1357d24b92f0fb49173ead82bae33a64.ppt
- Количество слайдов: 16
 Arterio-venous Fistula Scaffold Jonathan Jaffery, Ph. D – School of Medicine Naomi Chesler, Ph. D – Dept. of Biomedical Engineering Kristyn Masters, Ph. D – Dept. of Biomedical Engineering Brenda Ogle, Ph. D – Dept. of Biomedical Engineering Karen Chen Holly Liske Laura Piechura Kellen Sheedy
Arterio-venous Fistula Scaffold Jonathan Jaffery, Ph. D – School of Medicine Naomi Chesler, Ph. D – Dept. of Biomedical Engineering Kristyn Masters, Ph. D – Dept. of Biomedical Engineering Brenda Ogle, Ph. D – Dept. of Biomedical Engineering Karen Chen Holly Liske Laura Piechura Kellen Sheedy
 University of Wisconsin - Madison Biomedical Engineering Design Courses INTELLECTUAL PROPERTY STATEMENT All information provided by individuals or Design Project Groups during this or subsequent presentations is the property of the researchers presenting this information. In addition, any information provided herein may include results sponsored by and provided to a member company of the Biomedical Engineering Student Design Consortium (SDC). Anyone to whom this information is disclosed: 1) Agrees to use this information solely for purposes related to this review; 2) Agrees not to use this information for any other purpose unless given written approval in advance by the Project Group, the Client / SDC, and the Advisor. 3) Agrees to keep this information in confidence until the relevant parties listed in Part (2) above have evaluated and secured any applicable intellectual property rights in this information. 4) Continued attendance at this presentation constitutes compliance with this agreement.
University of Wisconsin - Madison Biomedical Engineering Design Courses INTELLECTUAL PROPERTY STATEMENT All information provided by individuals or Design Project Groups during this or subsequent presentations is the property of the researchers presenting this information. In addition, any information provided herein may include results sponsored by and provided to a member company of the Biomedical Engineering Student Design Consortium (SDC). Anyone to whom this information is disclosed: 1) Agrees to use this information solely for purposes related to this review; 2) Agrees not to use this information for any other purpose unless given written approval in advance by the Project Group, the Client / SDC, and the Advisor. 3) Agrees to keep this information in confidence until the relevant parties listed in Part (2) above have evaluated and secured any applicable intellectual property rights in this information. 4) Continued attendance at this presentation constitutes compliance with this agreement.
 Presentation Overview Background information n Problem statement n Design criteria n Overview of four materials n Testing n Matrix n Future work n
Presentation Overview Background information n Problem statement n Design criteria n Overview of four materials n Testing n Matrix n Future work n
 Background - Hemodialysis n Renal failure n 300, 000+ Americans n To cleanse the blood n Methods ¨ Catheter ¨ Graft ¨ Arterio-venous (AV) fistula
Background - Hemodialysis n Renal failure n 300, 000+ Americans n To cleanse the blood n Methods ¨ Catheter ¨ Graft ¨ Arterio-venous (AV) fistula
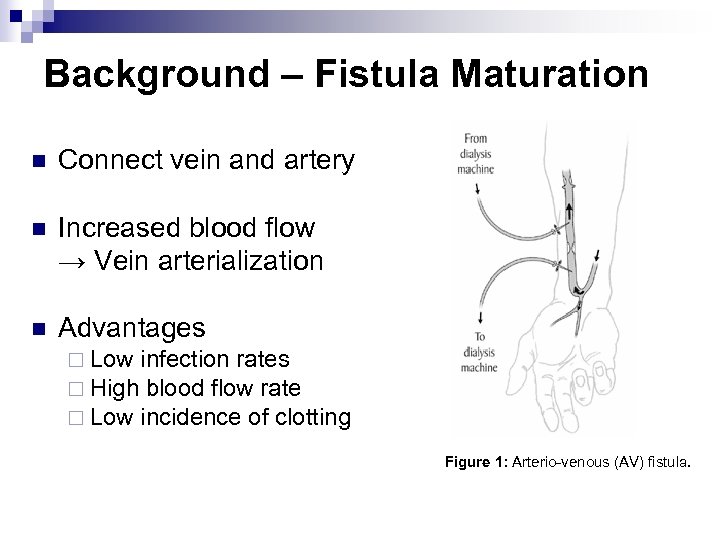 Background – Fistula Maturation n Connect vein and artery n Increased blood flow → Vein arterialization n Advantages ¨ Low infection rates ¨ High blood flow rate ¨ Low incidence of clotting Figure 1: Arterio-venous (AV) fistula.
