39c4f53e21fd7bba248db84f4a55f157.ppt
- Количество слайдов: 56
 ASCO 2006 Update Novel Therapeutics in Prevention and Treatment for Breast Cancer Hope S. Rugo, MD Clinical Professor of Medicine Director, Breast Oncology Clinical Trials Program UCSF Comprehensive Cancer Center
ASCO 2006 Update Novel Therapeutics in Prevention and Treatment for Breast Cancer Hope S. Rugo, MD Clinical Professor of Medicine Director, Breast Oncology Clinical Trials Program UCSF Comprehensive Cancer Center
 Discussion Outline • Endocrine Therapy § Prevention • § 5: STAR NSABP P-2 Adjuvant therapy • • • Metronomic chemotherapy (SWOG 0012) Adjuvant trastuzumab § • Bone health substudy from ATAC Neoadjuvant § • Updates from IES, ARNO 95, GROCTA 4/ITA, MA 17 Update on the HERA trial New treatments for HER 2+ disease – Lapatanib § Phase III trial with capecitabine § 502: Inflammatory breast cancer § 503: Treatment of CNS disease § 583: Risk of cardiac toxicity
Discussion Outline • Endocrine Therapy § Prevention • § 5: STAR NSABP P-2 Adjuvant therapy • • • Metronomic chemotherapy (SWOG 0012) Adjuvant trastuzumab § • Bone health substudy from ATAC Neoadjuvant § • Updates from IES, ARNO 95, GROCTA 4/ITA, MA 17 Update on the HERA trial New treatments for HER 2+ disease – Lapatanib § Phase III trial with capecitabine § 502: Inflammatory breast cancer § 503: Treatment of CNS disease § 583: Risk of cardiac toxicity
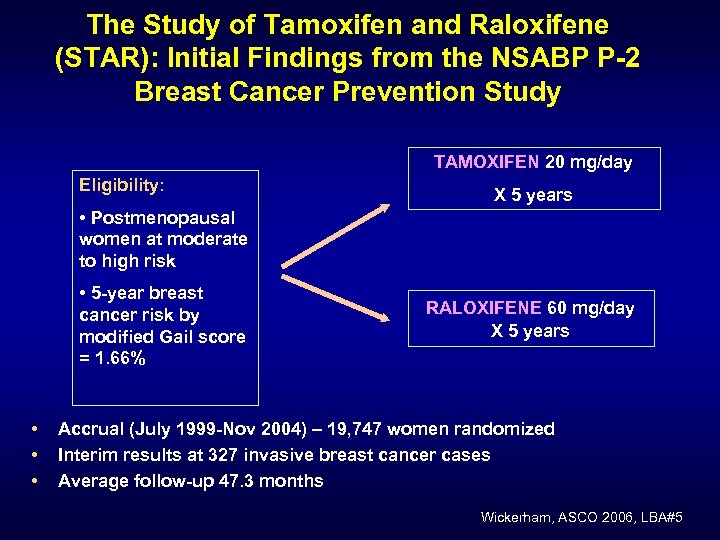 The Study of Tamoxifen and Raloxifene (STAR): Initial Findings from the NSABP P-2 Breast Cancer Prevention Study TAMOXIFEN 20 mg/day Eligibility: X 5 years • Postmenopausal women at moderate to high risk • 5 -year breast cancer risk by modified Gail score = 1. 66% • • • RALOXIFENE 60 mg/day X 5 years Accrual (July 1999 -Nov 2004) – 19, 747 women randomized Interim results at 327 invasive breast cancer cases Average follow-up 47. 3 months Wickerham, ASCO 2006, LBA#5
The Study of Tamoxifen and Raloxifene (STAR): Initial Findings from the NSABP P-2 Breast Cancer Prevention Study TAMOXIFEN 20 mg/day Eligibility: X 5 years • Postmenopausal women at moderate to high risk • 5 -year breast cancer risk by modified Gail score = 1. 66% • • • RALOXIFENE 60 mg/day X 5 years Accrual (July 1999 -Nov 2004) – 19, 747 women randomized Interim results at 327 invasive breast cancer cases Average follow-up 47. 3 months Wickerham, ASCO 2006, LBA#5
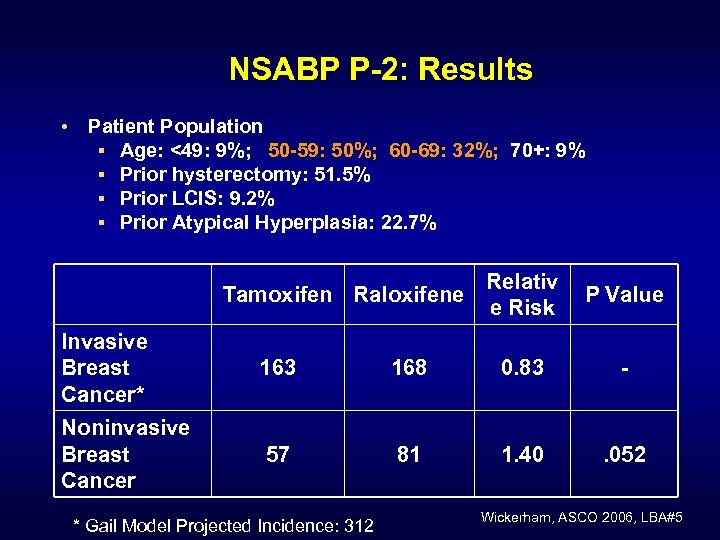 NSABP P-2: Results • Patient Population § Age: <49: 9%; 50 -59: 50%; 60 -69: 32%; 70+: 9% § Prior hysterectomy: 51. 5% § Prior LCIS: 9. 2% § Prior Atypical Hyperplasia: 22. 7% Tamoxifen Raloxifene Relativ e Risk P Value Invasive Breast Cancer* 163 168 0. 83 - Noninvasive Breast Cancer 57 81 1. 40 . 052 * Gail Model Projected Incidence: 312 Wickerham, ASCO 2006, LBA#5
NSABP P-2: Results • Patient Population § Age: <49: 9%; 50 -59: 50%; 60 -69: 32%; 70+: 9% § Prior hysterectomy: 51. 5% § Prior LCIS: 9. 2% § Prior Atypical Hyperplasia: 22. 7% Tamoxifen Raloxifene Relativ e Risk P Value Invasive Breast Cancer* 163 168 0. 83 - Noninvasive Breast Cancer 57 81 1. 40 . 052 * Gail Model Projected Incidence: 312 Wickerham, ASCO 2006, LBA#5
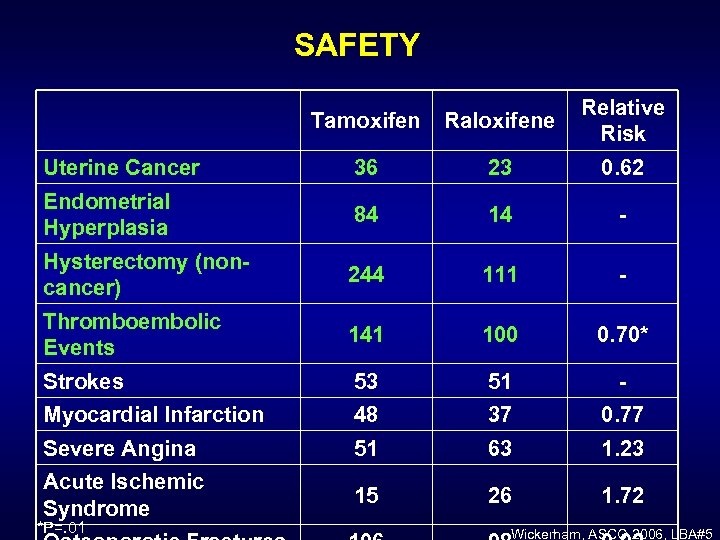 SAFETY Tamoxifen Raloxifene Relative Risk Uterine Cancer 36 23 0. 62 Endometrial Hyperplasia 84 14 - Hysterectomy (noncancer) 244 111 - Thromboembolic Events 141 100 0. 70* Strokes 53 51 - Myocardial Infarction 48 37 0. 77 Severe Angina 51 63 1. 23 Acute Ischemic Syndrome 15 26 1. 72 *P=. 01 Wickerham, ASCO 2006, LBA#5
SAFETY Tamoxifen Raloxifene Relative Risk Uterine Cancer 36 23 0. 62 Endometrial Hyperplasia 84 14 - Hysterectomy (noncancer) 244 111 - Thromboembolic Events 141 100 0. 70* Strokes 53 51 - Myocardial Infarction 48 37 0. 77 Severe Angina 51 63 1. 23 Acute Ischemic Syndrome 15 26 1. 72 *P=. 01 Wickerham, ASCO 2006, LBA#5
 STAR Trial: Summary • Raloxifene compared to tamoxifen for breast cancer prevention in postmenopausal women was § As effective in preventing invasive disease § Not as effective in preventing in situ disease • And resulted in: § Fewer thromboembolic events § Fewer cataracts § Fewer endometrial cancers • Patient reported outcomes § More musculoskeletal symptoms, weight gain, dyspareunia with raloxifene § More vasomotor symptoms, bladder problems, leg cramps, gynecologic symptoms with tamoxifen • The future of prevention trials? § Comparison of aromatase inhibitors to placebo • Map 3 – exemestane vs placebo (4560 pts) • IBIS-2 – anastrozole vs placebo (6000 pts)
STAR Trial: Summary • Raloxifene compared to tamoxifen for breast cancer prevention in postmenopausal women was § As effective in preventing invasive disease § Not as effective in preventing in situ disease • And resulted in: § Fewer thromboembolic events § Fewer cataracts § Fewer endometrial cancers • Patient reported outcomes § More musculoskeletal symptoms, weight gain, dyspareunia with raloxifene § More vasomotor symptoms, bladder problems, leg cramps, gynecologic symptoms with tamoxifen • The future of prevention trials? § Comparison of aromatase inhibitors to placebo • Map 3 – exemestane vs placebo (4560 pts) • IBIS-2 – anastrozole vs placebo (6000 pts)
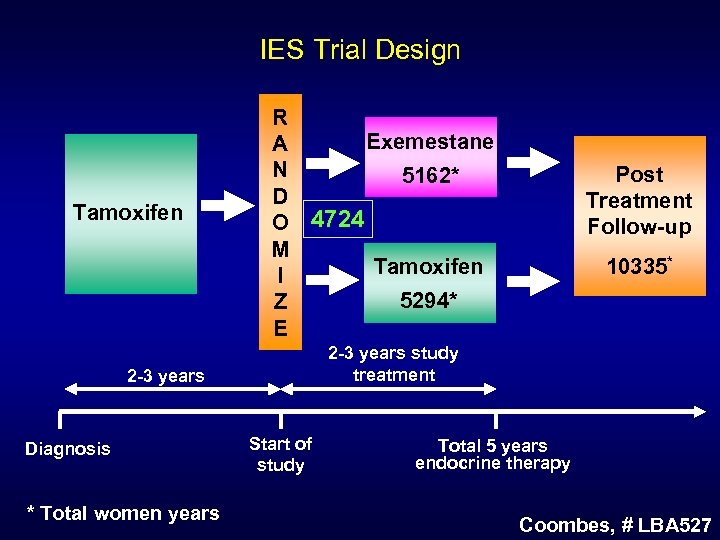 IES Trial Design Tamoxifen R Exemestane A N 5162* D O 4724 M Tamoxifen I 5294* Z E * Total women years 10335* 2 -3 years study treatment 2 -3 years Diagnosis Post Treatment Follow-up Start of study Total 5 years endocrine therapy Coombes, # LBA 527
IES Trial Design Tamoxifen R Exemestane A N 5162* D O 4724 M Tamoxifen I 5294* Z E * Total women years 10335* 2 -3 years study treatment 2 -3 years Diagnosis Post Treatment Follow-up Start of study Total 5 years endocrine therapy Coombes, # LBA 527
