81b1927b8f23a689ce7d0afbbfae8ea5.ppt
- Количество слайдов: 29
 Applying Computational Toxicology and Multicase (MCASE) Software to the FDA Mission Edwin J. Matthews, Ph. D. , Director Computational Toxicology Program Computational Toxicology Consultant Service Joseph F. Contrera, Ph. D. , Director Regulatory Research and Analysis Staff (RRAS) Disclaimer: This is not an official guidance or policy statement of the U. S. Food and Drug Administration (FDA) and Center for Drug Evaluation and Research (CDER) FDA/CDER/Office of Testing and Research (OTR) 1
Applying Computational Toxicology and Multicase (MCASE) Software to the FDA Mission Edwin J. Matthews, Ph. D. , Director Computational Toxicology Program Computational Toxicology Consultant Service Joseph F. Contrera, Ph. D. , Director Regulatory Research and Analysis Staff (RRAS) Disclaimer: This is not an official guidance or policy statement of the U. S. Food and Drug Administration (FDA) and Center for Drug Evaluation and Research (CDER) FDA/CDER/Office of Testing and Research (OTR) 1
 MISSION of OTR Programs to provide decision support to strengthen scientific basis of regulatory decisions Objectives are to provide: 4 A Reviewer Support Service 4 A Source of Scientific Information 4 An Institutional Memory 4 A Resource for Information Applications 4 A Vehicle for Regulatory and Applied Research 2
MISSION of OTR Programs to provide decision support to strengthen scientific basis of regulatory decisions Objectives are to provide: 4 A Reviewer Support Service 4 A Source of Scientific Information 4 An Institutional Memory 4 A Resource for Information Applications 4 A Vehicle for Regulatory and Applied Research 2
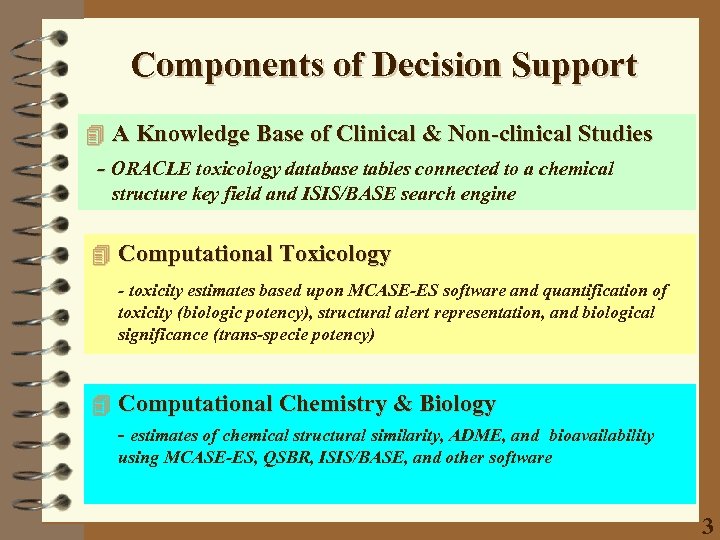 Components of Decision Support 4 A Knowledge Base of Clinical & Non-clinical Studies - ORACLE toxicology database tables connected to a chemical structure key field and ISIS/BASE search engine 4 Computational Toxicology - toxicity estimates based upon MCASE-ES software and quantification of toxicity (biologic potency), structural alert representation, and biological significance (trans-specie potency) 4 Computational Chemistry & Biology - estimates of chemical structural similarity, ADME, and bioavailability using MCASE-ES, QSBR, ISIS/BASE, and other software 3
Components of Decision Support 4 A Knowledge Base of Clinical & Non-clinical Studies - ORACLE toxicology database tables connected to a chemical structure key field and ISIS/BASE search engine 4 Computational Toxicology - toxicity estimates based upon MCASE-ES software and quantification of toxicity (biologic potency), structural alert representation, and biological significance (trans-specie potency) 4 Computational Chemistry & Biology - estimates of chemical structural similarity, ADME, and bioavailability using MCASE-ES, QSBR, ISIS/BASE, and other software 3
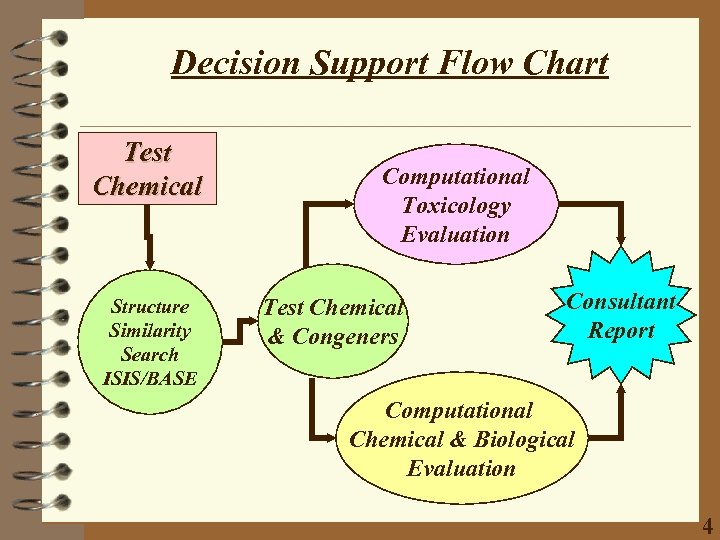 Decision Support Flow Chart Test Chemical Structure Similarity Search ISIS/BASE Computational Toxicology Evaluation Test Chemical & Congeners Consultant Report Computational Chemical & Biological Evaluation 4