Background – Fistula Maturation n Connect vein and artery n Increased blood flow → Vein arterialization n Advantages ¨ Low infection rates ¨ High blood flow rate ¨ Low incidence of clotting Figure 1: Arterio-venous (AV) fistula.
 Problem Statement n 45% success rate of current AV fistula n Veins collapse during hemodialysis n External scaffolding to prevent vein collapse n Shorter maturation period
Problem Statement n 45% success rate of current AV fistula n Veins collapse during hemodialysis n External scaffolding to prevent vein collapse n Shorter maturation period
 Design Criteria n Injectable liquid n Polymerizable in situ n Adhere and tether to the vein n Withstand puncture and tension n Biocompatibility
Design Criteria n Injectable liquid n Polymerizable in situ n Adhere and tether to the vein n Withstand puncture and tension n Biocompatibility
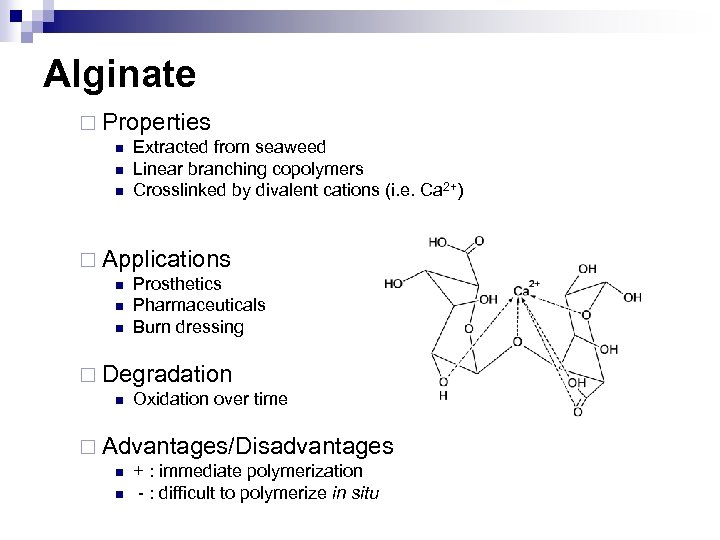 Alginate ¨ Properties n n n Extracted from seaweed Linear branching copolymers Crosslinked by divalent cations (i. e. Ca 2+) ¨ Applications n n n Prosthetics Pharmaceuticals Burn dressing ¨ Degradation n Oxidation over time ¨ Advantages/Disadvantages n n + : immediate polymerization - : difficult to polymerize in situ
Alginate ¨ Properties n n n Extracted from seaweed Linear branching copolymers Crosslinked by divalent cations (i. e. Ca 2+) ¨ Applications n n n Prosthetics Pharmaceuticals Burn dressing ¨ Degradation n Oxidation over time ¨ Advantages/Disadvantages n n + : immediate polymerization - : difficult to polymerize in situ
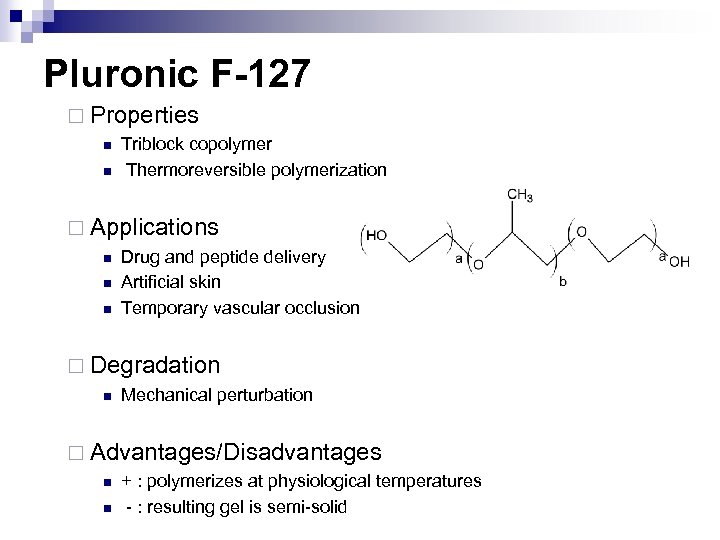 Pluronic F-127 ¨ Properties n n Triblock copolymer Thermoreversible polymerization ¨ Applications n n n Drug and peptide delivery Artificial skin Temporary vascular occlusion ¨ Degradation n Mechanical perturbation ¨ Advantages/Disadvantages n n + : polymerizes at physiological temperatures - : resulting gel is semi-solid