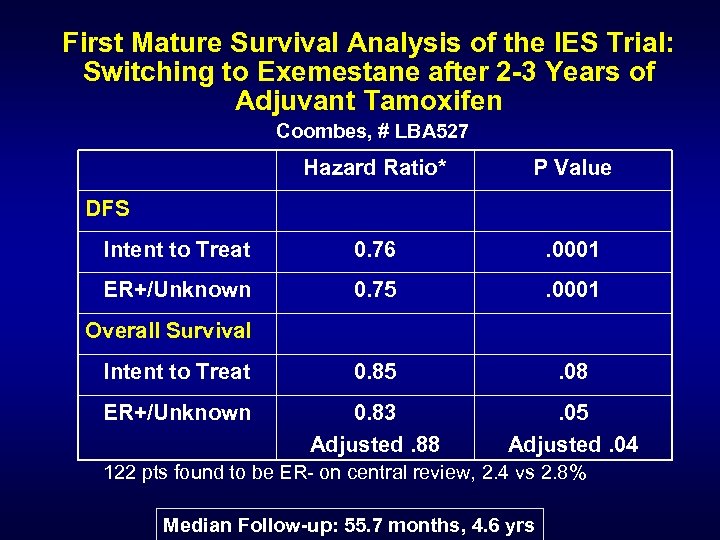 First Mature Survival Analysis of the IES Trial: Switching to Exemestane after 2 -3 Years of Adjuvant Tamoxifen Coombes, # LBA 527 Hazard Ratio* P Value Intent to Treat 0. 76 . 0001 ER+/Unknown 0. 75 . 0001 Intent to Treat 0. 85 . 08 ER+/Unknown 0. 83 Adjusted. 88 . 05 Adjusted. 04 DFS Overall Survival 122 pts found to be ER- on central review, 2. 4 vs 2. 8% Median Follow-up: 55. 7 months, 4. 6 yrs
First Mature Survival Analysis of the IES Trial: Switching to Exemestane after 2 -3 Years of Adjuvant Tamoxifen Coombes, # LBA 527 Hazard Ratio* P Value Intent to Treat 0. 76 . 0001 ER+/Unknown 0. 75 . 0001 Intent to Treat 0. 85 . 08 ER+/Unknown 0. 83 Adjusted. 88 . 05 Adjusted. 04 DFS Overall Survival 122 pts found to be ER- on central review, 2. 4 vs 2. 8% Median Follow-up: 55. 7 months, 4. 6 yrs
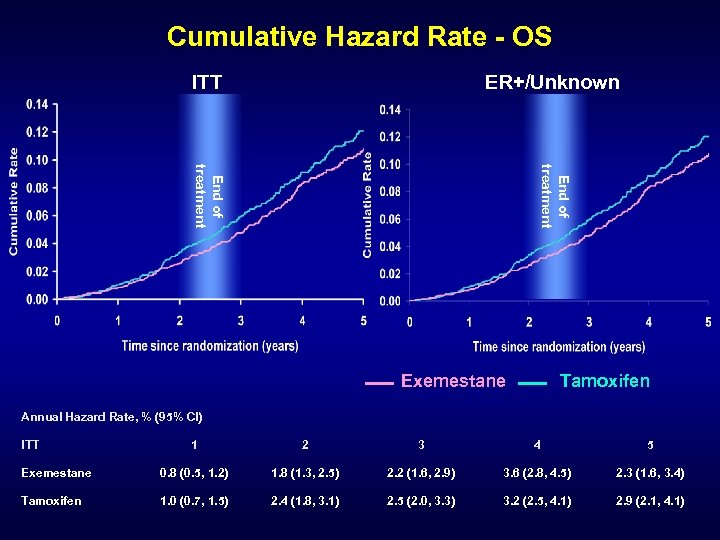 Cumulative Hazard Rate - OS ITT ER+/Unknown End of treatment Exemestane Tamoxifen Annual Hazard Rate, % (95% CI) ITT 1 2 3 4 5 Exemestane 0. 8 (0. 5, 1. 2) 1. 8 (1. 3, 2. 5) 2. 2 (1. 6, 2. 9) 3. 6 (2. 8, 4. 5) 2. 3 (1. 6, 3. 4) Tamoxifen 1. 0 (0. 7, 1. 5) 2. 4 (1. 8, 3. 1) 2. 5 (2. 0, 3. 3) 3. 2 (2. 5, 4. 1) 2. 9 (2. 1, 4. 1)
Cumulative Hazard Rate - OS ITT ER+/Unknown End of treatment Exemestane Tamoxifen Annual Hazard Rate, % (95% CI) ITT 1 2 3 4 5 Exemestane 0. 8 (0. 5, 1. 2) 1. 8 (1. 3, 2. 5) 2. 2 (1. 6, 2. 9) 3. 6 (2. 8, 4. 5) 2. 3 (1. 6, 3. 4) Tamoxifen 1. 0 (0. 7, 1. 5) 2. 4 (1. 8, 3. 1) 2. 5 (2. 0, 3. 3) 3. 2 (2. 5, 4. 1) 2. 9 (2. 1, 4. 1)
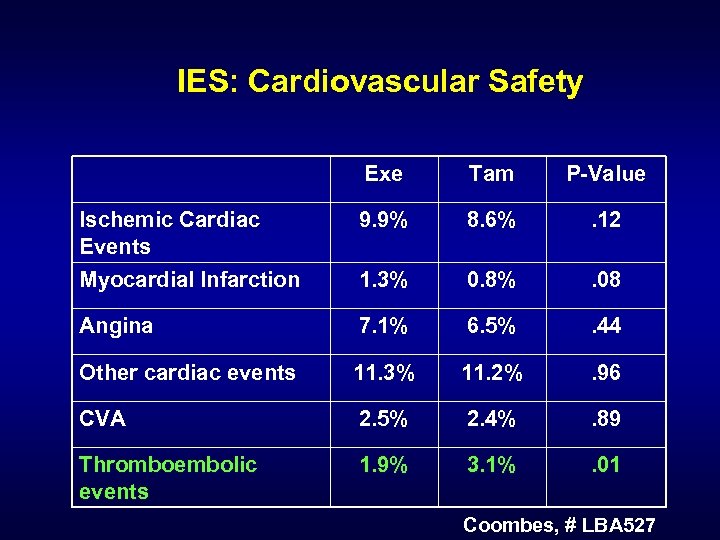 IES: Cardiovascular Safety Exe Tam P-Value Ischemic Cardiac Events 9. 9% 8. 6% . 12 Myocardial Infarction 1. 3% 0. 8% . 08 Angina 7. 1% 6. 5% . 44 Other cardiac events 11. 3% 11. 2% . 96 CVA 2. 5% 2. 4% . 89 Thromboembolic events 1. 9% 3. 1% . 01 Coombes, # LBA 527
IES: Cardiovascular Safety Exe Tam P-Value Ischemic Cardiac Events 9. 9% 8. 6% . 12 Myocardial Infarction 1. 3% 0. 8% . 08 Angina 7. 1% 6. 5% . 44 Other cardiac events 11. 3% 11. 2% . 96 CVA 2. 5% 2. 4% . 89 Thromboembolic events 1. 9% 3. 1% . 01 Coombes, # LBA 527
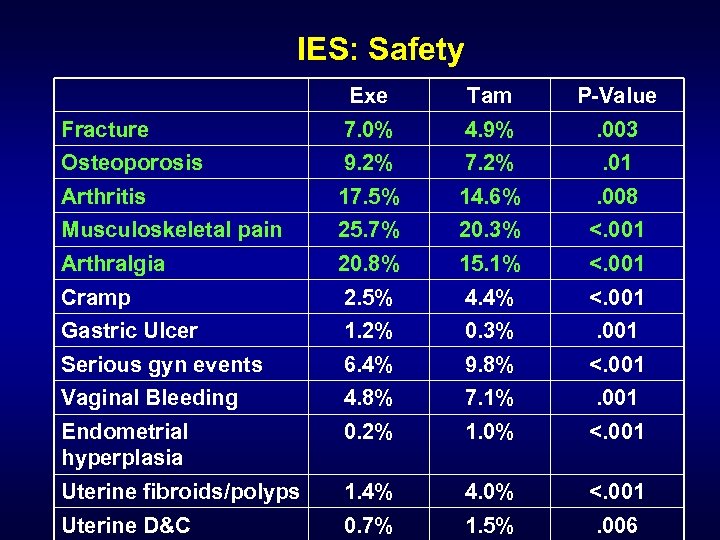 IES: Safety Exe Tam P-Value Fracture Osteoporosis 7. 0% 9. 2% 4. 9% 7. 2% . 003. 01 Arthritis Musculoskeletal pain 17. 5% 25. 7% 14. 6% 20. 3% . 008 <. 001 Arthralgia Cramp 20. 8% 2. 5% 15. 1% 4. 4% <. 001 Gastric Ulcer 1. 2% 0. 3% . 001 Serious gyn events 6. 4% 9. 8% <. 001 Vaginal Bleeding 4. 8% 7. 1% . 001 Endometrial hyperplasia 0. 2% 1. 0% <. 001 Uterine fibroids/polyps 1. 4% 4. 0% <. 001 Uterine D&C 0. 7% 1. 5% . 006
IES: Safety Exe Tam P-Value Fracture Osteoporosis 7. 0% 9. 2% 4. 9% 7. 2% . 003. 01 Arthritis Musculoskeletal pain 17. 5% 25. 7% 14. 6% 20. 3% . 008 <. 001 Arthralgia Cramp 20. 8% 2. 5% 15. 1% 4. 4% <. 001 Gastric Ulcer 1. 2% 0. 3% . 001 Serious gyn events 6. 4% 9. 8% <. 001 Vaginal Bleeding 4. 8% 7. 1% . 001 Endometrial hyperplasia 0. 2% 1. 0% <. 001 Uterine fibroids/polyps 1. 4% 4. 0% <. 001 Uterine D&C 0. 7% 1. 5% . 006
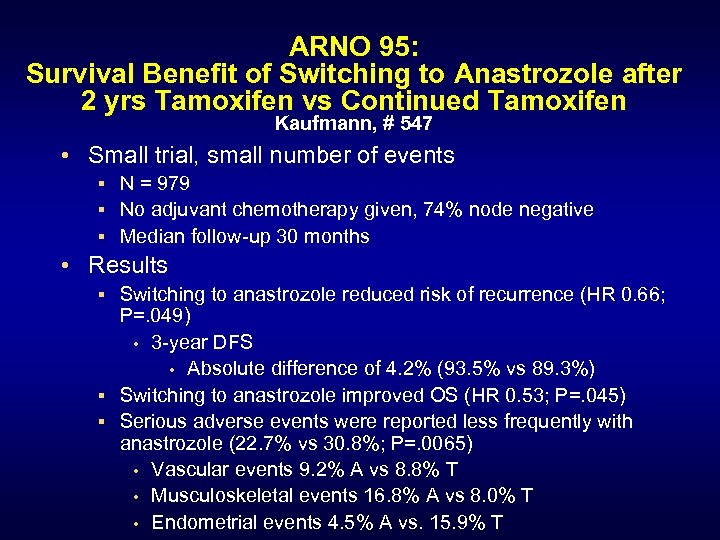 ARNO 95: Survival Benefit of Switching to Anastrozole after 2 yrs Tamoxifen vs Continued Tamoxifen Kaufmann, # 547 • Small trial, small number of events § N = 979 § No adjuvant chemotherapy given, 74% node negative § Median follow-up 30 months • Results § Switching to anastrozole reduced risk of recurrence (HR 0. 66; P=. 049) • 3 -year DFS • Absolute difference of 4. 2% (93. 5% vs 89. 3%) § Switching to anastrozole improved OS (HR 0. 53; P=. 045) § Serious adverse events were reported less frequently with anastrozole (22. 7% vs 30. 8%; P=. 0065) • Vascular events 9. 2% A vs 8. 8% T • Musculoskeletal events 16. 8% A vs 8. 0% T • Endometrial events 4. 5% A vs. 15. 9% T
ARNO 95: Survival Benefit of Switching to Anastrozole after 2 yrs Tamoxifen vs Continued Tamoxifen Kaufmann, # 547 • Small trial, small number of events § N = 979 § No adjuvant chemotherapy given, 74% node negative § Median follow-up 30 months • Results § Switching to anastrozole reduced risk of recurrence (HR 0. 66; P=. 049) • 3 -year DFS • Absolute difference of 4. 2% (93. 5% vs 89. 3%) § Switching to anastrozole improved OS (HR 0. 53; P=. 045) § Serious adverse events were reported less frequently with anastrozole (22. 7% vs 30. 8%; P=. 0065) • Vascular events 9. 2% A vs 8. 8% T • Musculoskeletal events 16. 8% A vs 8. 0% T • Endometrial events 4. 5% A vs. 15. 9% T
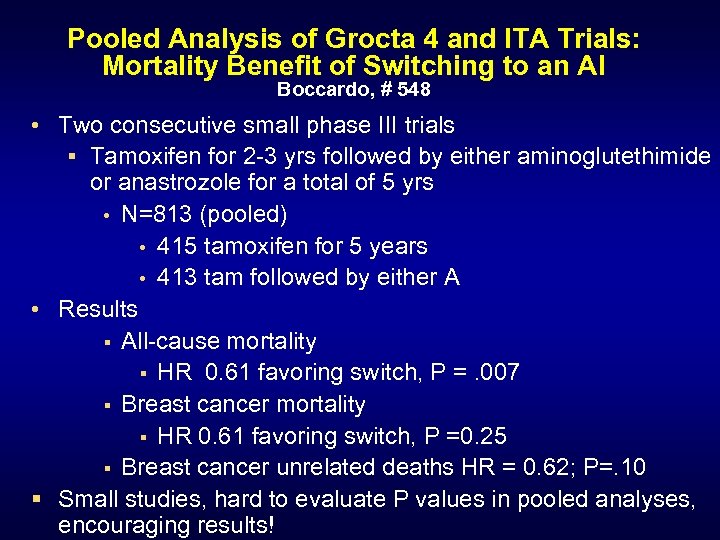 Pooled Analysis of Grocta 4 and ITA Trials: Mortality Benefit of Switching to an AI Boccardo, # 548 • Two consecutive small phase III trials § Tamoxifen for 2 -3 yrs followed by either aminoglutethimide or anastrozole for a total of 5 yrs • N=813 (pooled) • 415 tamoxifen for 5 years • 413 tam followed by either A • Results § All-cause mortality § HR 0. 61 favoring switch, P =. 007 § Breast cancer mortality § HR 0. 61 favoring switch, P =0. 25 § Breast cancer unrelated deaths HR = 0. 62; P=. 10 § Small studies, hard to evaluate P values in pooled analyses, encouraging results!
Pooled Analysis of Grocta 4 and ITA Trials: Mortality Benefit of Switching to an AI Boccardo, # 548 • Two consecutive small phase III trials § Tamoxifen for 2 -3 yrs followed by either aminoglutethimide or anastrozole for a total of 5 yrs • N=813 (pooled) • 415 tamoxifen for 5 years • 413 tam followed by either A • Results § All-cause mortality § HR 0. 61 favoring switch, P =. 007 § Breast cancer mortality § HR 0. 61 favoring switch, P =0. 25 § Breast cancer unrelated deaths HR = 0. 62; P=. 10 § Small studies, hard to evaluate P values in pooled analyses, encouraging results!
