque reduction or plaque inhibition using known antisera. • Real Time PCR
 African Horse Sickness Laboratory Diagnosis: Serology • The antibody to AHS can be detected starting about 10 days after infection. • Group-specific tests are complement fixation (CF antibody present 4 to 6 months) • Immunofluorescent assay (IFA) • ELISA • Immunodiffusion • Detectable antibodies are present for 1 to 4 years after infection.
African Horse Sickness Laboratory Diagnosis: Serology • The antibody to AHS can be detected starting about 10 days after infection. • Group-specific tests are complement fixation (CF antibody present 4 to 6 months) • Immunofluorescent assay (IFA) • ELISA • Immunodiffusion • Detectable antibodies are present for 1 to 4 years after infection.
 Equine Infectious anemia (EIA) Laboratory diagnosis • Antemortem specimens – Serum (Serology – AGID, ELISA Ab) – Whole blood (VI, PCR, viral detection not routine) • Postmortem specimens – Serum (Serology – AGID, ELISA Ab) – Whole blood (VI, PCR, viral detection not routine • Clinicopathology – “swamp fever” of equines; typically sub clinical; primary infection febrile respiratory; chronic infection and shedding eventually glomerulonephritis
Equine Infectious anemia (EIA) Laboratory diagnosis • Antemortem specimens – Serum (Serology – AGID, ELISA Ab) – Whole blood (VI, PCR, viral detection not routine) • Postmortem specimens – Serum (Serology – AGID, ELISA Ab) – Whole blood (VI, PCR, viral detection not routine • Clinicopathology – “swamp fever” of equines; typically sub clinical; primary infection febrile respiratory; chronic infection and shedding eventually glomerulonephritis
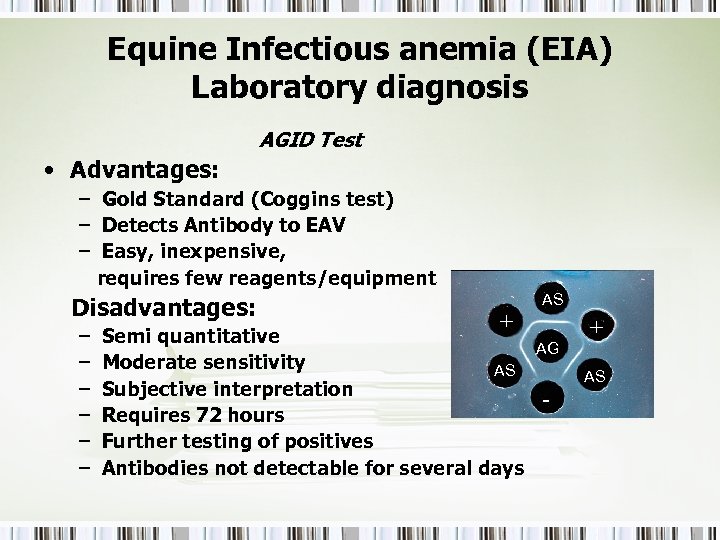 Equine Infectious anemia (EIA) Laboratory diagnosis AGID Test • Advantages: – Gold Standard (Coggins test) – Detects Antibody to EAV – Easy, inexpensive, requires few reagents/equipment Disadvantages: – – – + Semi quantitative Moderate sensitivity AS Subjective interpretation Requires 72 hours Further testing of positives Antibodies not detectable for several days AS AG - + AS