Pluronic F-127 ¨ Properties n n Triblock copolymer Thermoreversible polymerization ¨ Applications n n n Drug and peptide delivery Artificial skin Temporary vascular occlusion ¨ Degradation n Mechanical perturbation ¨ Advantages/Disadvantages n n + : polymerizes at physiological temperatures - : resulting gel is semi-solid
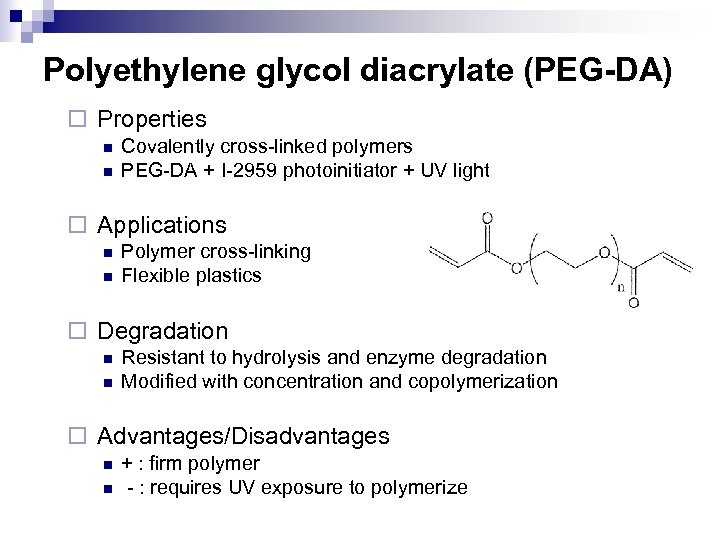 Polyethylene glycol diacrylate (PEG-DA) ¨ Properties n n Covalently cross-linked polymers PEG-DA + I-2959 photoinitiator + UV light ¨ Applications n n Polymer cross-linking Flexible plastics ¨ Degradation n n Resistant to hydrolysis and enzyme degradation Modified with concentration and copolymerization ¨ Advantages/Disadvantages n n + : firm polymer - : requires UV exposure to polymerize
Polyethylene glycol diacrylate (PEG-DA) ¨ Properties n n Covalently cross-linked polymers PEG-DA + I-2959 photoinitiator + UV light ¨ Applications n n Polymer cross-linking Flexible plastics ¨ Degradation n n Resistant to hydrolysis and enzyme degradation Modified with concentration and copolymerization ¨ Advantages/Disadvantages n n + : firm polymer - : requires UV exposure to polymerize
 Fibrin gel ¨ Properties n n Fibrinogen zymogen activated by thrombin Meshwork involved in blood clotting ¨ Applications n n Vascular sealant in surgery Tissue engineering of cartilage ¨ Degradation n Fibrinolysis ¨ Advantages/Disadvantages n n + : employs biological processes - : requires multiple polymerizing agents
Fibrin gel ¨ Properties n n Fibrinogen zymogen activated by thrombin Meshwork involved in blood clotting ¨ Applications n n Vascular sealant in surgery Tissue engineering of cartilage ¨ Degradation n Fibrinolysis ¨ Advantages/Disadvantages n n + : employs biological processes - : requires multiple polymerizing agents
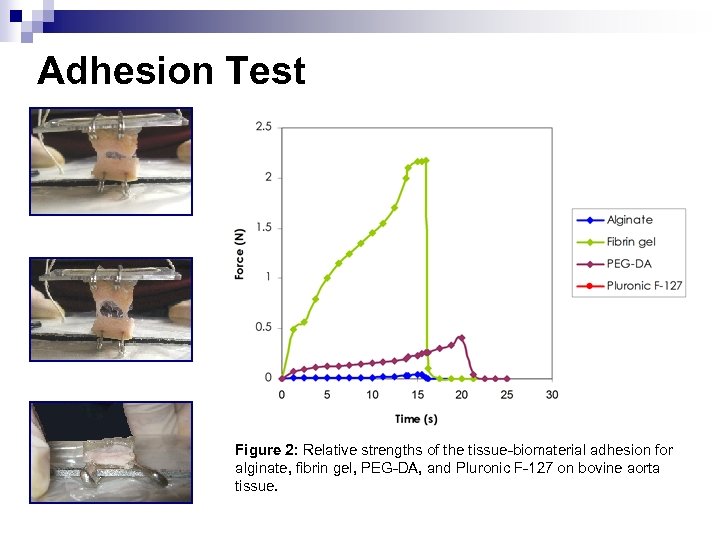 Adhesion Test Figure 2: Relative strengths of the tissue-biomaterial adhesion for alginate, fibrin gel, PEG-DA, and Pluronic F-127 on bovine aorta tissue.