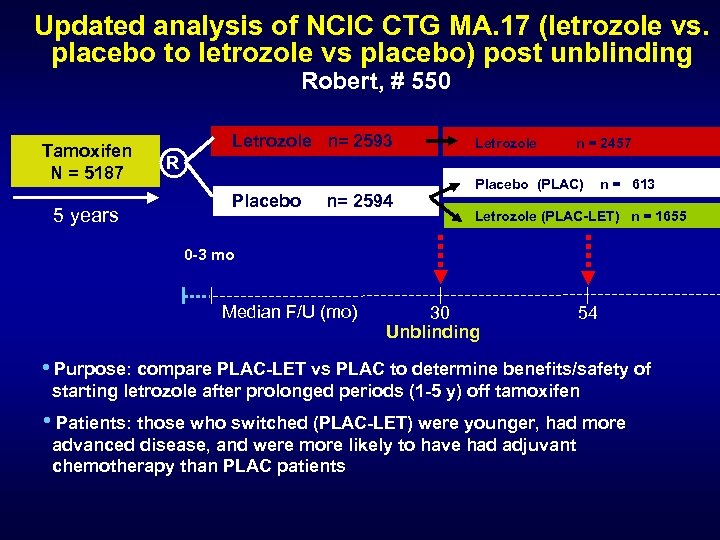 Updated analysis of NCIC CTG MA. 17 (letrozole vs. placebo to letrozole vs placebo) post unblinding Robert, # 550 Tamoxifen N = 5187 5 years Letrozole n= 2593 Letrozole n = 2457 R Placebo n= 2594 Placebo (PLAC) n = 613 Letrozole (PLAC-LET) n = 1655 0 -3 mo Median F/U (mo) 30 Unblinding 54 • Purpose: compare PLAC-LET vs PLAC to determine benefits/safety of starting letrozole after prolonged periods (1 -5 y) off tamoxifen • Patients: those who switched (PLAC-LET) were younger, had more advanced disease, and were more likely to have had adjuvant chemotherapy than PLAC patients
Updated analysis of NCIC CTG MA. 17 (letrozole vs. placebo to letrozole vs placebo) post unblinding Robert, # 550 Tamoxifen N = 5187 5 years Letrozole n= 2593 Letrozole n = 2457 R Placebo n= 2594 Placebo (PLAC) n = 613 Letrozole (PLAC-LET) n = 1655 0 -3 mo Median F/U (mo) 30 Unblinding 54 • Purpose: compare PLAC-LET vs PLAC to determine benefits/safety of starting letrozole after prolonged periods (1 -5 y) off tamoxifen • Patients: those who switched (PLAC-LET) were younger, had more advanced disease, and were more likely to have had adjuvant chemotherapy than PLAC patients
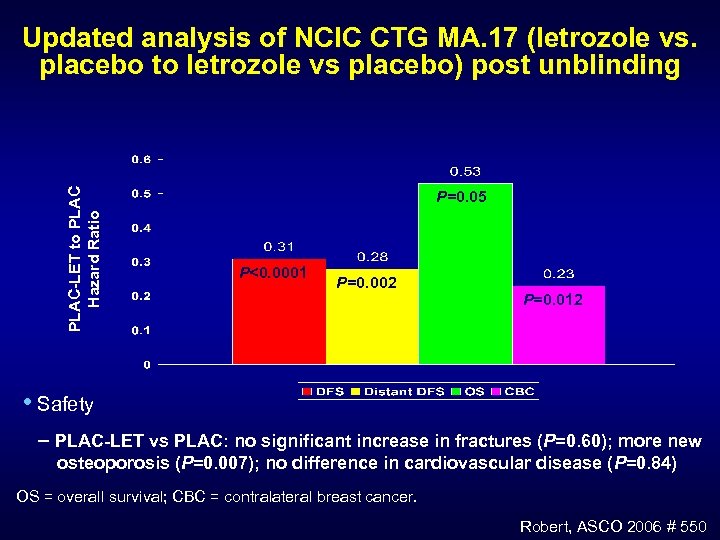 PLAC-LET to PLAC Hazard Ratio Updated analysis of NCIC CTG MA. 17 (letrozole vs. placebo to letrozole vs placebo) post unblinding P=0. 05 P<0. 0001 P=0. 002 P=0. 012 • Safety PLAC-LET vs PLAC: no significant increase in fractures (P=0. 60); more new osteoporosis (P=0. 007); no difference in cardiovascular disease (P=0. 84) OS = overall survival; CBC = contralateral breast cancer. Robert, ASCO 2006 # 550
PLAC-LET to PLAC Hazard Ratio Updated analysis of NCIC CTG MA. 17 (letrozole vs. placebo to letrozole vs placebo) post unblinding P=0. 05 P<0. 0001 P=0. 002 P=0. 012 • Safety PLAC-LET vs PLAC: no significant increase in fractures (P=0. 60); more new osteoporosis (P=0. 007); no difference in cardiovascular disease (P=0. 84) OS = overall survival; CBC = contralateral breast cancer. Robert, ASCO 2006 # 550
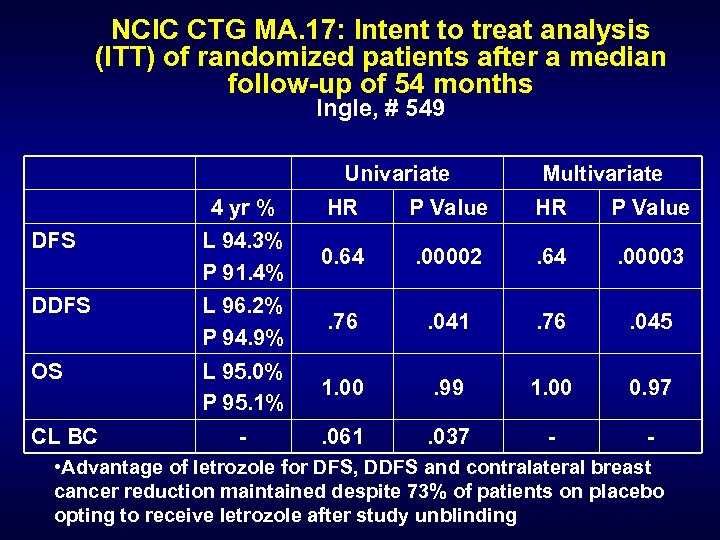 NCIC CTG MA. 17: Intent to treat analysis (ITT) of randomized patients after a median follow-up of 54 months Ingle, # 549 Univariate Multivariate 4 yr % HR P Value DFS L 94. 3% P 91. 4% 0. 64 . 00002 . 64 . 00003 DDFS L 96. 2% P 94. 9% . 76 . 041 . 76 . 045 OS L 95. 0% P 95. 1% 1. 00 . 99 1. 00 0. 97 - . 061 . 037 - - CL BC • Advantage of letrozole for DFS, DDFS and contralateral breast cancer reduction maintained despite 73% of patients on placebo opting to receive letrozole after study unblinding
NCIC CTG MA. 17: Intent to treat analysis (ITT) of randomized patients after a median follow-up of 54 months Ingle, # 549 Univariate Multivariate 4 yr % HR P Value DFS L 94. 3% P 91. 4% 0. 64 . 00002 . 64 . 00003 DDFS L 96. 2% P 94. 9% . 76 . 041 . 76 . 045 OS L 95. 0% P 95. 1% 1. 00 . 99 1. 00 0. 97 - . 061 . 037 - - CL BC • Advantage of letrozole for DFS, DDFS and contralateral breast cancer reduction maintained despite 73% of patients on placebo opting to receive letrozole after study unblinding
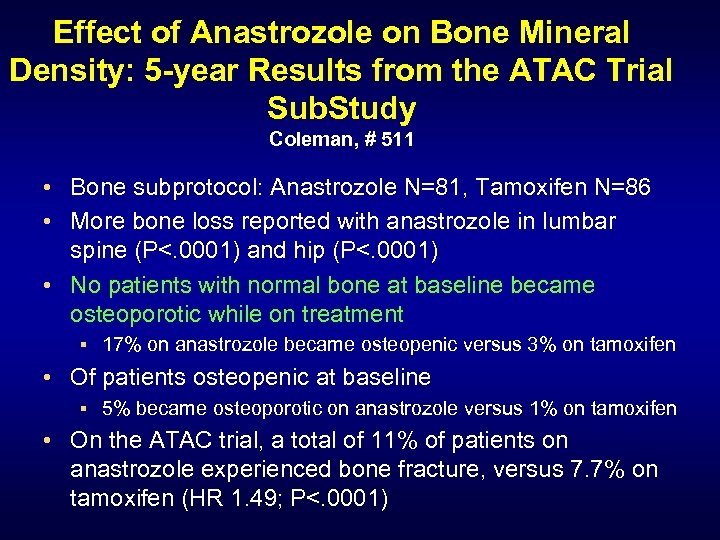 Effect of Anastrozole on Bone Mineral Density: 5 -year Results from the ATAC Trial Sub. Study Coleman, # 511 • Bone subprotocol: Anastrozole N=81, Tamoxifen N=86 • More bone loss reported with anastrozole in lumbar spine (P<. 0001) and hip (P<. 0001) • No patients with normal bone at baseline became osteoporotic while on treatment § 17% on anastrozole became osteopenic versus 3% on tamoxifen • Of patients osteopenic at baseline § 5% became osteoporotic on anastrozole versus 1% on tamoxifen • On the ATAC trial, a total of 11% of patients on anastrozole experienced bone fracture, versus 7. 7% on tamoxifen (HR 1. 49; P<. 0001)
Effect of Anastrozole on Bone Mineral Density: 5 -year Results from the ATAC Trial Sub. Study Coleman, # 511 • Bone subprotocol: Anastrozole N=81, Tamoxifen N=86 • More bone loss reported with anastrozole in lumbar spine (P<. 0001) and hip (P<. 0001) • No patients with normal bone at baseline became osteoporotic while on treatment § 17% on anastrozole became osteopenic versus 3% on tamoxifen • Of patients osteopenic at baseline § 5% became osteoporotic on anastrozole versus 1% on tamoxifen • On the ATAC trial, a total of 11% of patients on anastrozole experienced bone fracture, versus 7. 7% on tamoxifen (HR 1. 49; P<. 0001)
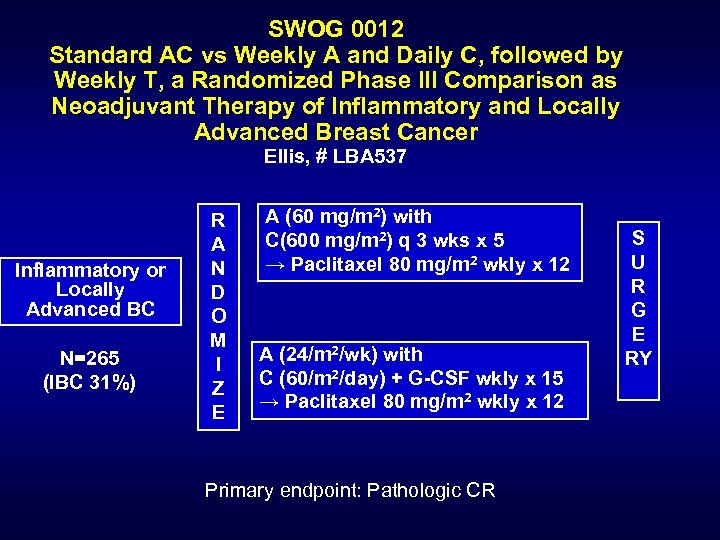 SWOG 0012 Standard AC vs Weekly A and Daily C, followed by Weekly T, a Randomized Phase III Comparison as Neoadjuvant Therapy of Inflammatory and Locally Advanced Breast Cancer Ellis, # LBA 537 Inflammatory or Locally Advanced BC N=265 (IBC 31%) R A N D O M I Z E A (60 mg/m 2) with C(600 mg/m 2) q 3 wks x 5 → Paclitaxel 80 mg/m 2 wkly x 12 A (24/m 2/wk) with C (60/m 2/day) + G-CSF wkly x 15 → Paclitaxel 80 mg/m 2 wkly x 12 Primary endpoint: Pathologic CR S U R G E RY
SWOG 0012 Standard AC vs Weekly A and Daily C, followed by Weekly T, a Randomized Phase III Comparison as Neoadjuvant Therapy of Inflammatory and Locally Advanced Breast Cancer Ellis, # LBA 537 Inflammatory or Locally Advanced BC N=265 (IBC 31%) R A N D O M I Z E A (60 mg/m 2) with C(600 mg/m 2) q 3 wks x 5 → Paclitaxel 80 mg/m 2 wkly x 12 A (24/m 2/wk) with C (60/m 2/day) + G-CSF wkly x 15 → Paclitaxel 80 mg/m 2 wkly x 12 Primary endpoint: Pathologic CR S U R G E RY
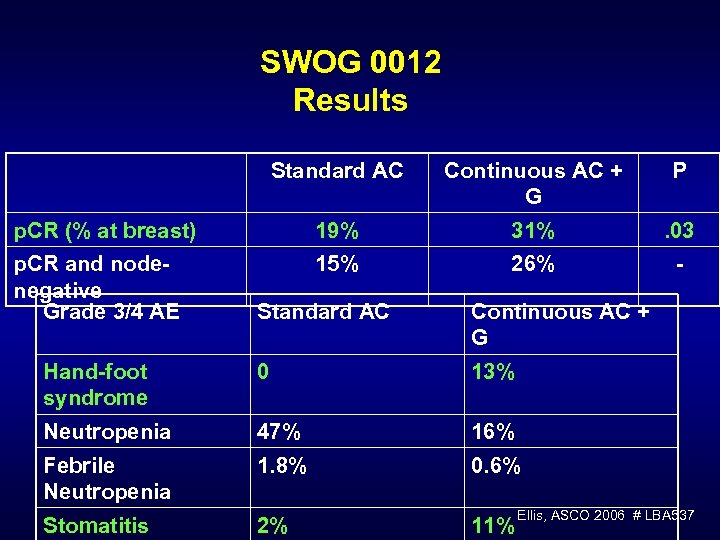 SWOG 0012 Results Standard AC p. CR (% at breast) p. CR and nodenegative Grade 3/4 AE Continuous AC + G P 19% 15% 31% 26% . 03 - Standard AC Continuous AC + G Hand-foot syndrome Neutropenia 0 13% 47% 16% Febrile Neutropenia 1. 8% 0. 6% Stomatitis 2% 11% Ellis, ASCO 2006 # LBA 537
SWOG 0012 Results Standard AC p. CR (% at breast) p. CR and nodenegative Grade 3/4 AE Continuous AC + G P 19% 15% 31% 26% . 03 - Standard AC Continuous AC + G Hand-foot syndrome Neutropenia 0 13% 47% 16% Febrile Neutropenia 1. 8% 0. 6% Stomatitis 2% 11% Ellis, ASCO 2006 # LBA 537