Equine Infectious anemia (EIA) Laboratory diagnosis AGID Test • Advantages: – Gold Standard (Coggins test) – Detects Antibody to EAV – Easy, inexpensive, requires few reagents/equipment Disadvantages: – – – + Semi quantitative Moderate sensitivity AS Subjective interpretation Requires 72 hours Further testing of positives Antibodies not detectable for several days AS AG - + AS
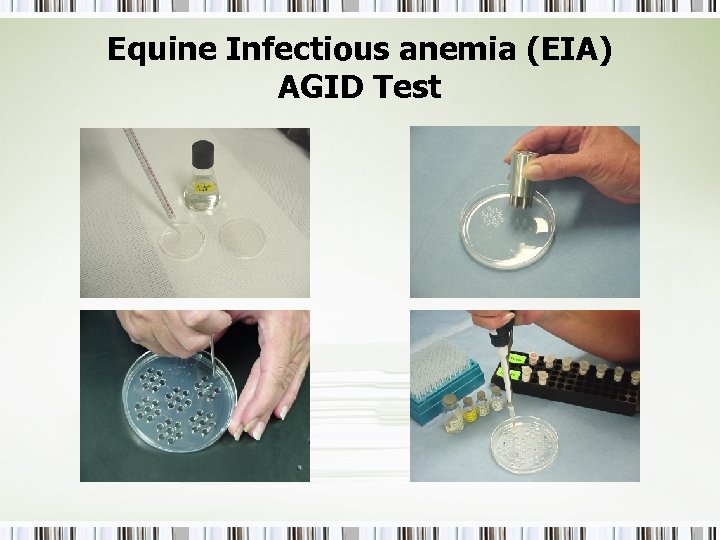 Equine Infectious anemia (EIA) AGID Test
Equine Infectious anemia (EIA) AGID Test
 Equine Viral Arteritis (EVA, EAV) Laboratory diagnosis • Virus isolation • ELISA for antibodies • Virus Neutralization Test • Real Time PCR
Equine Viral Arteritis (EVA, EAV) Laboratory diagnosis • Virus isolation • ELISA for antibodies • Virus Neutralization Test • Real Time PCR
 Equine Viral Arteritis (EVA, EAV) Laboratory diagnosis • Antemortem speciments – Paired serum (Serology – modified SN, IFA, ELISA) – Nasopharyngeal, conjuctival swab or wash, citrated blood, semen (PCR, VI, viral detection not routine) • Postmortem speciments – Part of lung, trachea, spleen, colon, cecum&associated lymph nodes, small and medium sized arteries (FA, PCR, VI, viral detection not routine) • Clinicopathology – subclinical febrile illness with leucopenia, depression, edema, panvasculitis, abortion storms on breeding farms • Virus culturable only for first 2 weeks post-infection
Equine Viral Arteritis (EVA, EAV) Laboratory diagnosis • Antemortem speciments – Paired serum (Serology – modified SN, IFA, ELISA) – Nasopharyngeal, conjuctival swab or wash, citrated blood, semen (PCR, VI, viral detection not routine) • Postmortem speciments – Part of lung, trachea, spleen, colon, cecum&associated lymph nodes, small and medium sized arteries (FA, PCR, VI, viral detection not routine) • Clinicopathology – subclinical febrile illness with leucopenia, depression, edema, panvasculitis, abortion storms on breeding farms • Virus culturable only for first 2 weeks post-infection