Decision Support Flow Chart Test Chemical Structure Similarity Search ISIS/BASE Computational Toxicology Evaluation Test Chemical & Congeners Consultant Report Computational Chemical & Biological Evaluation 4
 Ultimate Goals of OTR Programs 4 A new IND Therapeutic MOL-structure file(s) is entered in the Center’s Substance Inventory 4 Structurally Similarity Chemicals are Identified 4 Computational Toxicology Analyses are Performed 4 Computational Chemical & Biological Analyses are Conducted 4 Data is made available to Center Scientists at the time the IND is Assigned & Reviewed via CDER-net 4
Ultimate Goals of OTR Programs 4 A new IND Therapeutic MOL-structure file(s) is entered in the Center’s Substance Inventory 4 Structurally Similarity Chemicals are Identified 4 Computational Toxicology Analyses are Performed 4 Computational Chemical & Biological Analyses are Conducted 4 Data is made available to Center Scientists at the time the IND is Assigned & Reviewed via CDER-net 4
 ARCHITECTURE of a Centralized Client (CDER Reviewer) Support Service 4 Consistent Decision Support - using standardized study & endpoint evaluation criteria 4 Easy Access - using web-base service (CDER-net) and simple on screen request forms 4 Rapid Response (2 -3 weeks) 4 Limited Requirements - requires only chemical structures - requires NO new software to learn! 6
ARCHITECTURE of a Centralized Client (CDER Reviewer) Support Service 4 Consistent Decision Support - using standardized study & endpoint evaluation criteria 4 Easy Access - using web-base service (CDER-net) and simple on screen request forms 4 Rapid Response (2 -3 weeks) 4 Limited Requirements - requires only chemical structures - requires NO new software to learn! 6
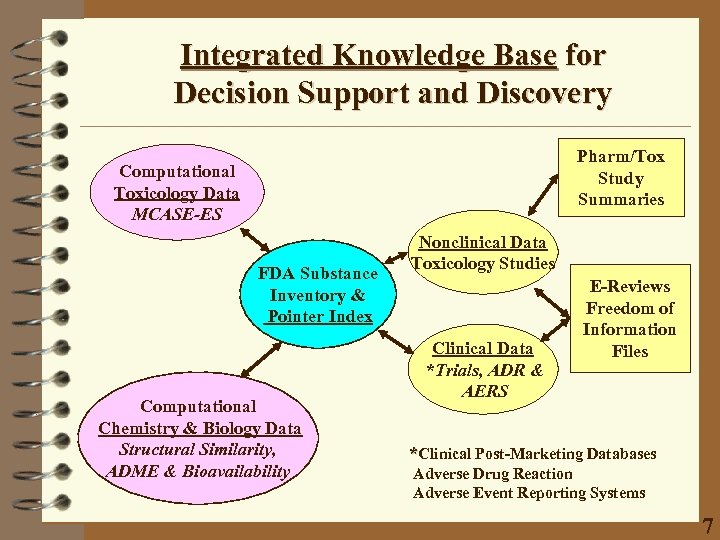 Integrated Knowledge Base for Decision Support and Discovery Pharm/Tox Study Summaries Computational Toxicology Data MCASE-ES FDA Substance Inventory & Pointer Index Computational Chemistry & Biology Data Structural Similarity, ADME & Bioavailability Nonclinical Data Toxicology Studies Clinical Data *Trials, ADR & AERS E-Reviews Freedom of Information Files *Clinical Post-Marketing Databases Adverse Drug Reaction Adverse Event Reporting Systems 7
Integrated Knowledge Base for Decision Support and Discovery Pharm/Tox Study Summaries Computational Toxicology Data MCASE-ES FDA Substance Inventory & Pointer Index Computational Chemistry & Biology Data Structural Similarity, ADME & Bioavailability Nonclinical Data Toxicology Studies Clinical Data *Trials, ADR & AERS E-Reviews Freedom of Information Files *Clinical Post-Marketing Databases Adverse Drug Reaction Adverse Event Reporting Systems 7
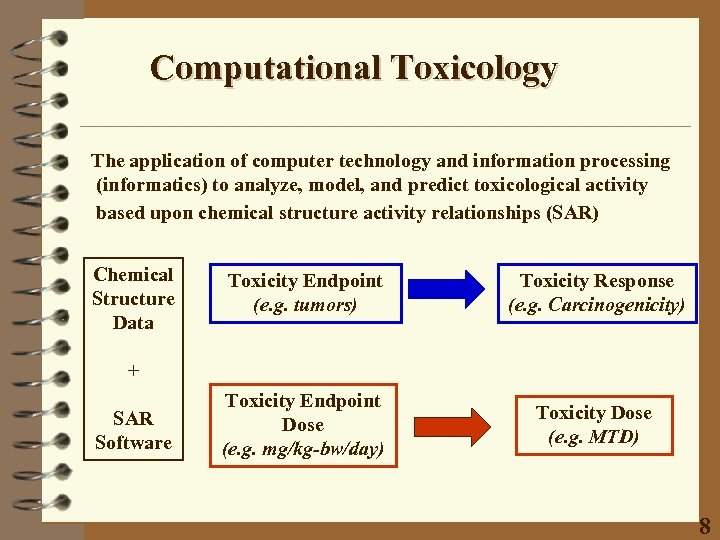 Computational Toxicology The application of computer technology and information processing (informatics) to analyze, model, and predict toxicological activity based upon chemical structure activity relationships (SAR) Chemical Structure Data Toxicity Endpoint (e. g. tumors) Toxicity Response (e. g. Carcinogenicity) Toxicity Endpoint Dose (e. g. mg/kg-bw/day) Toxicity Dose (e. g. MTD) + SAR Software 8