 Conclusions • We need to carefully select patients for adjuvant clinical trials testing different chemotherapy regimens § Differences in regimens will be magnified in specific patient groups • Topoisomerase II gene amplifications and deletions associated with response to anthracyclines • Genes associated with cellular proliferation correlate with response to chemotherapy • Aromatase inhibitors should be part of adjuvant hormonal therapy for postmenopausal women and are beneficial even late after diagnosis § Careful attention should be paid not only to side effects, but risk factors that predispose women to adverse events
Conclusions • We need to carefully select patients for adjuvant clinical trials testing different chemotherapy regimens § Differences in regimens will be magnified in specific patient groups • Topoisomerase II gene amplifications and deletions associated with response to anthracyclines • Genes associated with cellular proliferation correlate with response to chemotherapy • Aromatase inhibitors should be part of adjuvant hormonal therapy for postmenopausal women and are beneficial even late after diagnosis § Careful attention should be paid not only to side effects, but risk factors that predispose women to adverse events
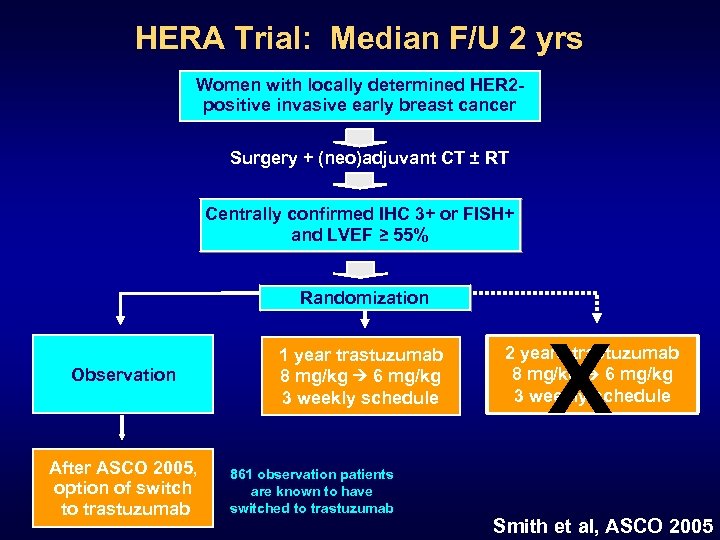 HERA Trial: Median F/U 2 yrs Women with locally determined HER 2 positive invasive early breast cancer Surgery + (neo)adjuvant CT ± RT Centrally confirmed IHC 3+ or FISH+ and LVEF ≥ 55% Randomization Observation After ASCO 2005, option of switch to trastuzumab 1 year trastuzumab 8 mg/kg 6 mg/kg 3 weekly schedule 861 observation patients are known to have switched to trastuzumab X 2 years trastuzumab 8 mg/kg 6 mg/kg 3 weekly schedule Smith et al, ASCO 2005
HERA Trial: Median F/U 2 yrs Women with locally determined HER 2 positive invasive early breast cancer Surgery + (neo)adjuvant CT ± RT Centrally confirmed IHC 3+ or FISH+ and LVEF ≥ 55% Randomization Observation After ASCO 2005, option of switch to trastuzumab 1 year trastuzumab 8 mg/kg 6 mg/kg 3 weekly schedule 861 observation patients are known to have switched to trastuzumab X 2 years trastuzumab 8 mg/kg 6 mg/kg 3 weekly schedule Smith et al, ASCO 2005
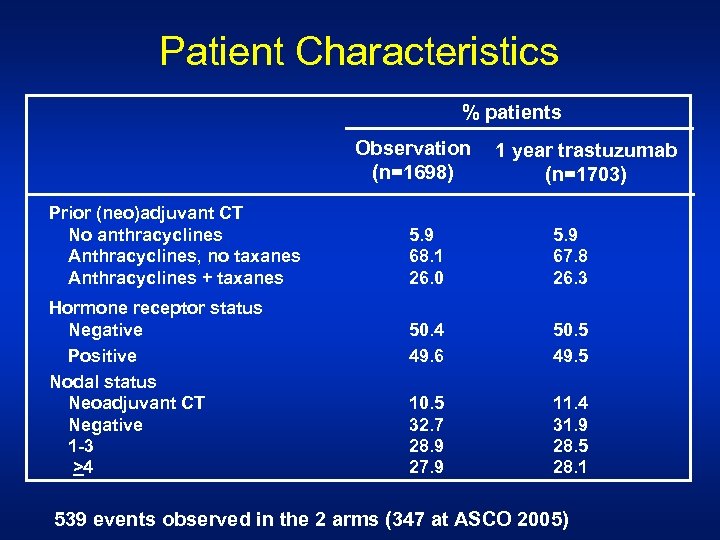 Patient Characteristics % patients Observation (n=1698) Prior (neo)adjuvant CT No anthracyclines Anthracyclines, no taxanes Anthracyclines + taxanes Hormone receptor status Negative Positive Nodal status Neoadjuvant CT Negative 1 -3 >4 1 year trastuzumab (n=1703) 5. 9 68. 1 26. 0 5. 9 67. 8 26. 3 50. 4 49. 6 50. 5 49. 5 10. 5 32. 7 28. 9 27. 9 11. 4 31. 9 28. 5 28. 1 539 events observed in the 2 arms (347 at ASCO 2005)
Patient Characteristics % patients Observation (n=1698) Prior (neo)adjuvant CT No anthracyclines Anthracyclines, no taxanes Anthracyclines + taxanes Hormone receptor status Negative Positive Nodal status Neoadjuvant CT Negative 1 -3 >4 1 year trastuzumab (n=1703) 5. 9 68. 1 26. 0 5. 9 67. 8 26. 3 50. 4 49. 6 50. 5 49. 5 10. 5 32. 7 28. 9 27. 9 11. 4 31. 9 28. 5 28. 1 539 events observed in the 2 arms (347 at ASCO 2005)
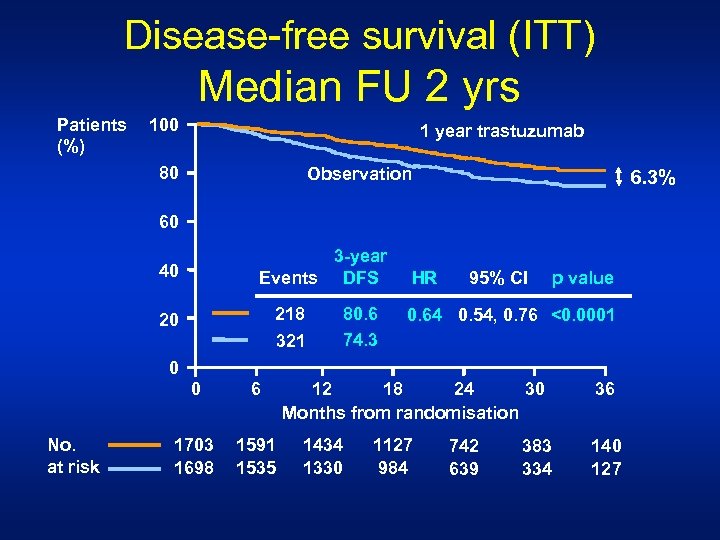 Disease-free survival (ITT) Median FU 2 yrs Patients (%) 100 1 year trastuzumab 80 Observation 6. 3% 60 3 -year Events DFS 40 218 321 20 80. 6 74. 3 HR 95% CI p value 0. 64 0. 54, 0. 76 <0. 0001 0 0 No. at risk 6 1703 1698 1591 1535 12 18 24 30 Months from randomisation 1434 1330 1127 984 742 639 383 334 36 140 127
Disease-free survival (ITT) Median FU 2 yrs Patients (%) 100 1 year trastuzumab 80 Observation 6. 3% 60 3 -year Events DFS 40 218 321 20 80. 6 74. 3 HR 95% CI p value 0. 64 0. 54, 0. 76 <0. 0001 0 0 No. at risk 6 1703 1698 1591 1535 12 18 24 30 Months from randomisation 1434 1330 1127 984 742 639 383 334 36 140 127
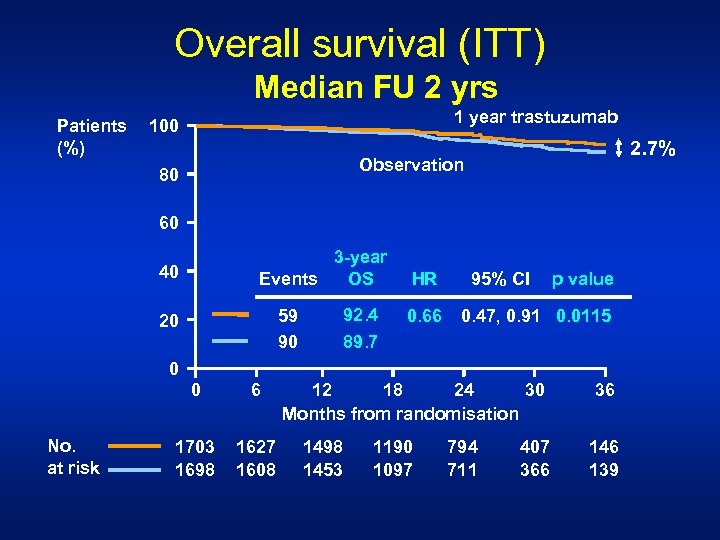 Overall survival (ITT) Median FU 2 yrs Patients (%) 1 year trastuzumab 100 2. 7% Observation 80 60 3 -year OS Events 40 92. 4 89. 7 59 90 20 HR 0. 66 95% CI p value 0. 47, 0. 91 0. 0115 0 0 No. at risk 6 1703 1698 1627 1608 12 18 24 30 Months from randomisation 1498 1453 1190 1097 794 711 407 366 36 146 139
Overall survival (ITT) Median FU 2 yrs Patients (%) 1 year trastuzumab 100 2. 7% Observation 80 60 3 -year OS Events 40 92. 4 89. 7 59 90 20 HR 0. 66 95% CI p value 0. 47, 0. 91 0. 0115 0 0 No. at risk 6 1703 1698 1627 1608 12 18 24 30 Months from randomisation 1498 1453 1190 1097 794 711 407 366 36 146 139
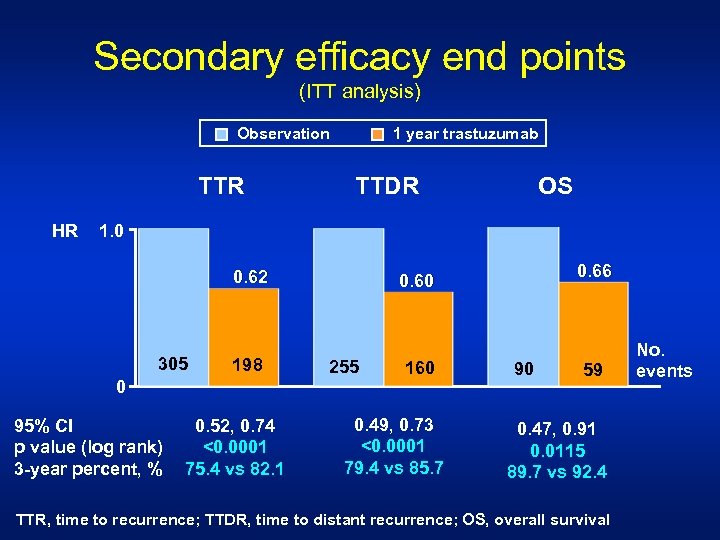 Secondary efficacy end points (ITT analysis) 1 year trastuzumab Observation TTR HR TTDR OS 1. 0 0. 62 305 198 0 95% CI p value (log rank) 3 -year percent, % 0. 52, 0. 74 <0. 0001 75. 4 vs 82. 1 0. 66 0. 60 255 160 0. 49, 0. 73 <0. 0001 79. 4 vs 85. 7 90 59 0. 47, 0. 91 0. 0115 89. 7 vs 92. 4 TTR, time to recurrence; TTDR, time to distant recurrence; OS, overall survival No. events
Secondary efficacy end points (ITT analysis) 1 year trastuzumab Observation TTR HR TTDR OS 1. 0 0. 62 305 198 0 95% CI p value (log rank) 3 -year percent, % 0. 52, 0. 74 <0. 0001 75. 4 vs 82. 1 0. 66 0. 60 255 160 0. 49, 0. 73 <0. 0001 79. 4 vs 85. 7 90 59 0. 47, 0. 91 0. 0115 89. 7 vs 92. 4 TTR, time to recurrence; TTDR, time to distant recurrence; OS, overall survival No. events
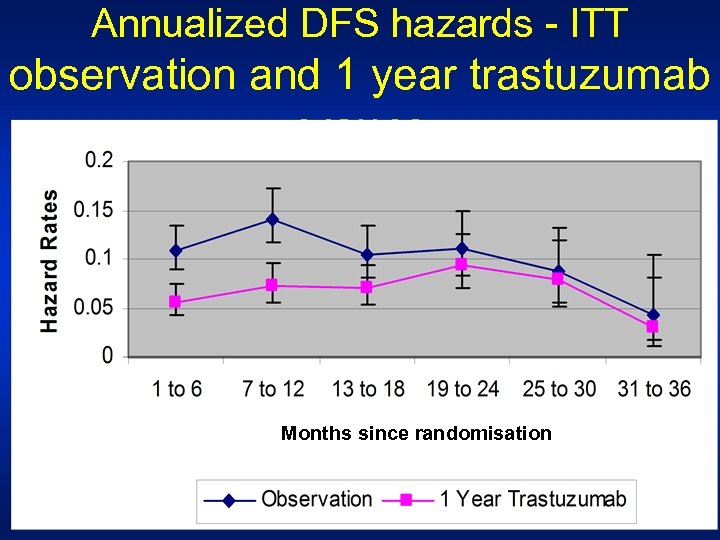 Annualized DFS hazards - ITT observation and 1 year trastuzumab groups Months since randomisation
Annualized DFS hazards - ITT observation and 1 year trastuzumab groups Months since randomisation
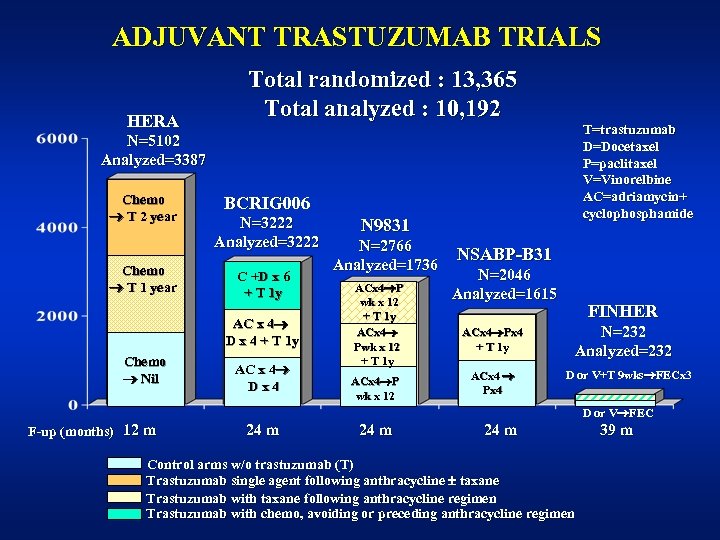 ADJUVANT TRASTUZUMAB TRIALS HERA Total randomized : 13, 365 Total analyzed : 10, 192 T=trastuzumab D=Docetaxel P=paclitaxel V=Vinorelbine AC=adriamycin+ cyclophosphamide N=5102 Analyzed=3387 Chemo T 2 year Chemo T 1 year BCRIG 006 N=3222 Analyzed=3222 C +D x 6 + T 1 y AC x 4 D x 4 + T 1 y Chemo Nil AC x 4 Dx 4 N 9831 N=2766 Analyzed=1736 ACx 4 P wk x 12 + T 1 y ACx 4 P wk x 12 NSABP-B 31 N=2046 Analyzed=1615 ACx 4 Px 4 + T 1 y ACx 4 Px 4 FINHER N=232 Analyzed=232 D or V+T 9 wks FECx 3 D or V FEC F-up (months) 12 m 24 m Control arms w/o trastuzumab (T) Trastuzumab single agent following anthracycline taxane Trastuzumab with taxane following anthracycline regimen Trastuzumab with chemo, avoiding or preceding anthracycline regimen 39 m