 Equine Viral Arteritis (EVA, EAV) ELISA • Advantages – Commercial kits available – Rapid (same day) – Can be semi-automated • Disadvantages – Requires expensive equipment – False positive reactions – Positives require confirmation
Equine Viral Arteritis (EVA, EAV) ELISA • Advantages – Commercial kits available – Rapid (same day) – Can be semi-automated • Disadvantages – Requires expensive equipment – False positive reactions – Positives require confirmation
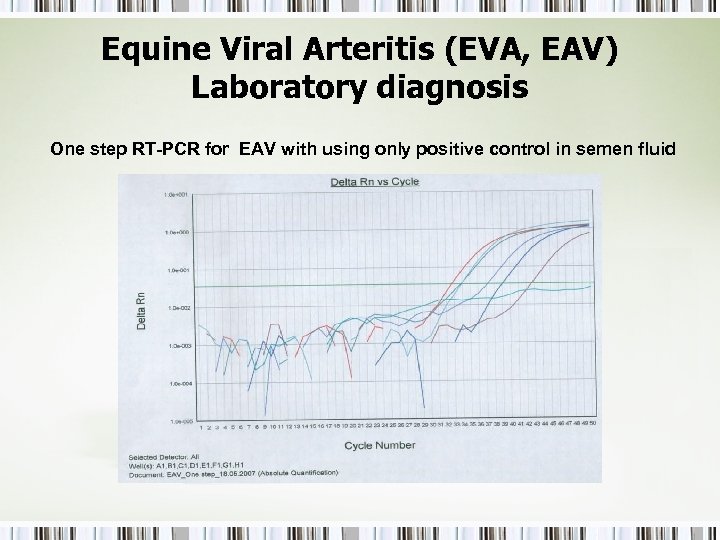 Equine Viral Arteritis (EVA, EAV) Laboratory diagnosis One step RT-PCR for EAV with using only positive control in semen fluid
Equine Viral Arteritis (EVA, EAV) Laboratory diagnosis One step RT-PCR for EAV with using only positive control in semen fluid
 Equine Herpes type 1 (EHV 1) and (EHV 4) DIAGNOSIS • Virus isolation • ELISA for antibodies and antigens • Virus Neutralization Test • Real Time PCR
Equine Herpes type 1 (EHV 1) and (EHV 4) DIAGNOSIS • Virus isolation • ELISA for antibodies and antigens • Virus Neutralization Test • Real Time PCR
 Equine Herpes type 1 (EHV 1) and (EHV 4) DIAGNOSIS • • • Methods available for the laboratory diagnosis of equine herpesvirus respiratory infections include: Virus isolation Polymerase chain reaction (PCR) Immunofluorescent detection of viral antigens Serologic testing (ELISA, CF, SN) The cytopathic effect of EHV-1 and EHV-4 is characteristic, and seroidentification of the two herpesviruses can be made with type-specific monoclonal antibodies. • Amplification of viral DNA using PCR is a rapid, sensitive and increasing utilized assay for detection of EHV-1 or EHV-4 respiratory tract infection. • When direct antigen detection methods are used for a rapid laboratory diagnosis of EHV-1 or EHV-4, it is important to confirm the direct test results by virus isolation.
Equine Herpes type 1 (EHV 1) and (EHV 4) DIAGNOSIS • • • Methods available for the laboratory diagnosis of equine herpesvirus respiratory infections include: Virus isolation Polymerase chain reaction (PCR) Immunofluorescent detection of viral antigens Serologic testing (ELISA, CF, SN) The cytopathic effect of EHV-1 and