Computational Toxicology The application of computer technology and information processing (informatics) to analyze, model, and predict toxicological activity based upon chemical structure activity relationships (SAR) Chemical Structure Data Toxicity Endpoint (e. g. tumors) Toxicity Response (e. g. Carcinogenicity) Toxicity Endpoint Dose (e. g. mg/kg-bw/day) Toxicity Dose (e. g. MTD) + SAR Software 8
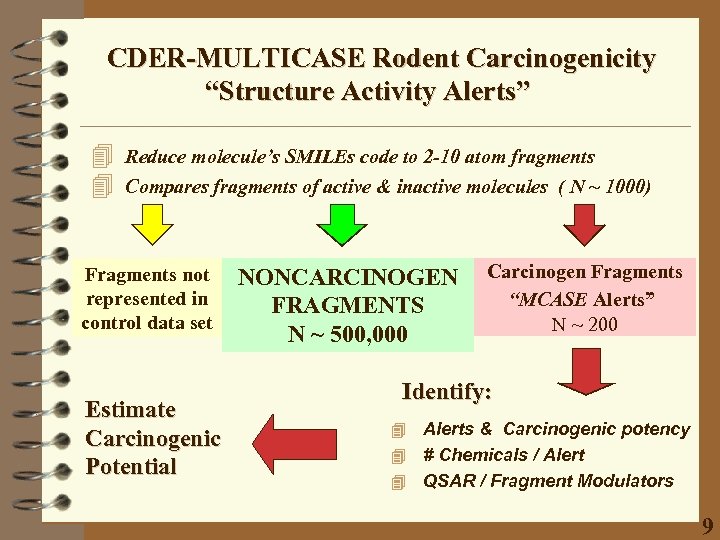 CDER-MULTICASE Rodent Carcinogenicity “Structure Activity Alerts” 4 4 Reduce molecule’s SMILEs code to 2 -10 atom fragments Compares fragments of active & inactive molecules ( N ~ 1000) Fragments not represented in control data set Estimate Carcinogenic Potential NONCARCINOGEN FRAGMENTS N ~ 500, 000 Carcinogen Fragments “MCASE Alerts” N ~ 200 Identify: Alerts & Carcinogenic potency 4 # Chemicals / Alert 4 QSAR / Fragment Modulators 4 9
CDER-MULTICASE Rodent Carcinogenicity “Structure Activity Alerts” 4 4 Reduce molecule’s SMILEs code to 2 -10 atom fragments Compares fragments of active & inactive molecules ( N ~ 1000) Fragments not represented in control data set Estimate Carcinogenic Potential NONCARCINOGEN FRAGMENTS N ~ 500, 000 Carcinogen Fragments “MCASE Alerts” N ~ 200 Identify: Alerts & Carcinogenic potency 4 # Chemicals / Alert 4 QSAR / Fragment Modulators 4 9
 SUCCESS! New: FDA MCASE: ES Software developed under FDA and Multicase, Inc. CRADA (1997 -2002) Old: MCASE / CASETOX / CASE Software developed at Case Western Reserve University (~1985 -1997) MCASE QSAR ES CRADA multiple computer automated structure evaluation quantitative structure activity relationship (human) expert system cooperative research and development agreement 10
SUCCESS! New: FDA MCASE: ES Software developed under FDA and Multicase, Inc. CRADA (1997 -2002) Old: MCASE / CASETOX / CASE Software developed at Case Western Reserve University (~1985 -1997) MCASE QSAR ES CRADA multiple computer automated structure evaluation quantitative structure activity relationship (human) expert system cooperative research and development agreement 10
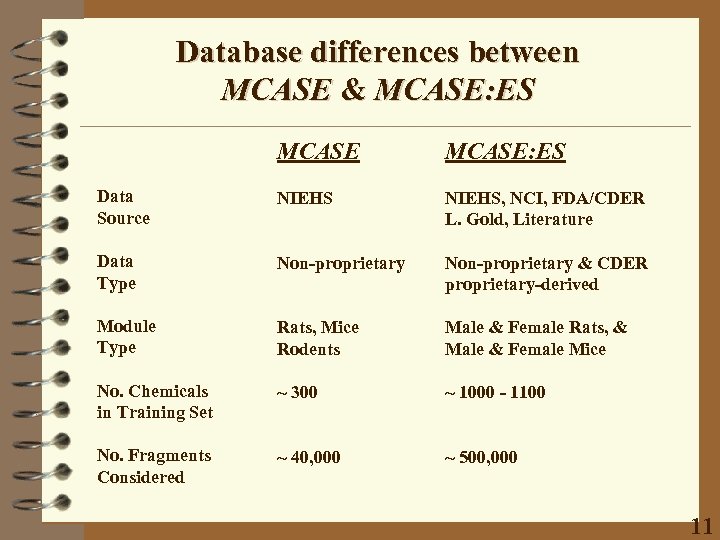 Database differences between MCASE & MCASE: ES Data Source NIEHS, NCI, FDA/CDER L. Gold, Literature Data Type Non-proprietary & CDER proprietary-derived Module Type Rats, Mice Rodents Male & Female Rats, & Male & Female Mice No. Chemicals in Training Set ~ 300 ~ 1000 - 1100 No. Fragments Considered ~ 40, 000 ~ 500, 000 11
Database differences between MCASE & MCASE: ES Data Source NIEHS, NCI, FDA/CDER L. Gold, Literature Data Type Non-proprietary & CDER proprietary-derived Module Type Rats, Mice Rodents Male & Female Rats, & Male & Female Mice No. Chemicals in Training Set ~ 300 ~ 1000 - 1100 No. Fragments Considered ~ 40, 000 ~ 500, 000 11