Adhesion Test Figure 2: Relative strengths of the tissue-biomaterial adhesion for alginate, fibrin gel, PEG-DA, and Pluronic F-127 on bovine aorta tissue.
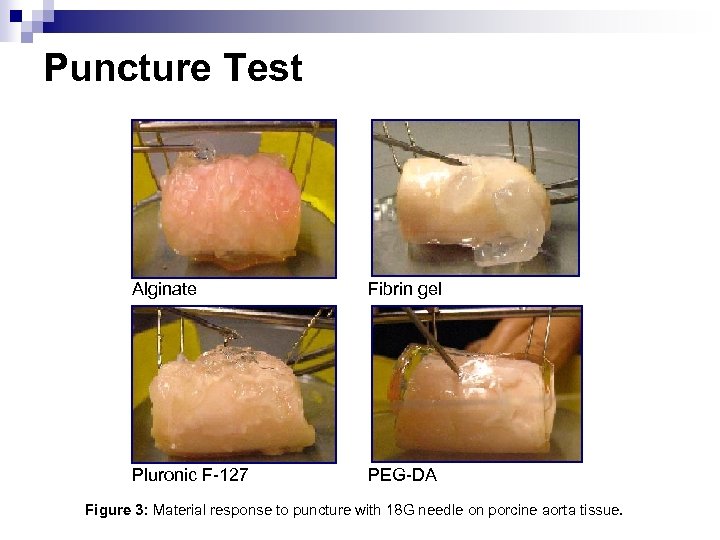 Puncture Test Alginate Fibrin gel Pluronic F-127 PEG-DA Figure 3: Material response to puncture with 18 G needle on porcine aorta tissue.
Puncture Test Alginate Fibrin gel Pluronic F-127 PEG-DA Figure 3: Material response to puncture with 18 G needle on porcine aorta tissue.
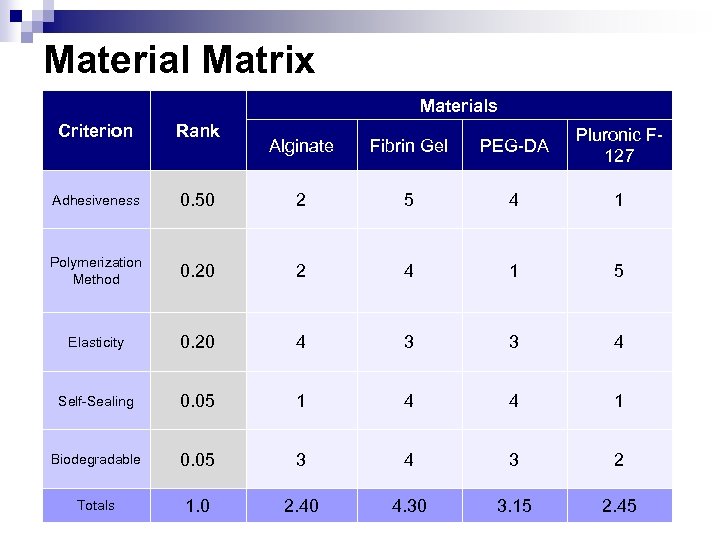 Material Matrix Materials Criterion Rank Adhesiveness Alginate Fibrin Gel PEG-DA Pluronic F 127 0. 50 2 5 4 1 Polymerization Method 0. 20 2 4 1 5 Elasticity 0. 20 4 3 3 4 Self-Sealing 0. 05 1 4 4 1 Biodegradable 0. 05 3 4 3 2 Totals 1. 0 2. 40 4. 30 3. 15 2. 45
Material Matrix Materials Criterion Rank Adhesiveness Alginate Fibrin Gel PEG-DA Pluronic F 127 0. 50 2 5 4 1 Polymerization Method 0. 20 2 4 1 5 Elasticity 0. 20 4 3 3 4 Self-Sealing 0. 05 1 4 4 1 Biodegradable 0. 05 3 4 3 2 Totals 1. 0 2. 40 4. 30 3. 15 2. 45
 Future Work n Optimize properties of fibrin gel n Test material in vitro Adhesion test ¨ Puncture test ¨ Perfusion chamber ¨ n Test material in vivo ¨ Pig model system
Future Work n Optimize properties of fibrin gel n Test material in vitro Adhesion test ¨ Puncture test ¨ Perfusion chamber ¨ n Test material in vivo ¨ Pig model system
 References Ching, A. L. , Liew, C. V. , Heng, P. W. S. and Chan, L. W. 2008. Impact of cross-linker on alginate matrix integrity and drug release. Int’l J. Pharmaceutics. Escobar-Chavez, J. J. , Lopez-Cervantez, M. , Maik, A. , Kalia, Y. N. , Quintanar-Guerrero, D. , Ganem-Quintanar, A. 2006. Applications of thermoreversible pluronic F-127 gels in pharmaceutical formulations. Journal of Pharmacy and Pharmaceutical Sciences. 9 (3): 339 -358. Eyrich, D. , Brandl, F. , Appel, B. , Wiese, H. , Maier, G. , Wenzel, Ml, Staudenmair, R. , Goepferich, A. , Blunk, T. 2007. Long-term stable fibrin gels for cartilage engineering. Biomaterials 28: 55 -65. Mao, Jeremy. 