EHV-4 is characteristic, and seroidentification of the two herpesviruses can be made with type-specific monoclonal antibodies. • Amplification of viral DNA using PCR is a rapid, sensitive and increasing utilized assay for detection of EHV-1 or EHV-4 respiratory tract infection. • When direct antigen detection methods are used for a rapid laboratory diagnosis of EHV-1 or EHV-4, it is important to confirm the direct test results by virus isolation.
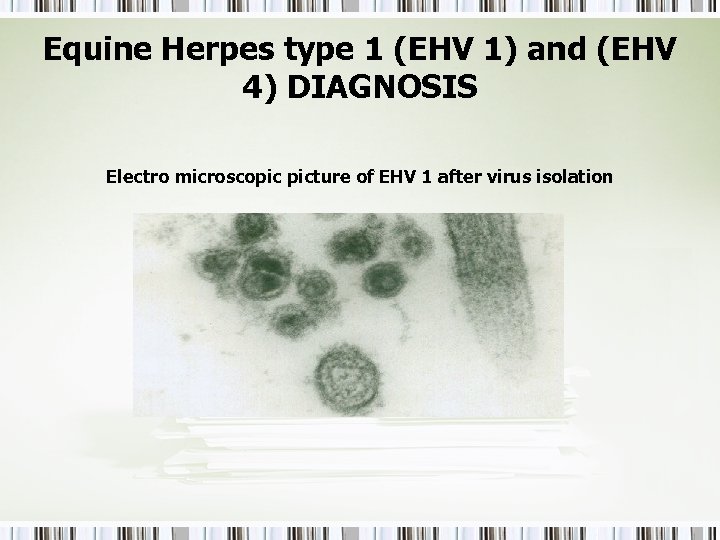 Equine Herpes type 1 (EHV 1) and (EHV 4) DIAGNOSIS Electro microscopic picture of EHV 1 after virus isolation
Equine Herpes type 1 (EHV 1) and (EHV 4) DIAGNOSIS Electro microscopic picture of EHV 1 after virus isolation
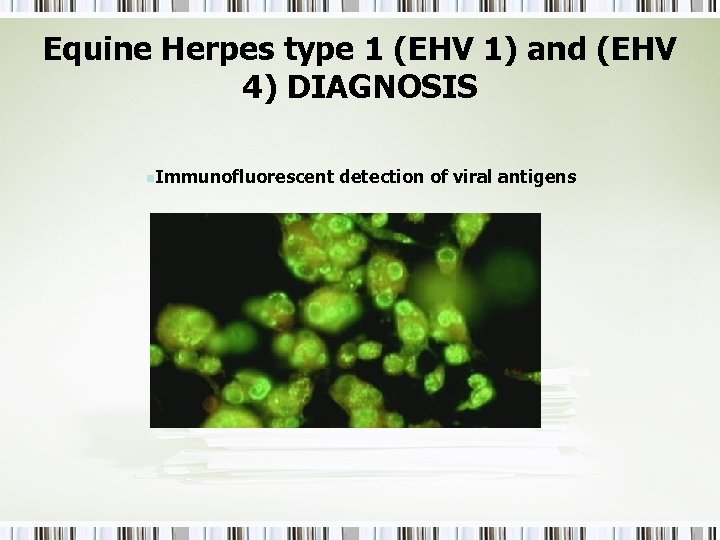 Equine Herpes type 1 (EHV 1) and (EHV 4) DIAGNOSIS n. Immunofluorescent detection of viral antigens
Equine Herpes type 1 (EHV 1) and (EHV 4) DIAGNOSIS n. Immunofluorescent detection of viral antigens
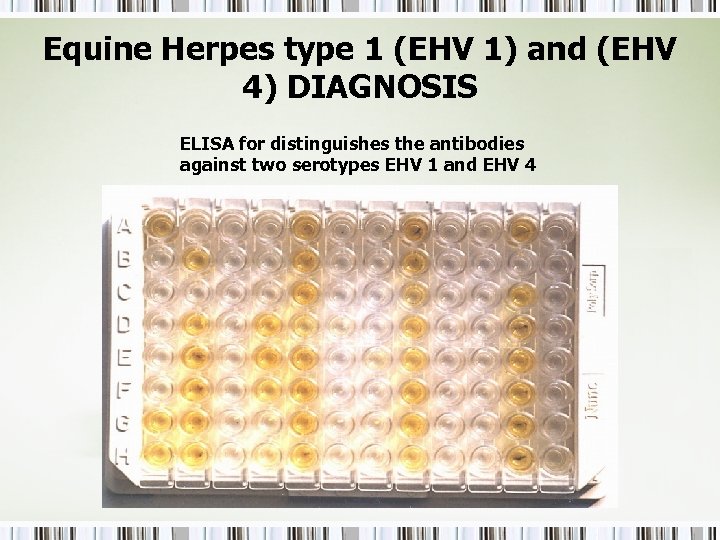 Equine Herpes type 1 (EHV 1) and (EHV 4) DIAGNOSIS ELISA for distinguishes the antibodies against two serotypes EHV 1 and EHV 4
Equine Herpes type 1 (EHV 1) and (EHV 4) DIAGNOSIS ELISA for distinguishes the antibodies against two serotypes EHV 1 and EHV 4
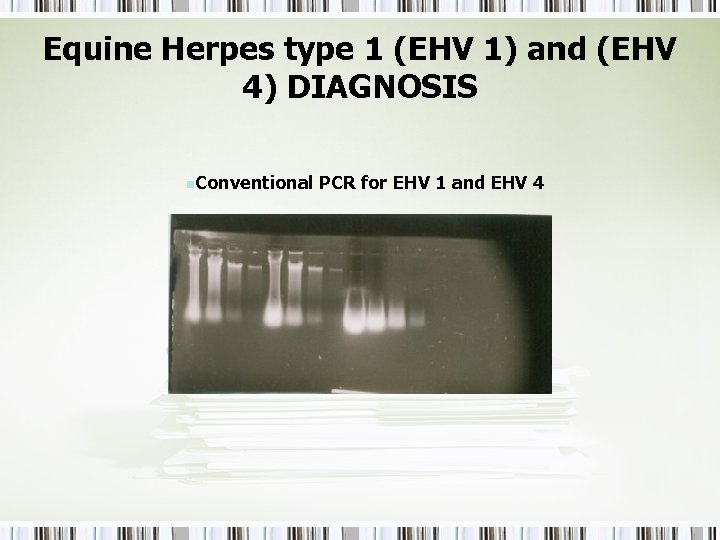 Equine Herpes type 1 (EHV 1) and (EHV 4) DIAGNOSIS n. Conventional PCR for EHV 1 and EHV 4
Equine Herpes type 1 (EHV 1) and (EHV 4) DIAGNOSIS n. Conventional PCR for EHV 1 and EHV 4
 Equine Influenza virus (EIV) Laboratory Diagnosis • Presumptive diagnosis – Serologic diagnosis – Clinical signs/lesions – Antigen capture tests • Definitive diagnosis – Isolation and characterization of the virus – Molecular detection with subtyping/pathotyping
Equine Influenza virus (EIV) Laboratory Diagnosis • Presumptive diagnosis – Serologic diagnosis – Clinical signs/lesions – Antigen capture tests • Definitive diagnosis – Isolation and characterization of the virus – Molecular detection with subtyping/pathotyping