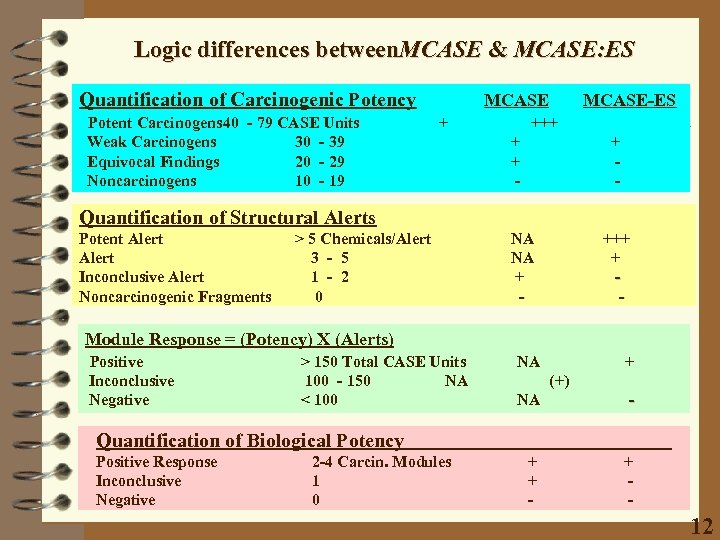 Logic differences between. MCASE & MCASE: ES Quantification of Carcinogenic Potency Potent Carcinogens 40 - 79 CASE Units Weak Carcinogens 30 - 39 Equivocal Findings 20 - 29 Noncarcinogens 10 - 19 MCASE + MCASE-ES +++ + + - Quantification of Structural Alerts Potent Alert Inconclusive Alert Noncarcinogenic Fragments > 5 Chemicals/Alert 3 - 5 1 - 2 0 NA NA + - +++ + - Module Response = (Potency) X (Alerts) Positive Inconclusive Negative > 150 Total CASE Units 100 - 150 NA < 100 NA + (+) NA - + + - Quantification of Biological Potency Positive Response Inconclusive Negative 2 -4 Carcin. Modules 1 0 12
Logic differences between. MCASE & MCASE: ES Quantification of Carcinogenic Potency Potent Carcinogens 40 - 79 CASE Units Weak Carcinogens 30 - 39 Equivocal Findings 20 - 29 Noncarcinogens 10 - 19 MCASE + MCASE-ES +++ + + - Quantification of Structural Alerts Potent Alert Inconclusive Alert Noncarcinogenic Fragments > 5 Chemicals/Alert 3 - 5 1 - 2 0 NA NA + - +++ + - Module Response = (Potency) X (Alerts) Positive Inconclusive Negative > 150 Total CASE Units 100 - 150 NA < 100 NA + (+) NA - + + - Quantification of Biological Potency Positive Response Inconclusive Negative 2 -4 Carcin. Modules 1 0 12
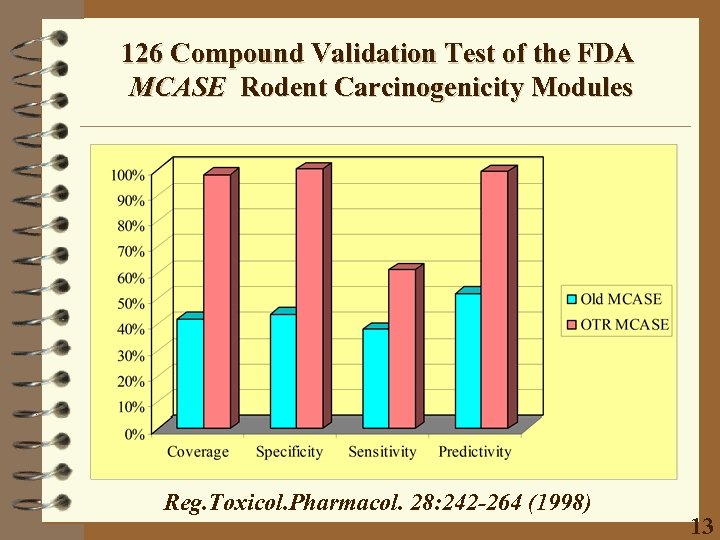 126 Compound Validation Test of the FDA MCASE Rodent Carcinogenicity Modules Reg. Toxicol. Pharmacol. 28: 242 -264 (1998) 13
126 Compound Validation Test of the FDA MCASE Rodent Carcinogenicity Modules Reg. Toxicol. Pharmacol. 28: 242 -264 (1998) 13
 RISK IDENTIFICATION: Advantages when MCASE-ES is optimized for High Specificity & High Predictive Value False Positives False Negatives 4 MCASE-ES false negatives are correctable {enhancement of data set improves software sensitivity} 4 MCASE-ES predictions often reflect known mechanisms and are defensible {studies from knowledge base support conclusions} 4 MCASE-ES predictions provide new insights 4 Program is optimal for lead chemical selection and is possible alternative for In Vitro/In Vivo studies 14
RISK IDENTIFICATION: Advantages when MCASE-ES is optimized for High Specificity & High Predictive Value False Positives False Negatives 4 MCASE-ES false negatives are correctable {enhancement of data set improves software sensitivity} 4 MCASE-ES predictions often reflect known mechanisms and are defensible {studies from knowledge base support conclusions} 4 MCASE-ES predictions provide new insights 4 Program is optimal for lead chemical selection and is possible alternative for In Vitro/In Vivo studies 14
 RISK MANAGEMENT Disadvantages when MCASE-ES is optimized for High Sensitivity False Positives False Negatives 4 MCASE-ES false positives are not correctable {model is flawed; whimsical predictions of chemical toxicity} 4 MCASE-ES predictions are not defensible and usually do not reflect known mechanisms {increased probability of controversy; knowledge base studies do not support conclusions} 4 Predictions do not provide insights to unknown 4 Program is not useful for lead chemical selection or as a possible substitute for animal studies 15