ADJUVANT TRASTUZUMAB TRIALS HERA Total randomized : 13, 365 Total analyzed : 10, 192 T=trastuzumab D=Docetaxel P=paclitaxel V=Vinorelbine AC=adriamycin+ cyclophosphamide N=5102 Analyzed=3387 Chemo T 2 year Chemo T 1 year BCRIG 006 N=3222 Analyzed=3222 C +D x 6 + T 1 y AC x 4 D x 4 + T 1 y Chemo Nil AC x 4 Dx 4 N 9831 N=2766 Analyzed=1736 ACx 4 P wk x 12 + T 1 y ACx 4 P wk x 12 NSABP-B 31 N=2046 Analyzed=1615 ACx 4 Px 4 + T 1 y ACx 4 Px 4 FINHER N=232 Analyzed=232 D or V+T 9 wks FECx 3 D or V FEC F-up (months) 12 m 24 m Control arms w/o trastuzumab (T) Trastuzumab single agent following anthracycline taxane Trastuzumab with taxane following anthracycline regimen Trastuzumab with chemo, avoiding or preceding anthracycline regimen 39 m
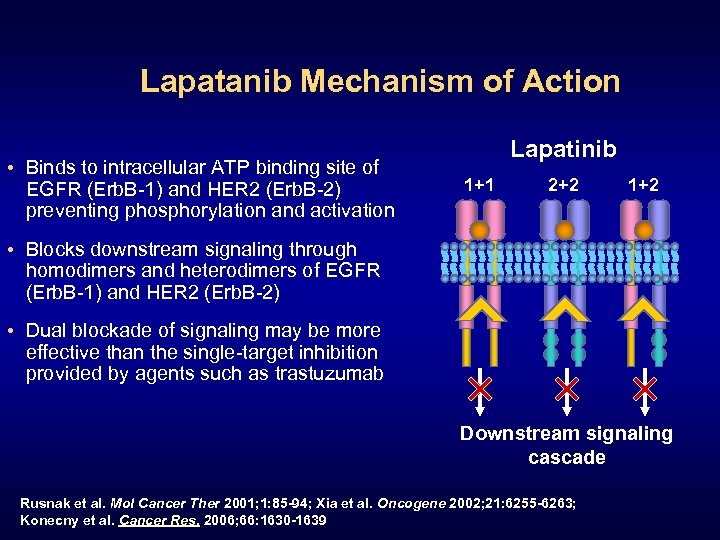 Lapatanib Mechanism of Action • Binds to intracellular ATP binding site of EGFR (Erb. B-1) and HER 2 (Erb. B-2) preventing phosphorylation and activation Lapatinib 1+1 2+2 1+2 • Blocks downstream signaling through homodimers and heterodimers of EGFR (Erb. B-1) and HER 2 (Erb. B-2) • Dual blockade of signaling may be more effective than the single-target inhibition provided by agents such as trastuzumab Downstream signaling cascade Rusnak et al. Mol Cancer Ther 2001; 1: 85 -94; Xia et al. Oncogene 2002; 21: 6255 -6263; Konecny et al. Cancer Res. 2006; 66: 1630 -1639
Lapatanib Mechanism of Action • Binds to intracellular ATP binding site of EGFR (Erb. B-1) and HER 2 (Erb. B-2) preventing phosphorylation and activation Lapatinib 1+1 2+2 1+2 • Blocks downstream signaling through homodimers and heterodimers of EGFR (Erb. B-1) and HER 2 (Erb. B-2) • Dual blockade of signaling may be more effective than the single-target inhibition provided by agents such as trastuzumab Downstream signaling cascade Rusnak et al. Mol Cancer Ther 2001; 1: 85 -94; Xia et al. Oncogene 2002; 21: 6255 -6263; Konecny et al. Cancer Res. 2006; 66: 1630 -1639
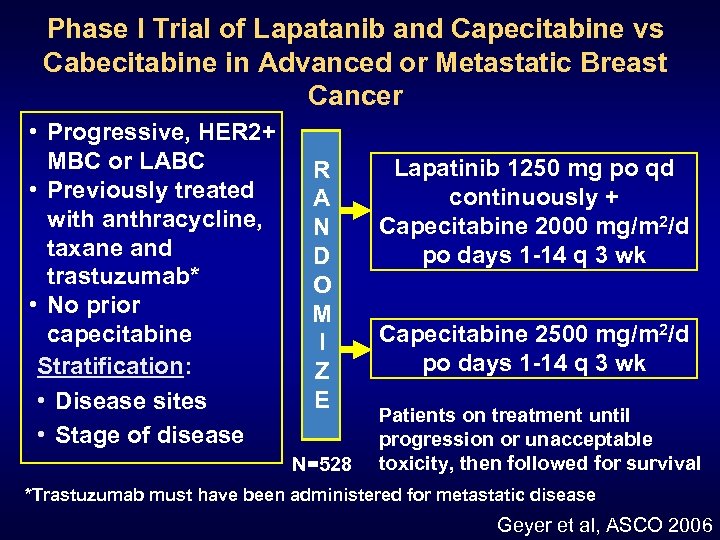 Phase I Trial of Lapatanib and Capecitabine vs Cabecitabine in Advanced or Metastatic Breast Cancer • Progressive, HER 2+ MBC or LABC • Previously treated with anthracycline, taxane and trastuzumab* • No prior capecitabine Stratification: • Disease sites • Stage of disease R A N D O M I Z E N=528 Lapatinib 1250 mg po qd continuously + Capecitabine 2000 mg/m 2/d po days 1 -14 q 3 wk Capecitabine 2500 mg/m 2/d po days 1 -14 q 3 wk Patients on treatment until progression or unacceptable toxicity, then followed for survival *Trastuzumab must have been administered for metastatic disease Geyer et al, ASCO 2006
Phase I Trial of Lapatanib and Capecitabine vs Cabecitabine in Advanced or Metastatic Breast Cancer • Progressive, HER 2+ MBC or LABC • Previously treated with anthracycline, taxane and trastuzumab* • No prior capecitabine Stratification: • Disease sites • Stage of disease R A N D O M I Z E N=528 Lapatinib 1250 mg po qd continuously + Capecitabine 2000 mg/m 2/d po days 1 -14 q 3 wk Capecitabine 2500 mg/m 2/d po days 1 -14 q 3 wk Patients on treatment until progression or unacceptable toxicity, then followed for survival *Trastuzumab must have been administered for metastatic disease Geyer et al, ASCO 2006
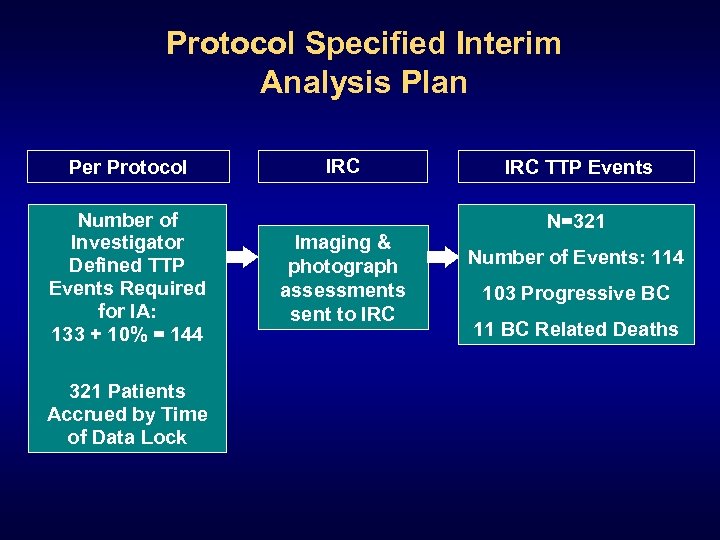 Protocol Specified Interim Analysis Plan Per Protocol Number of Investigator Defined TTP Events Required for IA: 133 + 10% = 144 321 Patients Accrued by Time of Data Lock IRC Imaging & photograph assessments sent to IRC TTP Events N=321 Number of Events: 114 103 Progressive BC 11 BC Related Deaths
Protocol Specified Interim Analysis Plan Per Protocol Number of Investigator Defined TTP Events Required for IA: 133 + 10% = 144 321 Patients Accrued by Time of Data Lock IRC Imaging & photograph assessments sent to IRC TTP Events N=321 Number of Events: 114 103 Progressive BC 11 BC Related Deaths
 • IDMC Recommendations March futility, boundaries 20 th 2006 Agreed with superiority and • Unanimous recommendation for termination of study enrollment based on : “clinically meaningful, statistically significant advantage in primary endpoint (TTP) in the capecitabine + lapatinib arm vs capecitabine alone arm” • No safety or tolerability concerns • Accrual as of 3/20/06: 392 of 528
• IDMC Recommendations March futility, boundaries 20 th 2006 Agreed with superiority and • Unanimous recommendation for termination of study enrollment based on : “clinically meaningful, statistically significant advantage in primary endpoint (TTP) in the capecitabine + lapatinib arm vs capecitabine alone arm” • No safety or tolerability concerns • Accrual as of 3/20/06: 392 of 528
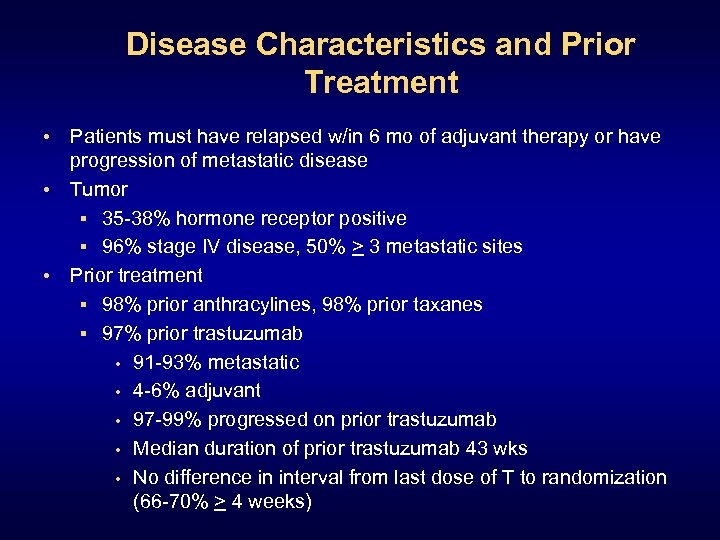 Disease Characteristics and Prior Treatment • Patients must have relapsed w/in 6 mo of adjuvant therapy or have progression of metastatic disease • Tumor § 35 -38% hormone receptor positive § 96% stage IV disease, 50% > 3 metastatic sites • Prior treatment § 98% prior anthracylines, 98% prior taxanes § 97% prior trastuzumab • 91 -93% metastatic • 4 -6% adjuvant • 97 -99% progressed on prior trastuzumab • Median duration of prior trastuzumab 43 wks • No difference in interval from last dose of T to randomization (66 -70% > 4 weeks)
Disease Characteristics and Prior Treatment • Patients must have relapsed w/in 6 mo of adjuvant therapy or have progression of metastatic disease • Tumor § 35 -38% hormone receptor positive § 96% stage IV disease, 50% > 3 metastatic sites • Prior treatment § 98% prior anthracylines, 98% prior taxanes § 97% prior trastuzumab • 91 -93% metastatic • 4 -6% adjuvant • 97 -99% progressed on prior trastuzumab • Median duration of prior trastuzumab 43 wks • No difference in interval from last dose of T to randomization (66 -70% > 4 weeks)