 Equine Influenza virus (EIV) Serologic Tests: • Type-Specific Tests (type A): – Enzyme-linked immunosorbent assay (ELISA) Ig. G – Detects all subtypes (H 3, H 7) • Subtype-Specific Tests (H or N subtype): – Hemaggltination-inhibition test – Neuraminidase-inhibition test – Detects only homologous subtype
Equine Influenza virus (EIV) Serologic Tests: • Type-Specific Tests (type A): – Enzyme-linked immunosorbent assay (ELISA) Ig. G – Detects all subtypes (H 3, H 7) • Subtype-Specific Tests (H or N subtype): – Hemaggltination-inhibition test – Neuraminidase-inhibition test – Detects only homologous subtype
 Equine Influenza virus (EIV) Serologic Tests • Limited value because of routine use of vaccine • Hemagglutination-inhibition test (HI) • Enzyme-linked immunosorbent assay (ELISA)
Equine Influenza virus (EIV) Serologic Tests • Limited value because of routine use of vaccine • Hemagglutination-inhibition test (HI) • Enzyme-linked immunosorbent assay (ELISA)
 Subtype-Specific Tests for EIV HI/NI (antibodies) • Advantages – Gold standard – Quantitative (titer) – Rapid (same day) • Disadvantages – Requires many reagents (antigens/antiserums) – Non-specific (steric) inhibition – Requires pre-treatment of serum to remove normal serum agglutinins (false negatives)
Subtype-Specific Tests for EIV HI/NI (antibodies) • Advantages – Gold standard – Quantitative (titer) – Rapid (same day) • Disadvantages – Requires many reagents (antigens/antiserums) – Non-specific (steric) inhibition – Requires pre-treatment of serum to remove normal serum agglutinins (false negatives)
 WNF - Clinical Signs in Horses • Paralysis of lips, facial muscles, or tongue • Head tilt, difficulty swallowing • Altered mentation • Sound sensitive • Blindness • Troubling righting • Drowsiness • Flu-like, anorexia, depression • Muscle and skin twitching • Hyperesthesia • Propulsive walking • Weakness, ataxia, recumbency • Seizures
WNF - Clinical Signs in Horses • Paralysis of lips, facial muscles, or tongue • Head tilt, difficulty swallowing • Altered mentation • Sound sensitive • Blindness • Troubling righting • Drowsiness • Flu-like, anorexia, depression • Muscle and skin twitching • Hyperesthesia • Propulsive walking • Weakness, ataxia, recumbency • Seizures