RISK MANAGEMENT Disadvantages when MCASE-ES is optimized for High Sensitivity False Positives False Negatives 4 MCASE-ES false positives are not correctable {model is flawed; whimsical predictions of chemical toxicity} 4 MCASE-ES predictions are not defensible and usually do not reflect known mechanisms {increased probability of controversy; knowledge base studies do not support conclusions} 4 Predictions do not provide insights to unknown 4 Program is not useful for lead chemical selection or as a possible substitute for animal studies 15
 Supportive Citations 4 MULTICASE SOFTWARE: A new highly specific method for predicting the carcinogenic potential of pharmaceuticals in rodents using enhanced MCASE QSAR-ES software. Edwin Matthews and Joseph Contrera (1998) Reg. Toxicol. Pharmacol. 28: 242 -264 4 CASE SOFTWARE: CASE-SAR Analysis of polycyclic aromatic hydrocarbon carcinogenicity. Ann Richard and Yin-tak Woo. (1990) Mutat. Res. 242: 285 -303. 4 TOXICOLOGIC POTENCY: Stratification of carcinogenicity bioassay results to reflect relative human hazard. Raymond Tennant. Mutat. Res. 286: 111 -118. 4 VALIDATION CRITERIA: Describing the validity of carcinogen screening tests. J. A. Cooper, R. Saracci, & P. Cole (1979) Br. J. Cancer 39: 87 -89 16
Supportive Citations 4 MULTICASE SOFTWARE: A new highly specific method for predicting the carcinogenic potential of pharmaceuticals in rodents using enhanced MCASE QSAR-ES software. Edwin Matthews and Joseph Contrera (1998) Reg. Toxicol. Pharmacol. 28: 242 -264 4 CASE SOFTWARE: CASE-SAR Analysis of polycyclic aromatic hydrocarbon carcinogenicity. Ann Richard and Yin-tak Woo. (1990) Mutat. Res. 242: 285 -303. 4 TOXICOLOGIC POTENCY: Stratification of carcinogenicity bioassay results to reflect relative human hazard. Raymond Tennant. Mutat. Res. 286: 111 -118. 4 VALIDATION CRITERIA: Describing the validity of carcinogen screening tests. J. A. Cooper, R. Saracci, & P. Cole (1979) Br. J. Cancer 39: 87 -89 16
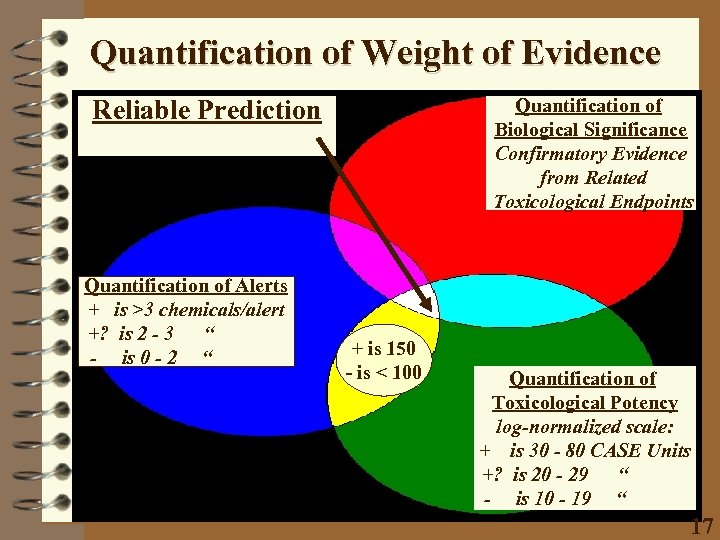 Quantification of Weight of Evidence Reliable Prediction Quantification of Alerts + is >3 chemicals/alert +? is 2 - 3 “ - is 0 - 2 “ Quantification of Biological Significance Confirmatory Evidence from Related Toxicological Endpoints + is 150 - is < 100 Quantification of Toxicological Potency log-normalized scale: + is 30 - 80 CASE Units +? is 20 - 29 “ - is 10 - 19 “ 17
Quantification of Weight of Evidence Reliable Prediction Quantification of Alerts + is >3 chemicals/alert +? is 2 - 3 “ - is 0 - 2 “ Quantification of Biological Significance Confirmatory Evidence from Related Toxicological Endpoints + is 150 - is < 100 Quantification of Toxicological Potency log-normalized scale: + is 30 - 80 CASE Units +? is 20 - 29 “ - is 10 - 19 “ 17
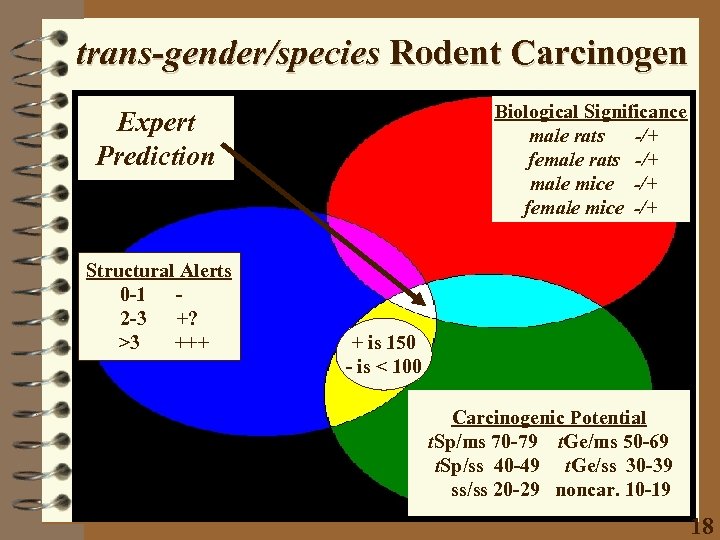 trans-gender/species Rodent Carcinogen Biological Significance male rats -/+ female rats -/+ male mice -/+ female mice -/+ Expert Prediction Structural Alerts 0 -1 2 -3 +? >3 +++ + is 150 - is < 100 Carcinogenic Potential t. Sp/ms 70 -79 t. Ge/ms 50 -69 t. Sp/ss 40 -49 t. Ge/ss 30 -39 ss/ss 20 -29 noncar. 10 -19 18
trans-gender/species Rodent Carcinogen Biological Significance male rats -/+ female rats -/+ male mice -/+ female mice -/+ Expert Prediction Structural Alerts 0 -1 2 -3 +? >3 +++ + is 150 - is < 100 Carcinogenic Potential t. Sp/ms 70 -79 t. Ge/ms 50 -69 t. Sp/ss 40 -49 t. Ge/ss 30 -39 ss/ss 20 -29 noncar. 10 -19 18