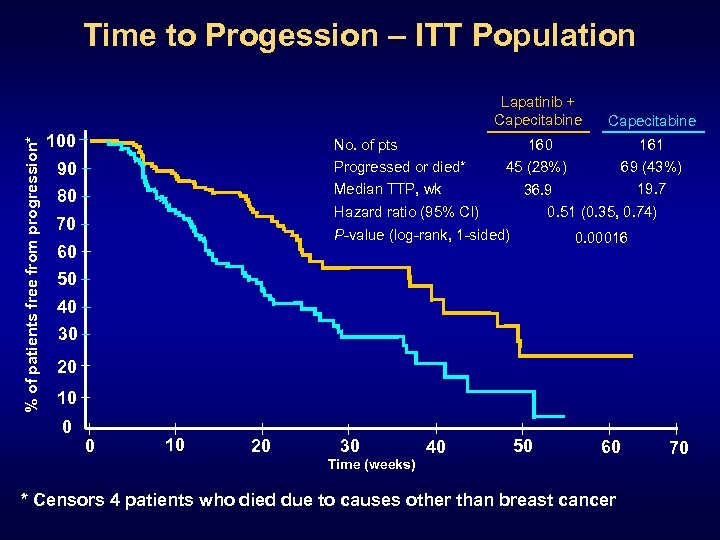 Time to Progession – ITT Population % of patients free from progression* Lapatinib + Capecitabine 100 No. of pts Progressed or died* Median TTP, wk 90 80 160 45 (28%) Hazard ratio (95% CI) 70 161 69 (43%) 19. 7 36. 9 0. 51 (0. 35, 0. 74) P-value (log-rank, 1 -sided) 60 50 Capecitabine 0. 00016 40 30 20 10 0 0 10 20 30 Time (weeks) 40 50 60 * Censors 4 patients who died due to causes other than breast cancer 70
Time to Progession – ITT Population % of patients free from progression* Lapatinib + Capecitabine 100 No. of pts Progressed or died* Median TTP, wk 90 80 160 45 (28%) Hazard ratio (95% CI) 70 161 69 (43%) 19. 7 36. 9 0. 51 (0. 35, 0. 74) P-value (log-rank, 1 -sided) 60 50 Capecitabine 0. 00016 40 30 20 10 0 0 10 20 30 Time (weeks) 40 50 60 * Censors 4 patients who died due to causes other than breast cancer 70
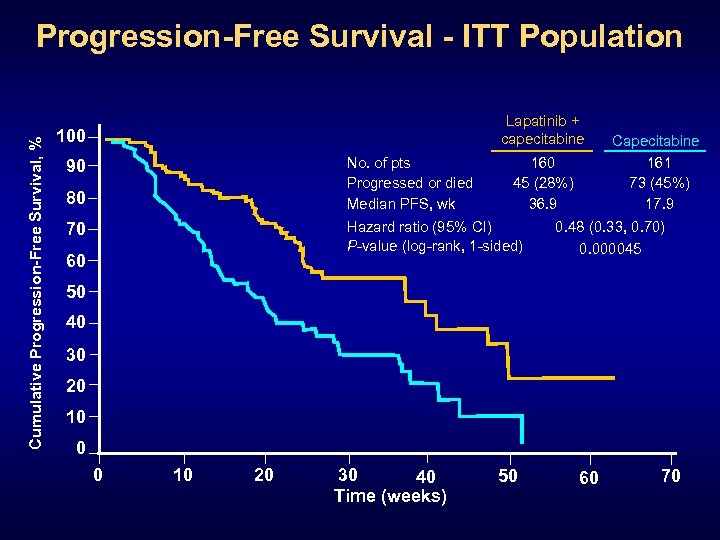 Cumulative Progression-Free Survival, % Progression-Free Survival - ITT Population Lapatinib + capecitabine 100 No. of pts Progressed or died Median PFS, wk 90 80 70 160 45 (28%) 36. 9 Hazard ratio (95% CI) P-value (log-rank, 1 -sided) 60 Capecitabine 161 73 (45%) 17. 9 0. 48 (0. 33, 0. 70) 0. 000045 50 40 30 20 10 0 0 10 20 30 40 Time (weeks) 50 60 70
Cumulative Progression-Free Survival, % Progression-Free Survival - ITT Population Lapatinib + capecitabine 100 No. of pts Progressed or died Median PFS, wk 90 80 70 160 45 (28%) 36. 9 Hazard ratio (95% CI) P-value (log-rank, 1 -sided) 60 Capecitabine 161 73 (45%) 17. 9 0. 48 (0. 33, 0. 70) 0. 000045 50 40 30 20 10 0 0 10 20 30 40 Time (weeks) 50 60 70
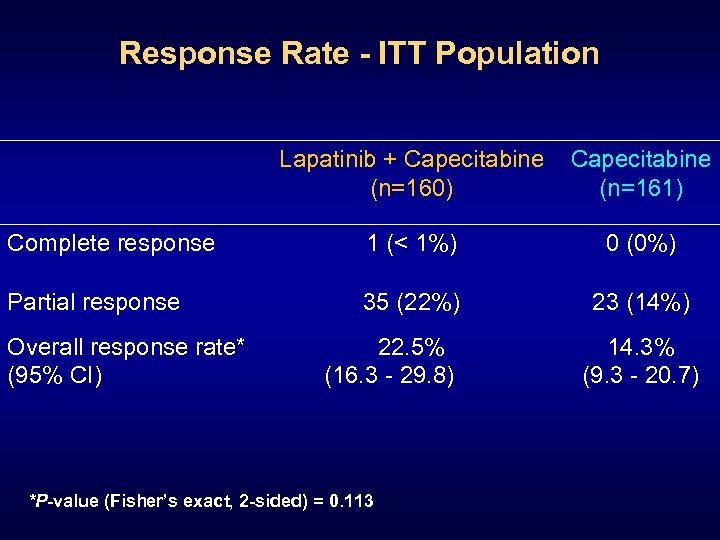 Response Rate - ITT Population Lapatinib + Capecitabine (n=160) Capecitabine (n=161) Complete response 1 (< 1%) 0 (0%) Partial response 35 (22%) 23 (14%) Overall response rate* (95% CI) 22. 5% (16. 3 - 29. 8) *P-value (Fisher’s exact, 2 -sided) = 0. 113 14. 3% (9. 3 - 20. 7)
Response Rate - ITT Population Lapatinib + Capecitabine (n=160) Capecitabine (n=161) Complete response 1 (< 1%) 0 (0%) Partial response 35 (22%) 23 (14%) Overall response rate* (95% CI) 22. 5% (16. 3 - 29. 8) *P-value (Fisher’s exact, 2 -sided) = 0. 113 14. 3% (9. 3 - 20. 7)
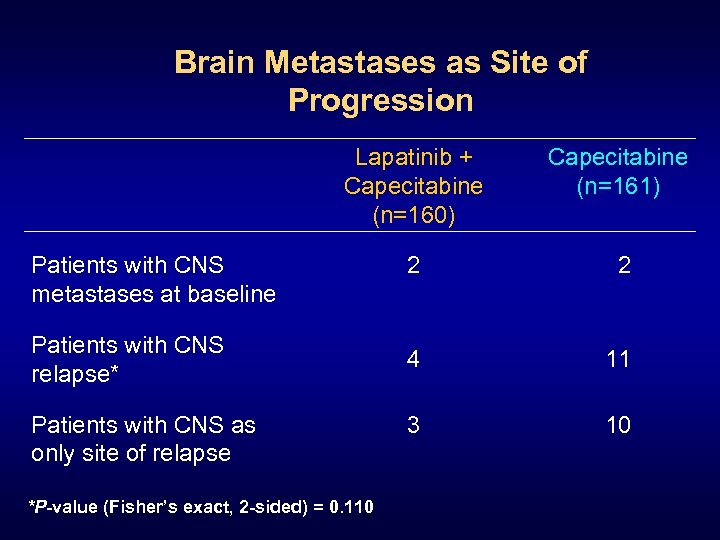 Brain Metastases as Site of Progression Lapatinib + Capecitabine (n=160) Capecitabine (n=161) 2 2 4 11 3 10 Patients with CNS metastases at baseline Patients with CNS relapse* Patients with CNS as only site of relapse *P-value (Fisher’s exact, 2 -sided) = 0. 110
Brain Metastases as Site of Progression Lapatinib + Capecitabine (n=160) Capecitabine (n=161) 2 2 4 11 3 10 Patients with CNS metastases at baseline Patients with CNS relapse* Patients with CNS as only site of relapse *P-value (Fisher’s exact, 2 -sided) = 0. 110
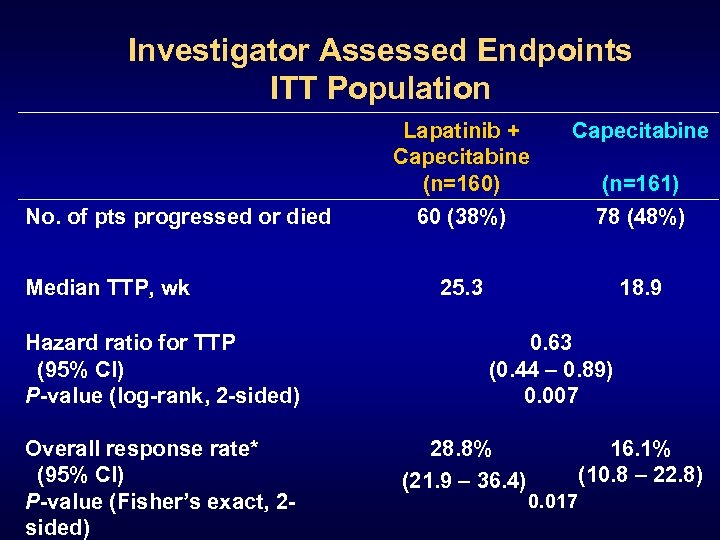 Investigator Assessed Endpoints ITT Population Lapatinib + Capecitabine (n=160) No. of pts progressed or died Median TTP, wk Hazard ratio for TTP (95% CI) P-value (log-rank, 2 -sided) Overall response rate* (95% CI) P-value (Fisher’s exact, 2 sided) Capecitabine 60 (38%) 78 (48%) 25. 3 18. 9 (n=161) 0. 63 (0. 44 – 0. 89) 0. 007 28. 8% (21. 9 – 36. 4) 16. 1% (10. 8 – 22. 8) 0. 017
Investigator Assessed Endpoints ITT Population Lapatinib + Capecitabine (n=160) No. of pts progressed or died Median TTP, wk Hazard ratio for TTP (95% CI) P-value (log-rank, 2 -sided) Overall response rate* (95% CI) P-value (Fisher’s exact, 2 sided) Capecitabine 60 (38%) 78 (48%) 25. 3 18. 9 (n=161) 0. 63 (0. 44 – 0. 89) 0. 007 28. 8% (21. 9 – 36. 4) 16. 1% (10. 8 – 22. 8) 0. 017
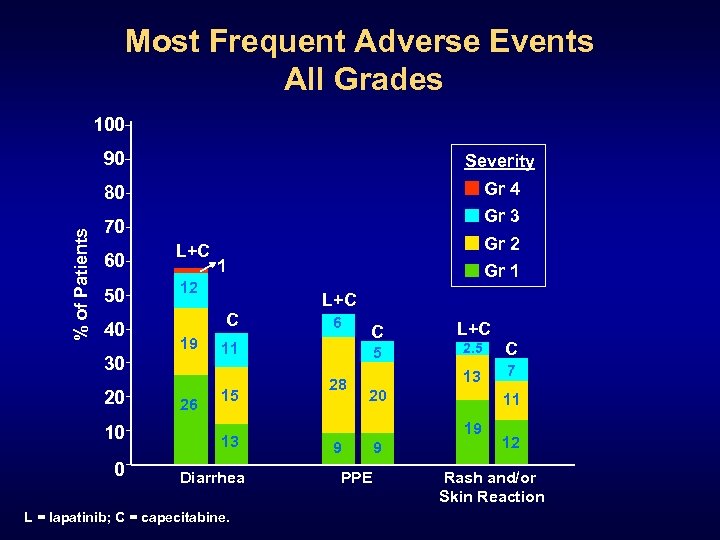 Most Frequent Adverse Events All Grades 100 Severity 80 % of Patients 90 Gr 4 Gr 3 70 60 50 40 L+C 10 0 1 12 C 19 30 20 Gr 2 26 Gr 1 L+C 6 11 15 13 Diarrhea L = lapatinib; C = capecitabine. C 2. 5 C 13 5 28 L+C 7 20 11 19 9 PPE 9 12 Rash and/or Skin Reaction
Most Frequent Adverse Events All Grades 100 Severity 80 % of Patients 90 Gr 4 Gr 3 70 60 50 40 L+C 10 0 1 12 C 19 30 20 Gr 2 26 Gr 1 L+C 6 11 15 13 Diarrhea L = lapatinib; C = capecitabine. C 2. 5 C 13 5 28 L+C 7 20 11 19 9 PPE 9 12 Rash and/or Skin Reaction
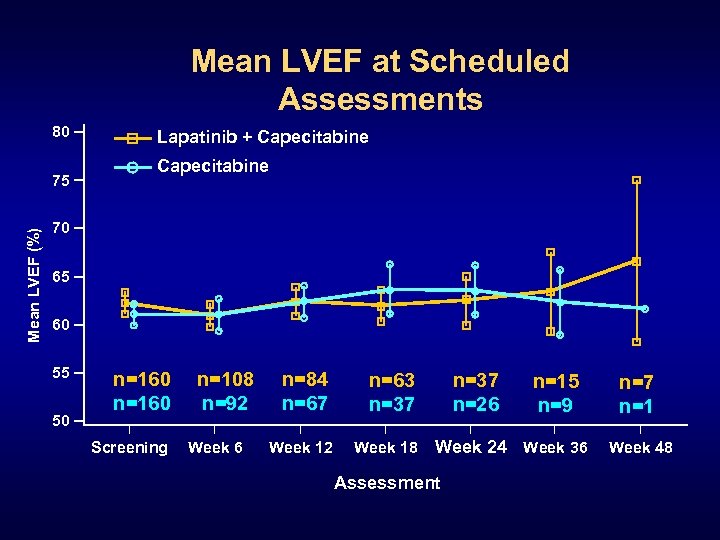 Mean LVEF at Scheduled Assessments 80 Mean LVEF (%) 75 Lapatinib + Capecitabine 70 65 60 55 50 n=160 Screening n=108 n=92 Week 6 n=84 n=67 n=63 n=37 Week 12 Week 18 n=37 n=26 n=15 n=9 Week 24 Week 36 Assessment n=7 n=1 Week 48
Mean LVEF at Scheduled Assessments 80 Mean LVEF (%) 75 Lapatinib + Capecitabine 70 65 60 55 50 n=160 Screening n=108 n=92 Week 6 n=84 n=67 n=63 n=37 Week 12 Week 18 n=37 n=26 n=15 n=9 Week 24 Week 36 Assessment n=7 n=1 Week 48
 Conclusions • The planned interim analysis crossed pre-specified reporting boundary and demonstrated a clinically meaningful and statistically significant improvement in median TTP § Lapatinib + capecitabine 8. 5 mo vs capecitabine 4. 5 mo • Lapatinib + capecitabine well tolerated; declines in LVEF were infrequent, asymptomatic, reversible • Fewer patients developed brain metastases as first site of progression in the group receiving lapatinib • Trials evaluating lapatinib in earlier stages of HER 2+ breast cancer are warranted
Conclusions • The planned interim analysis crossed pre-specified reporting boundary and demonstrated a clinically meaningful and statistically significant improvement in median TTP § Lapatinib + capecitabine 8. 5 mo vs capecitabine 4. 5 mo • Lapatinib + capecitabine well tolerated; declines in LVEF were infrequent, asymptomatic, reversible • Fewer patients developed brain metastases as first site of progression in the group receiving lapatinib • Trials evaluating lapatinib in earlier stages of HER 2+ breast cancer are warranted