 WNF – Laboratory Diagnosis in Horses • Serology: blood and CSF • ELISA Ig. M and Ig. G • Virus isolation • Isolation cell cultures (C 6/36 and VERO-6) • RT-PCR
WNF – Laboratory Diagnosis in Horses • Serology: blood and CSF • ELISA Ig. M and Ig. G • Virus isolation • Isolation cell cultures (C 6/36 and VERO-6) • RT-PCR
 ELISA - Principe • Enzyme Linked Immunosorbent Assay (ELISA) • Term was coined by Engvall and Pearlmann in 1971 • Different Types – Sandwich – Indirect – Competitive • • Similar To RIA, Except No Radiolabel Can Be Used To Detect Both Antibody and Antigen Very Sensitive, pg/m. L Relies on Monoclonal Abs
ELISA - Principe • Enzyme Linked Immunosorbent Assay (ELISA) • Term was coined by Engvall and Pearlmann in 1971 • Different Types – Sandwich – Indirect – Competitive • • Similar To RIA, Except No Radiolabel Can Be Used To Detect Both Antibody and Antigen Very Sensitive, pg/m. L Relies on Monoclonal Abs
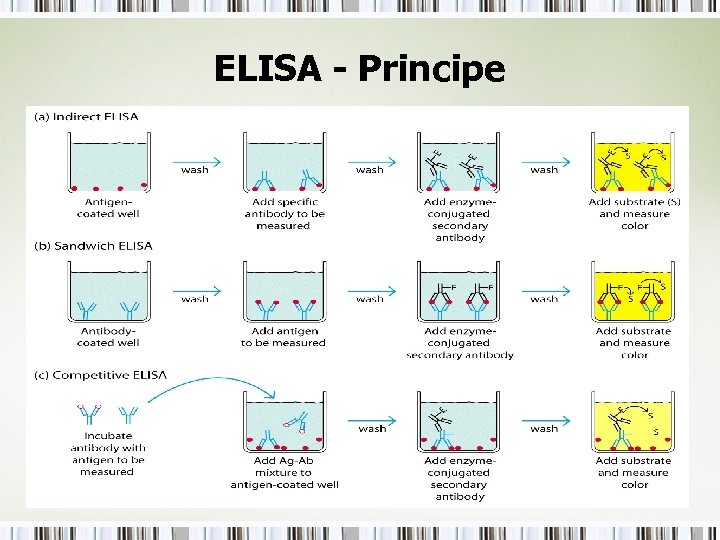 ELISA - Principe
ELISA - Principe
 Sandwich ELISA • • • 2 antibodies required Must recognize different epitopes 1 st antibody is referred to as capture Ab 2 nd antibody detection Ab 2 nd antibody is biotinylated Enzymes commonly used: HRP (Horse Radish Peroxidase) and AKP (Alkaline Phosphatase) • Substrate is TMB (Chromogen)
Sandwich ELISA • • • 2 antibodies required Must recognize different epitopes 1 st antibody is referred to as capture Ab 2 nd antibody detection Ab 2 nd antibody is biotinylated Enzymes commonly used: HRP (Horse Radish Peroxidase) and AKP (Alkaline Phosphatase) • Substrate is TMB (Chromogen)
 ELISA Plate • 96 well plate • Made of plastic on which protein can be adsorbed (bind) easily • Usually done overnight @ 4 C • Special buffer used that will not denature Ab and maximize binding • Blocking step ensures no empty spaces are left • Blocking reagent is often 10% FBS