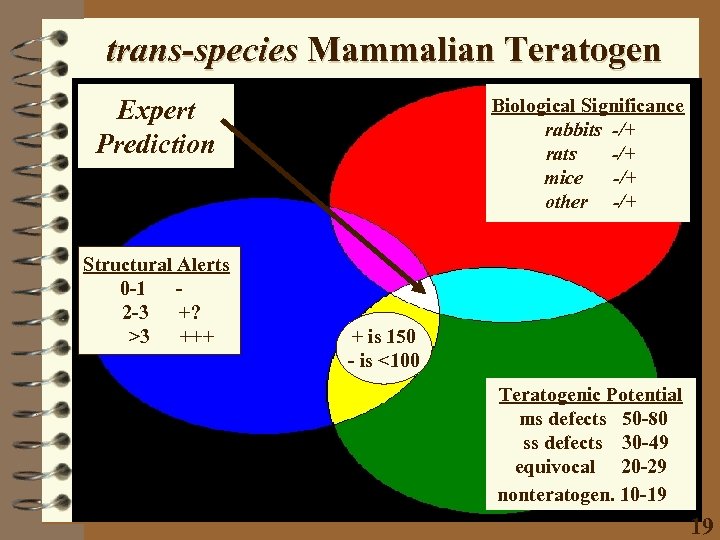 trans-species Mammalian Teratogen Expert Prediction Structural Alerts 0 -1 2 -3 +? >3 +++ Biological Significance rabbits -/+ rats -/+ mice -/+ other -/+ + is 150 - is <100 Teratogenic Potential ms defects 50 -80 ss defects 30 -49 equivocal 20 -29 nonteratogen. 10 -19 19
trans-species Mammalian Teratogen Expert Prediction Structural Alerts 0 -1 2 -3 +? >3 +++ Biological Significance rabbits -/+ rats -/+ mice -/+ other -/+ + is 150 - is <100 Teratogenic Potential ms defects 50 -80 ss defects 30 -49 equivocal 20 -29 nonteratogen. 10 -19 19
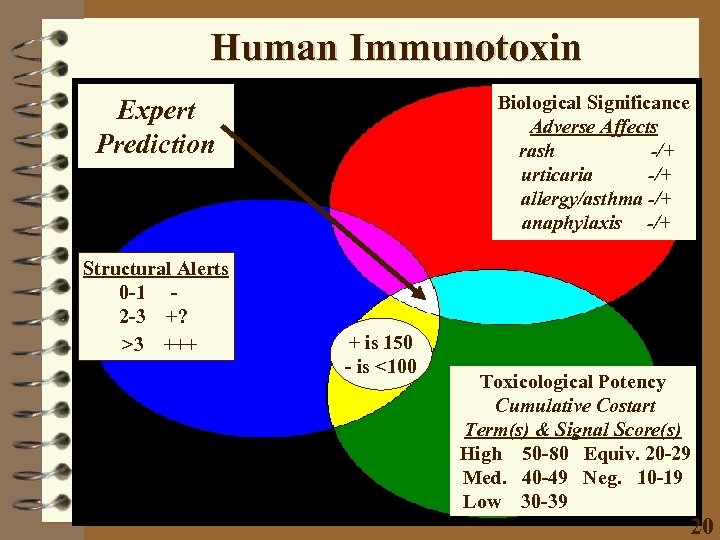 Human Immunotoxin Biological Significance Adverse Affects rash -/+ urticaria -/+ allergy/asthma -/+ anaphylaxis -/+ Expert Prediction Structural Alerts 0 -1 2 -3 +? >3 +++ + is 150 - is <100 Toxicological Potency Cumulative Costart Term(s) & Signal Score(s) High 50 -80 Equiv. 20 -29 Med. 40 -49 Neg. 10 -19 Low 30 -39 20
Human Immunotoxin Biological Significance Adverse Affects rash -/+ urticaria -/+ allergy/asthma -/+ anaphylaxis -/+ Expert Prediction Structural Alerts 0 -1 2 -3 +? >3 +++ + is 150 - is <100 Toxicological Potency Cumulative Costart Term(s) & Signal Score(s) High 50 -80 Equiv. 20 -29 Med. 40 -49 Neg. 10 -19 Low 30 -39 20
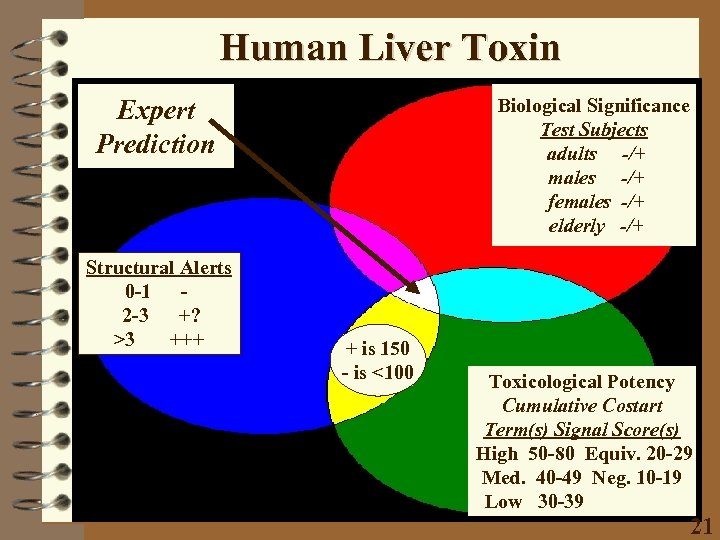 Human Liver Toxin Expert Prediction Structural Alerts 0 -1 2 -3 +? >3 +++ Biological Significance Test Subjects adults -/+ males -/+ females -/+ elderly -/+ + is 150 - is <100 Toxicological Potency Cumulative Costart Term(s) Signal Score(s) High 50 -80 Equiv. 20 -29 Med. 40 -49 Neg. 10 -19 Low 30 -39 21
Human Liver Toxin Expert Prediction Structural Alerts 0 -1 2 -3 +? >3 +++ Biological Significance Test Subjects adults -/+ males -/+ females -/+ elderly -/+ + is 150 - is <100 Toxicological Potency Cumulative Costart Term(s) Signal Score(s) High 50 -80 Equiv. 20 -29 Med. 40 -49 Neg. 10 -19 Low 30 -39 21
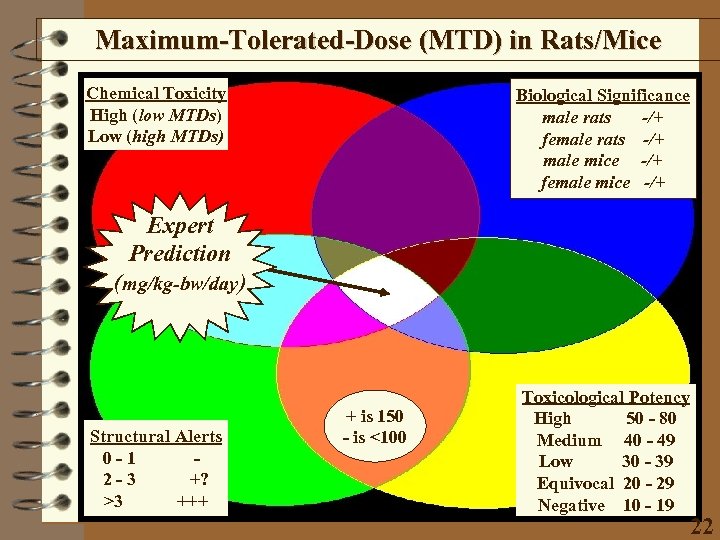 Maximum-Tolerated-Dose (MTD) in Rats/Mice Chemical Toxicity High (low MTDs) Low (high MTDs) Biological Significance male rats -/+ female rats -/+ male mice -/+ female mice -/+ Expert Prediction (mg/kg-bw/day) Structural Alerts 0 -1 2 -3 +? >3 +++ + is 150 - is <100 Toxicological Potency High 50 - 80 Medium 40 - 49 Low 30 - 39 Equivocal 20 - 29 Negative 10 - 19 22
Maximum-Tolerated-Dose (MTD) in Rats/Mice Chemical Toxicity High (low MTDs) Low (high MTDs) Biological Significance male rats -/+ female rats -/+ male mice -/+ female mice -/+ Expert Prediction (mg/kg-bw/day) Structural Alerts 0 -1 2 -3 +? >3 +++ + is 150 - is <100 Toxicological Potency High 50 - 80 Medium 40 - 49 Low 30 - 39 Equivocal 20 - 29 Negative 10 - 19 22
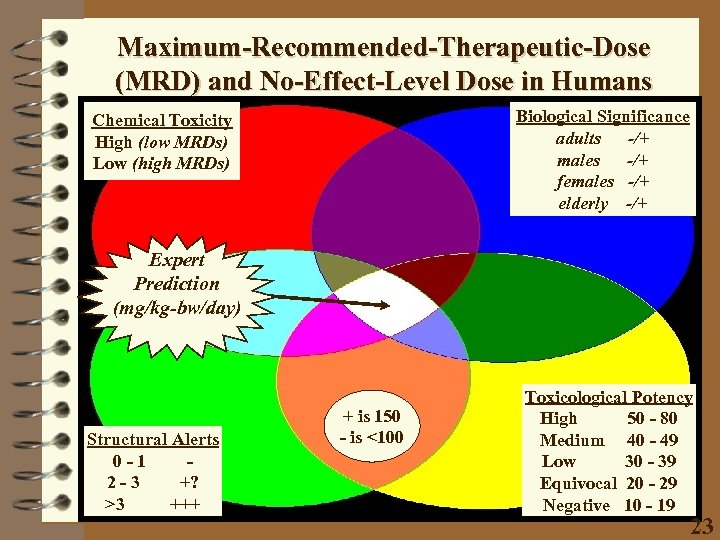 Maximum-Recommended-Therapeutic-Dose (MRD) and No-Effect-Level Dose in Humans Biological Significance adults -/+ males -/+ females -/+ elderly -/+ Chemical Toxicity High (low MRDs) Low (high MRDs) Expert Prediction (mg/kg-bw/day) Structural Alerts 0 -1 2 -3 +? >3 +++ + is 150 - is <100 Toxicological Potency High 50 - 80 Medium 40 - 49 Low 30 - 39 Equivocal 20 - 29 Negative 10 - 19 23