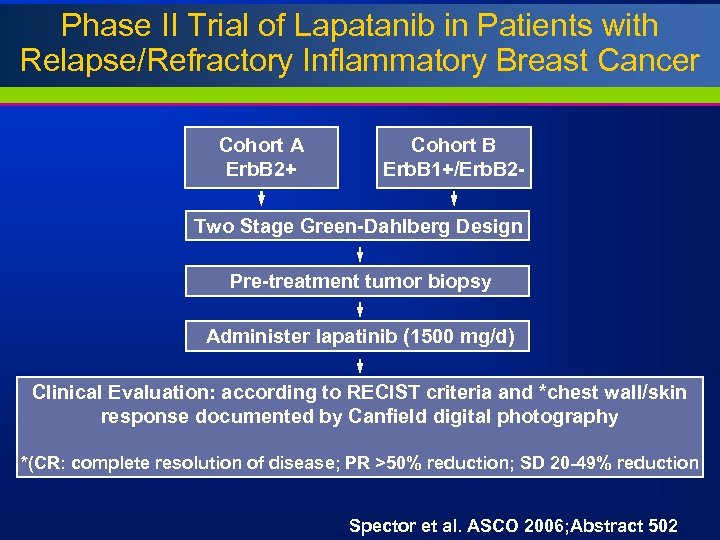 Phase II Trial of Lapatanib in Patients with Relapse/Refractory Inflammatory Breast Cancer Cohort A Erb. B 2+ Cohort B Erb. B 1+/Erb. B 2 - Two Stage Green-Dahlberg Design Pre-treatment tumor biopsy Administer lapatinib (1500 mg/d) Clinical Evaluation: according to RECIST criteria and *chest wall/skin response documented by Canfield digital photography *(CR: complete resolution of disease; PR >50% reduction; SD 20 -49% reduction Spector et al. ASCO 2006; Abstract 502
Phase II Trial of Lapatanib in Patients with Relapse/Refractory Inflammatory Breast Cancer Cohort A Erb. B 2+ Cohort B Erb. B 1+/Erb. B 2 - Two Stage Green-Dahlberg Design Pre-treatment tumor biopsy Administer lapatinib (1500 mg/d) Clinical Evaluation: according to RECIST criteria and *chest wall/skin response documented by Canfield digital photography *(CR: complete resolution of disease; PR >50% reduction; SD 20 -49% reduction Spector et al. ASCO 2006; Abstract 502
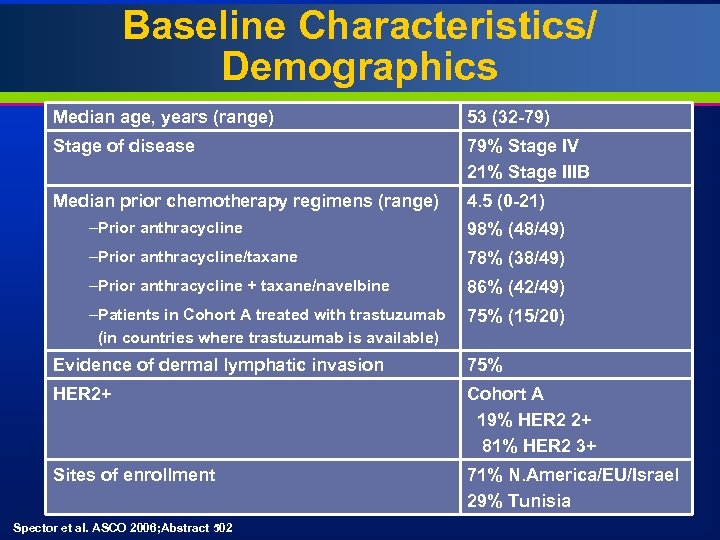 Baseline Characteristics/ Demographics Median age, years (range) 53 (32 -79) Stage of disease 79% Stage IV 21% Stage IIIB Median prior chemotherapy regimens (range) 4. 5 (0 -21) –Prior anthracycline 98% (48/49) –Prior anthracycline/taxane 78% (38/49) –Prior anthracycline + taxane/navelbine 86% (42/49) –Patients in Cohort A treated with trastuzumab (in countries where trastuzumab is available) 75% (15/20) Evidence of dermal lymphatic invasion 75% HER 2+ Cohort A 19% HER 2 2+ 81% HER 2 3+ Sites of enrollment 71% N. America/EU/Israel 29% Tunisia Spector et al. ASCO 2006; Abstract 502
Baseline Characteristics/ Demographics Median age, years (range) 53 (32 -79) Stage of disease 79% Stage IV 21% Stage IIIB Median prior chemotherapy regimens (range) 4. 5 (0 -21) –Prior anthracycline 98% (48/49) –Prior anthracycline/taxane 78% (38/49) –Prior anthracycline + taxane/navelbine 86% (42/49) –Patients in Cohort A treated with trastuzumab (in countries where trastuzumab is available) 75% (15/20) Evidence of dermal lymphatic invasion 75% HER 2+ Cohort A 19% HER 2 2+ 81% HER 2 3+ Sites of enrollment 71% N. America/EU/Israel 29% Tunisia Spector et al. ASCO 2006; Abstract 502
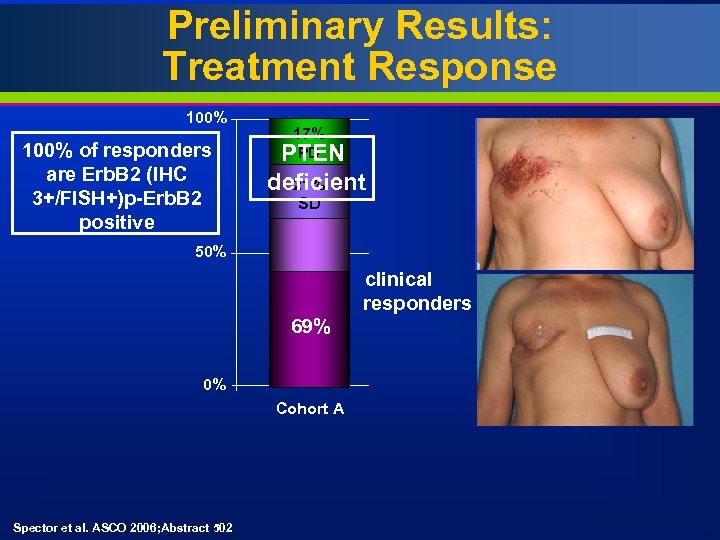 Preliminary Results: Treatment Response 100% of responders are Erb. B 2 (IHC 3+/FISH+)p-Erb. B 2 positive 17% PD PTEN 17% pending deficient 21% SD 58% PD 50% 62% 100% PR 69% 0% clinical responders 17% SD 8. 3% Cohort A Erb. B 2+ Cohort B Erb. B 1+/Erb. B 2 - 24 patients 12 patients 5 enrolled patients were not evaluable (did not express target or died prior to Day 28) Spector et al. ASCO 2006; Abstract 502
Preliminary Results: Treatment Response 100% of responders are Erb. B 2 (IHC 3+/FISH+)p-Erb. B 2 positive 17% PD PTEN 17% pending deficient 21% SD 58% PD 50% 62% 100% PR 69% 0% clinical responders 17% SD 8. 3% Cohort A Erb. B 2+ Cohort B Erb. B 1+/Erb. B 2 - 24 patients 12 patients 5 enrolled patients were not evaluable (did not express target or died prior to Day 28) Spector et al. ASCO 2006; Abstract 502
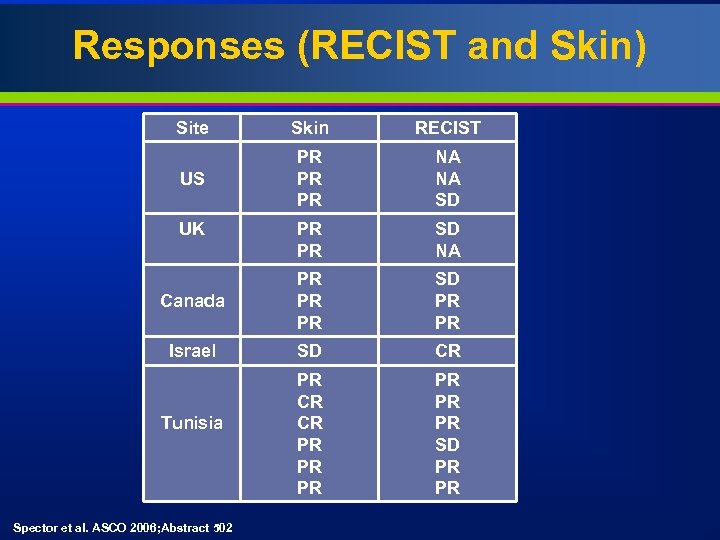 Responses (RECIST and Skin) Site Skin RECIST US PR PR PR NA NA SD PR PR SD NA Canada PR PR PR SD PR PR Israel SD CR PR CR CR PR PR PR SD PR PR UK Tunisia Spector et al. ASCO 2006; Abstract 502
Responses (RECIST and Skin) Site Skin RECIST US PR PR PR NA NA SD PR PR SD NA Canada PR PR PR SD PR PR Israel SD CR PR CR CR PR PR PR SD PR PR UK Tunisia Spector et al. ASCO 2006; Abstract 502
 Summary Lapatinib monotherapy is clinically active in heavily pre-treated IBC patients • 62% response rate in Erb. B 2 overexpressors Lapatinib is well tolerated • Generally grade 1/2 GI and skin toxicity Preliminary biomarker analysis suggests • Correlation of Erb. B 2 (IHC 3+ or FISH+) with response • Responders are more likely to be p-Erb. B 2 positive (which appears to correlate with FISH +) • Co-expression of IGF-IR does not appear to preclude response • PTEN deficiency does not appear to preclude response Spector et al. ASCO 2006; Abstract 502
Summary Lapatinib monotherapy is clinically active in heavily pre-treated IBC patients • 62% response rate in Erb. B 2 overexpressors Lapatinib is well tolerated • Generally grade 1/2 GI and skin toxicity Preliminary biomarker analysis suggests • Correlation of Erb. B 2 (IHC 3+ or FISH+) with response • Responders are more likely to be p-Erb. B 2 positive (which appears to correlate with FISH +) • Co-expression of IGF-IR does not appear to preclude response • PTEN deficiency does not appear to preclude response Spector et al. ASCO 2006; Abstract 502
 Phase 2 Trial of Lapatanib for Brain Metastases in Patients with HER 2+ Breast Cancer § Lapatinib given in four week cycles – 750 mg PO BID – response assessed every 2 cycles § Dose modifications – grade 3 or 4 hold until grade 0 or 1 and reduce one level – grade 3 or 4 LVEF dysfunction or interstitial pneumonitis off study Lin et al, ASCO 2006, #503
Phase 2 Trial of Lapatanib for Brain Metastases in Patients with HER 2+ Breast Cancer § Lapatinib given in four week cycles – 750 mg PO BID – response assessed every 2 cycles § Dose modifications – grade 3 or 4 hold until grade 0 or 1 and reduce one level – grade 3 or 4 LVEF dysfunction or interstitial pneumonitis off study Lin et al, ASCO 2006, #503
 Patients and Toxicity § 39 patients enrolled, all off study by 5/06 § Prior CNS radiation in 95% (37) – 51% WBRT, 15% SRS, 28% both § Toxicity – No grade 4 – 21% grade 3 diarrhea, 22% grade 2 – Other common toxicities § Fatigue § Headache § Mild rash
Patients and Toxicity § 39 patients enrolled, all off study by 5/06 § Prior CNS radiation in 95% (37) – 51% WBRT, 15% SRS, 28% both § Toxicity – No grade 4 – 21% grade 3 diarrhea, 22% grade 2 – Other common toxicities § Fatigue § Headache § Mild rash
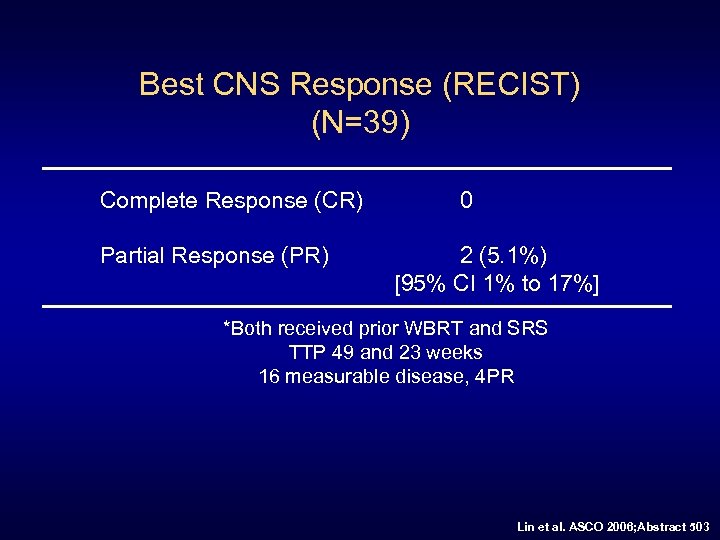 Best CNS Response (RECIST) (N=39) Complete Response (CR) Partial Response (PR) 0 2 (5. 1%) [95% CI 1% to 17%] *Both received prior WBRT and SRS TTP 49 and 23 weeks 16 measurable disease, 4 PR Lin et al. ASCO 2006; Abstract 503