ELISA Plate • 96 well plate • Made of plastic on which protein can be adsorbed (bind) easily • Usually done overnight @ 4 C • Special buffer used that will not denature Ab and maximize binding • Blocking step ensures no empty spaces are left • Blocking reagent is often 10% FBS
 PCR as diagnostic tool Advantages • Fast • Highly sensitive • Highly specific Disadvantages • Highly sensitive (contamination!!!) • Highly specific (mutants may escape detection due to mutations in primer or probe region
PCR as diagnostic tool Advantages • Fast • Highly sensitive • Highly specific Disadvantages • Highly sensitive (contamination!!!) • Highly specific (mutants may escape detection due to mutations in primer or probe region
 Steps in PCR • Preparation of sample (e. g. tissue homogenate) • Isolation of genetic material • Amplification of the target (PCR) • Detection of amplicons (gel / real-time)
Steps in PCR • Preparation of sample (e. g. tissue homogenate) • Isolation of genetic material • Amplification of the target (PCR) • Detection of amplicons (gel / real-time)
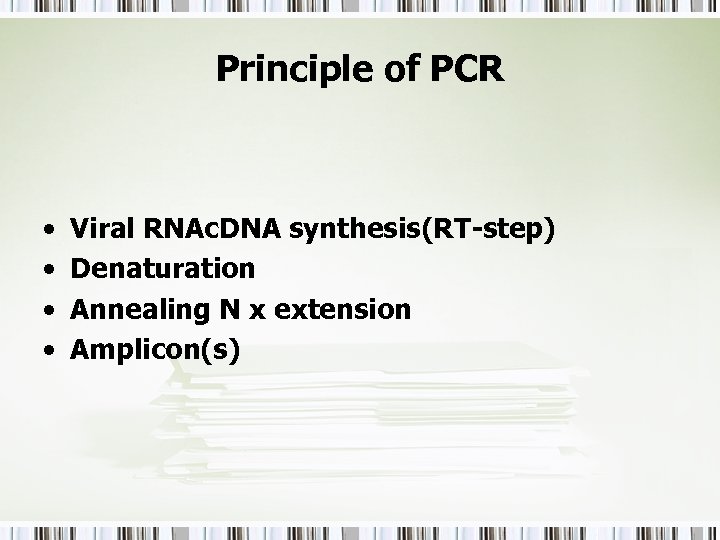 Principle of PCR • • Viral RNAc. DNA synthesis(RT-step) Denaturation Annealing N x extension Amplicon(s)
Principle of PCR • • Viral RNAc. DNA synthesis(RT-step) Denaturation Annealing N x extension Amplicon(s)
 Principle of PCR Real time detection Advantages • • • With probes additional spec ificity Low risk of contamination (closed system!) Quantification possible Fast Robotisation possible Disadvantages • SYBR Green less specific than probes (Specificity can been hanced through melting curves) • More sophisticated equipment needed
Principle of PCR Real time detection Advantages • • • With probes additional spec ificity Low risk of contamination (closed system!) Quantification possible Fast Robotisation possible Disadvantages • SYBR Green less specific than probes (Specificity can been hanced through melting curves) • More sophisticated equipment needed
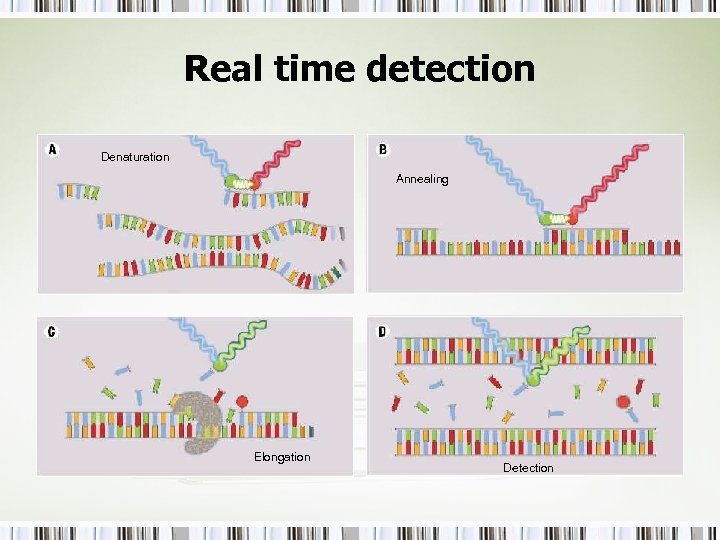 Real time detection Denaturation Annealing Elongation Detection
Real time detection Denaturation Annealing Elongation Detection