Maximum-Recommended-Therapeutic-Dose (MRD) and No-Effect-Level Dose in Humans Biological Significance adults -/+ males -/+ females -/+ elderly -/+ Chemical Toxicity High (low MRDs) Low (high MRDs) Expert Prediction (mg/kg-bw/day) Structural Alerts 0 -1 2 -3 +? >3 +++ + is 150 - is <100 Toxicological Potency High 50 - 80 Medium 40 - 49 Low 30 - 39 Equivocal 20 - 29 Negative 10 - 19 23
 Multicase Limitations 4 Non-organics (salts, metals) 4 Polymers (fibers, proteins, polysaccharides; however, <5000 mw substructures OK) 4 Organometallics (- metal OK) 4 Certain Organic Chemicals Mixtures, but individual components OK 2 or more unknown fragments, but <2 OK small molecules 1 -7 atoms, excluding H 25
Multicase Limitations 4 Non-organics (salts, metals) 4 Polymers (fibers, proteins, polysaccharides; however, <5000 mw substructures OK) 4 Organometallics (- metal OK) 4 Certain Organic Chemicals Mixtures, but individual components OK 2 or more unknown fragments, but <2 OK small molecules 1 -7 atoms, excluding H 25
 Pre-Market Applications for Computational Toxicology at CDER “when toxicology data is limited or absent!” 4 Potential Hazard(s) of Contaminants and Degradents in IND and NDA Therapeutics 4 Potential Hazard(s) of Excipients, Additives, and New Contaminants in Generic Therapeutics 4 Toxicological Profile of Newly Submitted Therapeutics Integrated knowledge base(OTR Programs); Proposed to support entry of women of child bearing potential into phase I clinical trials (FDA/Office of Women’s Health) 26
Pre-Market Applications for Computational Toxicology at CDER “when toxicology data is limited or absent!” 4 Potential Hazard(s) of Contaminants and Degradents in IND and NDA Therapeutics 4 Potential Hazard(s) of Excipients, Additives, and New Contaminants in Generic Therapeutics 4 Toxicological Profile of Newly Submitted Therapeutics Integrated knowledge base(OTR Programs); Proposed to support entry of women of child bearing potential into phase I clinical trials (FDA/Office of Women’s Health) 26
 Pre-Market Applications for Computational Toxicology at FDA “ when toxicology data is limited or absent!” 4 Potential Hazard(s) of Food Contact Substances {CFSAN/OPA (FDAMA, 1997; Dr. Cheeseman); FDA/Office of the Commissioner, Office of Science)} 4 Potential Hazard(s) of Lead Pharmaceuticals {IAG with National Institute for Drug Abuse, NIH: Drug Discovery Program for Medications Development for Addiction Treatment } 4 Potential Hazard(s) of Non-pharmaceutical Substances with Pharmacologic Properties {e. g. , EPA, RTP, NC; ATSDR, Atlanta, GA} 27
Pre-Market Applications for Computational Toxicology at FDA “ when toxicology data is limited or absent!” 4 Potential Hazard(s) of Food Contact Substances {CFSAN/OPA (FDAMA, 1997; Dr. Cheeseman); FDA/Office of the Commissioner, Office of Science)} 4 Potential Hazard(s) of Lead Pharmaceuticals {IAG with National Institute for Drug Abuse, NIH: Drug Discovery Program for Medications Development for Addiction Treatment } 4 Potential Hazard(s) of Non-pharmaceutical Substances with Pharmacologic Properties {e. g. , EPA, RTP, NC; ATSDR, Atlanta, GA} 27