2006. Poly(ethylene glycol)-diacrylate crosslinked hydrogels comprising adipogenic mesenchymal stem cells. International Patent Application PCT/US 2005/023318. Ohta, S. , Nitta, N. , Takahasi, M. , Sonoda, A. , Tanaka, T. , Yamasaki, M. , Furukawa, A. , Takazakura, R. , Murata, K. , Sakamoto, T. , Kushibiki, T. , Tabata, Y. 2006. Pluronic F-127: application in arterial embolization. Journal of vascular and interventional radiology. 17 (3): 533 -539. Polyethylene glycol diacrylate. http: //chemicalland 21. com/industrialchem/functional%20 Monomer/POLYETHYLENE%20 GLYCOL%20 DI ACRYLATE. htm. <4 March 2008> Raymond, J. , Metcalfe, A. , Salazkin, I. , Schwarz, A. 2004. Temporary vascular occlusion with poloxamer 407. Biomaterials 25 (18): 3983 -3989.
References Ching, A. L. , Liew, C. V. , Heng, P. W. S. and Chan, L. W. 2008. Impact of cross-linker on alginate matrix integrity and drug release. Int’l J. Pharmaceutics. Escobar-Chavez, J. J. , Lopez-Cervantez, M. , Maik, A. , Kalia, Y. N. , Quintanar-Guerrero, D. , Ganem-Quintanar, A. 2006. Applications of thermoreversible pluronic F-127 gels in pharmaceutical formulations. Journal of Pharmacy and Pharmaceutical Sciences. 9 (3): 339 -358. Eyrich, D. , Brandl, F. , Appel, B. , Wiese, H. , Maier, G. , Wenzel, Ml, Staudenmair, R. , Goepferich, A. , Blunk, T. 2007. Long-term stable fibrin gels for cartilage engineering. Biomaterials 28: 55 -65. Mao, Jeremy. 2006. Poly(ethylene glycol)-diacrylate crosslinked hydrogels comprising adipogenic mesenchymal stem cells. International Patent Application PCT/US 2005/023318. Ohta, S. , Nitta, N. , Takahasi, M. , Sonoda, A. , Tanaka, T. , Yamasaki, M. , Furukawa, A. , Takazakura, R. , Murata, K. , Sakamoto, T. , Kushibiki, T. , Tabata, Y. 2006. Pluronic F-127: application in arterial embolization. Journal of vascular and interventional radiology. 17 (3): 533 -539. Polyethylene glycol diacrylate. http: //chemicalland 21. com/industrialchem/functional%20 Monomer/POLYETHYLENE%20 GLYCOL%20 DI ACRYLATE. htm. <4 March 2008> Raymond, J. , Metcalfe, A. , Salazkin, I. , Schwarz, A. 2004. Temporary vascular occlusion with poloxamer 407. Biomaterials 25 (18): 3983 -3989.