Best CNS Response (RECIST) (N=39) Complete Response (CR) Partial Response (PR) 0 2 (5. 1%) [95% CI 1% to 17%] *Both received prior WBRT and SRS TTP 49 and 23 weeks 16 measurable disease, 4 PR Lin et al. ASCO 2006; Abstract 503
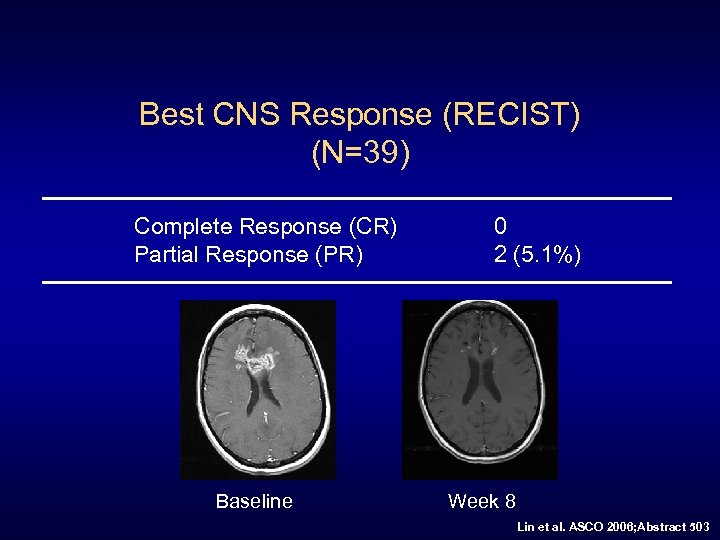 Best CNS Response (RECIST) (N=39) Complete Response (CR) Partial Response (PR) Baseline 0 2 (5. 1%) Week 8 Lin et al. ASCO 2006; Abstract 503
Best CNS Response (RECIST) (N=39) Complete Response (CR) Partial Response (PR) Baseline 0 2 (5. 1%) Week 8 Lin et al. ASCO 2006; Abstract 503
 Conclusions • Volumetric and PET changes also evaluated § Significance still unclear but both volume and PET changes noted § Volumetric declines greater than 30% in 4 pts, 6 pts 1030% • Quality of life generally stable at 8 weeks • Some evidence of clinical activity § 2 PR by RECIST § 8 progression free in CNS at 16 weeks • What do we know about Lapatanib? § Crosses the blood brain barrier § Has activity against HER 2+ brain metastases § Is generally well tolerated § May have activity in prevention of brain metastases?
Conclusions • Volumetric and PET changes also evaluated § Significance still unclear but both volume and PET changes noted § Volumetric declines greater than 30% in 4 pts, 6 pts 1030% • Quality of life generally stable at 8 weeks • Some evidence of clinical activity § 2 PR by RECIST § 8 progression free in CNS at 16 weeks • What do we know about Lapatanib? § Crosses the blood brain barrier § Has activity against HER 2+ brain metastases § Is generally well tolerated § May have activity in prevention of brain metastases?
 Cardiac Function in 3127 Patients Treated with Lapatanib • Pooled analysis of 10 monotherapy trials and 8 trials of lapatanib in combination with chemotherapy or anti-hormonal agents • All studies required EF within institutional range of normal § Monitoring at baseline, q 8 weeks on treatment • Length of exposure (3535 including placebo) § > 6 months in 1090 Perez E. , ASCO 2006, #583
Cardiac Function in 3127 Patients Treated with Lapatanib • Pooled analysis of 10 monotherapy trials and 8 trials of lapatanib in combination with chemotherapy or anti-hormonal agents • All studies required EF within institutional range of normal § Monitoring at baseline, q 8 weeks on treatment • Length of exposure (3535 including placebo) § > 6 months in 1090 Perez E. , ASCO 2006, #583
 Results and Conclusion • • • Symptomatic LVEF decrease § Breast cancer: 2 (0. 1%) § Non breast cancer: 2 (0. 1%) Asymptomatic LVEF decrease § 37 (1. 2%) Average duration of LVEF decrease was 40 days § 41% (9/22) with breast cancer recovered while continuing to receive lapatanib Avereage decrease relative to baseline was 29% Potential risk factors § Mediastinal/left sided radiation § LVEF on trastuzumab (only 2 pts) § Prior anthracyclines § Past significant cardiac history Overall risk appears to be very low § 1. 3% across breast and other malignancies § Further followup is ongoing
Results and Conclusion • • • Symptomatic LVEF decrease § Breast cancer: 2 (0. 1%) § Non breast cancer: 2 (0. 1%) Asymptomatic LVEF decrease § 37 (1. 2%) Average duration of LVEF decrease was 40 days § 41% (9/22) with breast cancer recovered while continuing to receive lapatanib Avereage decrease relative to baseline was 29% Potential risk factors § Mediastinal/left sided radiation § LVEF on trastuzumab (only 2 pts) § Prior anthracyclines § Past significant cardiac history Overall risk appears to be very low § 1. 3% across breast and other malignancies § Further followup is ongoing
 Sponsored and Cooperative Group Trials with Lapatanib • First line metastatic § § § • Trastuzumb resistant § § • Lapatanib alone vs lapatanib combined with trastuzumab Lapatanib with bevacizumab (MSKCC and UCSF collaboration) Brain metastases § • Paclitaxel with trastuzumab with or without lapatanib Aromatase inhibitors with lapatanib Other chemotherapy combinations Progression after radiation with lapatanib alone Cooperative group (pending, will open in 2008) Neoadjuvant chemotherapy with trastuzumab vs lapatanib vs the combination (3 arm study) § Adjuvant trial (Perez et al) • Chemotherapy with: • Lapatanib • Trastuzumab • The combination § • Other interesting research directions for HER 2+ disease § § § 17 AAG (HSP inhibitor) IGFR inhibitors MTOR inhibitors (inhibition of the downstream pathway)
Sponsored and Cooperative Group Trials with Lapatanib • First line metastatic § § § • Trastuzumb resistant § § • Lapatanib alone vs lapatanib combined with trastuzumab Lapatanib with bevacizumab (MSKCC and UCSF collaboration) Brain metastases § • Paclitaxel with trastuzumab with or without lapatanib Aromatase inhibitors with lapatanib Other chemotherapy combinations Progression after radiation with lapatanib alone Cooperative group (pending, will open in 2008) Neoadjuvant chemotherapy with trastuzumab vs lapatanib vs the combination (3 arm study) § Adjuvant trial (Perez et al) • Chemotherapy with: • Lapatanib • Trastuzumab • The combination § • Other interesting research directions for HER 2+ disease § § § 17 AAG (HSP inhibitor) IGFR inhibitors MTOR inhibitors (inhibition of the downstream pathway)
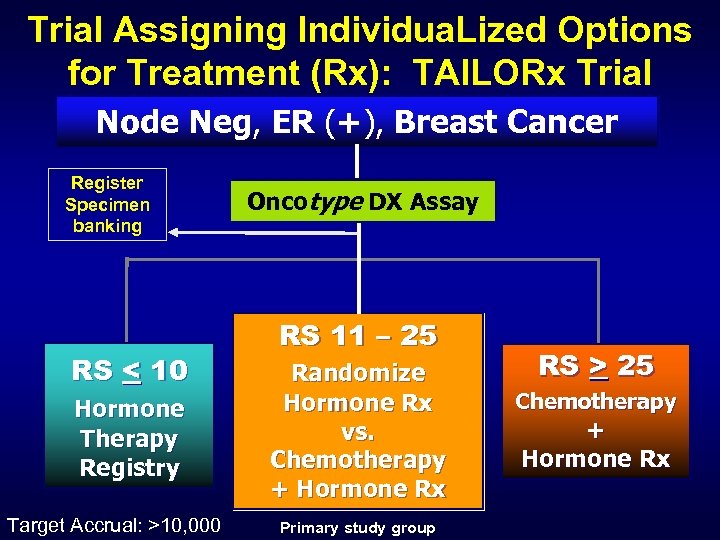 Trial Assigning Individua. Lized Options for Treatment (Rx): TAILORx Trial Node Neg, ER (+), Breast Cancer Register Specimen banking RS < 10 Hormone Therapy Registry Target Accrual: >10, 000 Oncotype DX Assay RS 11 – 25 Randomize Hormone Rx vs. Chemotherapy + Hormone Rx Primary study group RS > 25 Chemotherapy + Hormone Rx
Trial Assigning Individua. Lized Options for Treatment (Rx): TAILORx Trial Node Neg, ER (+), Breast Cancer Register Specimen banking RS < 10 Hormone Therapy Registry Target Accrual: >10, 000 Oncotype DX Assay RS 11 – 25 Randomize Hormone Rx vs. Chemotherapy + Hormone Rx Primary study group RS > 25 Chemotherapy + Hormone Rx
 The Changing Paradigm of Clinical Trials in Breast Cancer Non Selected Adjuvant Trials in Breast Cancer
The Changing Paradigm of Clinical Trials in Breast Cancer Non Selected Adjuvant Trials in Breast Cancer