 Post-Market Applications for Computational Toxicology at FDA “when required toxicology data is limited or absent” 4 Potential Hazard(s) of Active Ingredients of Cosmetics {CFSAN/OCAC (Dr. Milstein); Offices of Commissioner, Science, & Women’s Health} 4 Potential Hazards of Therapeutics in Humans {Model data in CDER’s Adverse Drug Reaction (ADR) and Adverse Event Reporting System (AERS) databases; Dr. Szarfman, {CDER/OB/QMRS ; Drs. Hanig, Weaver (OTR} 4 Potential Hazards of Mixtures of Concern to FDA {Evaluation of components of dietary and nutritional supplements, flavors, herbs, spices, herbal medicines, etc. } 28
Post-Market Applications for Computational Toxicology at FDA “when required toxicology data is limited or absent” 4 Potential Hazard(s) of Active Ingredients of Cosmetics {CFSAN/OCAC (Dr. Milstein); Offices of Commissioner, Science, & Women’s Health} 4 Potential Hazards of Therapeutics in Humans {Model data in CDER’s Adverse Drug Reaction (ADR) and Adverse Event Reporting System (AERS) databases; Dr. Szarfman, {CDER/OB/QMRS ; Drs. Hanig, Weaver (OTR} 4 Potential Hazards of Mixtures of Concern to FDA {Evaluation of components of dietary and nutritional supplements, flavors, herbs, spices, herbal medicines, etc. } 28
 OTR Computational Toxicology and Toxicology Database Programs (2002) Non-clinical Endpoint Projects 4 Behavioral toxicity (rats) 4 Reproductive toxicity (male & female rats) 4 Genetic Toxicity {Salmonella t. Mutagenicity (Multicase, Inc. ); Chromosome aberrations; Mouse micronucleus; Mouse lymphoma, Cell transformation (BALB/c-3 T 3 & SHE)} 4 90 -Day Organ Toxicity (rats, mice, rabbits, dogs) 4 Acute Toxicity (rats, mice, rabbits) 29
OTR Computational Toxicology and Toxicology Database Programs (2002) Non-clinical Endpoint Projects 4 Behavioral toxicity (rats) 4 Reproductive toxicity (male & female rats) 4 Genetic Toxicity {Salmonella t. Mutagenicity (Multicase, Inc. ); Chromosome aberrations; Mouse micronucleus; Mouse lymphoma, Cell transformation (BALB/c-3 T 3 & SHE)} 4 90 -Day Organ Toxicity (rats, mice, rabbits, dogs) 4 Acute Toxicity (rats, mice, rabbits) 29
 OTR Computational Toxicology and Toxicology Database Programs (2002) Clinical Endpoint Projects 4 Neurotoxicity 4 Organ and organ system toxicities Computational Chemistry Projects 4 Metabolism {MTA with MDL (Elsevier) to add FDA/CDER drug metabolism data to ISIS/BASE: Metabolite} 4 ADME and Bioavailability {Dr. Saiakhov, Multicase, Inc. ; Dr. Yu, OTR; MTA with Camitro Corporation, Inc. }) 30
OTR Computational Toxicology and Toxicology Database Programs (2002) Clinical Endpoint Projects 4 Neurotoxicity 4 Organ and organ system toxicities Computational Chemistry Projects 4 Metabolism {MTA with MDL (Elsevier) to add FDA/CDER drug metabolism data to ISIS/BASE: Metabolite} 4 ADME and Bioavailability {Dr. Saiakhov, Multicase, Inc. ; Dr. Yu, OTR; MTA with Camitro Corporation, Inc. }) 30


