Part III_XRF_XRD_Version 2.pptx
- Количество слайдов: 21
 Applications of X-rays to Geology Part III. XRF and XRD analyses.
Applications of X-rays to Geology Part III. XRF and XRD analyses.
 Part III. XRF and XRD analyses. • X-ray fluorescence • Moseley's law • Sample preparation for XRF analyses • X-ray diffraction and X-ray crystallography • Wave dispersive XRF
Part III. XRF and XRD analyses. • X-ray fluorescence • Moseley's law • Sample preparation for XRF analyses • X-ray diffraction and X-ray crystallography • Wave dispersive XRF
 X-ray Fluorescence (XRF) X-ray fluorescence (XRF) is the emission of characteristic "secondary" (or fluorescent) Xrays from a material that has been excited by bombarding with high-energy X-rays. X-ray source X-ray detector Energy of characteristic x-rays Type of elements present (qualitative analysis) Number of x-rays for each element Concentration (quantitative analysis)
X-ray Fluorescence (XRF) X-ray fluorescence (XRF) is the emission of characteristic "secondary" (or fluorescent) Xrays from a material that has been excited by bombarding with high-energy X-rays. X-ray source X-ray detector Energy of characteristic x-rays Type of elements present (qualitative analysis) Number of x-rays for each element Concentration (quantitative analysis)
 What is Good About XRF? • There is no “bremsstrahlung“ • There is no vacuum required • Elemental analysis of solids and liquids • Multi-element capability • Non-destructive • Minimal preparation • Fast • Easy to use • Cost-effective
What is Good About XRF? • There is no “bremsstrahlung“ • There is no vacuum required • Elemental analysis of solids and liquids • Multi-element capability • Non-destructive • Minimal preparation • Fast • Easy to use • Cost-effective
 X-ray Fluorescence (XRF)
X-ray Fluorescence (XRF)
 Different types of XRF analysers Based on the excitation • Tube excited XRF • Radio-isotope excited XRF • Secondary target, • Synchrotron, • Total reflection. . . Based on the detection • Wavelength dispersive (WD-XRF) • Energy dispersive (ED-XRF) • Filter instruments (proportional counter) 15 000 XRF instruments in operation world wide
Different types of XRF analysers Based on the excitation • Tube excited XRF • Radio-isotope excited XRF • Secondary target, • Synchrotron, • Total reflection. . . Based on the detection • Wavelength dispersive (WD-XRF) • Energy dispersive (ED-XRF) • Filter instruments (proportional counter) 15 000 XRF instruments in operation world wide
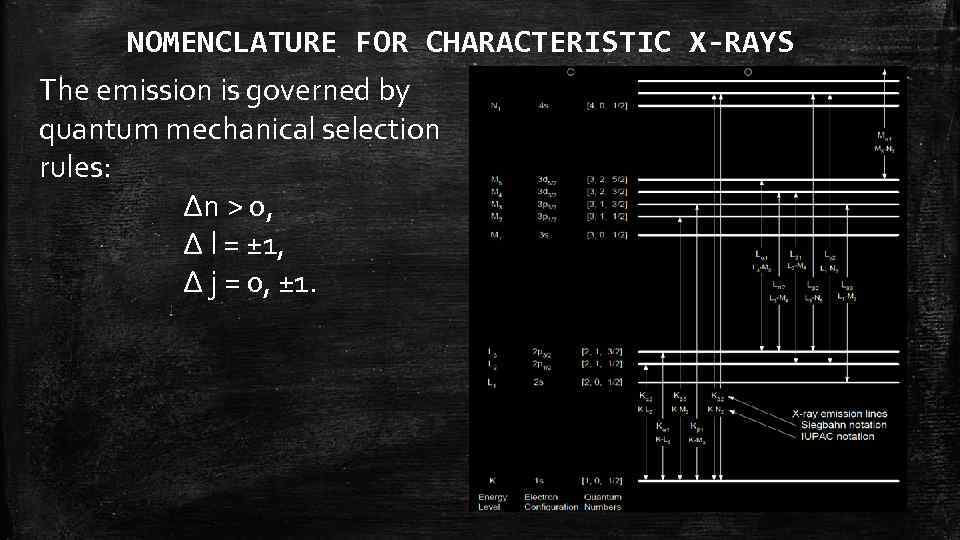 NOMENCLATURE FOR CHARACTERISTIC X-RAYS The emission is governed by quantum mechanical selection rules: Δn > 0, Δ l = ± 1, Δ j = 0, ± 1.
NOMENCLATURE FOR CHARACTERISTIC X-RAYS The emission is governed by quantum mechanical selection rules: Δn > 0, Δ l = ± 1, Δ j = 0, ± 1.
 Moseley’s law Moseley's law is an empirical law concerning the characteristic x -rays that are emitted by atoms:
Moseley’s law Moseley's law is an empirical law concerning the characteristic x -rays that are emitted by atoms:
 Moseley’s law The energy of the characteristic radiation within a given series of lines varies monotonically with atomic number.
Moseley’s law The energy of the characteristic radiation within a given series of lines varies monotonically with atomic number.
 X-ray Energies of Elements www. bruker. com/hhxrf
X-ray Energies of Elements www. bruker. com/hhxrf
 X-Ray Spectroscopy and Moseley’s Law : Required Knowledge • Basic atomic physics of inner shells, concepts and terms, characteristic numbers • Roentgen fluorescence yield, binding energy in the shell model of the atom, Auger electron emission • Production of X-rays • Bremsstrahlung • Characteristic X-rays, selection rules, K- and L- series • Absorption and scattering of X-rays
X-Ray Spectroscopy and Moseley’s Law : Required Knowledge • Basic atomic physics of inner shells, concepts and terms, characteristic numbers • Roentgen fluorescence yield, binding energy in the shell model of the atom, Auger electron emission • Production of X-rays • Bremsstrahlung • Characteristic X-rays, selection rules, K- and L- series • Absorption and scattering of X-rays
 X-ray Diffraction. . . Why “Phase” Matters 1. The atomic (chemical) composition is obtained from XRF 2. The same atoms may arrange in different geometries or crystal structures the resulting natural materials are called minerals or phases 3. The crystal structure determines the physical properties of a material. Therefore, minerals with the same/similar chemistry may differ in: • Hardness • Colour or spectral absorption of light • Durability or wear resistance • Solubility • Cleavability, grindability • many more. . . 4. Knowing the phases has immediate financial impact: • The economic value of a material • Costs of mining, transport, processability • Properties of a final product http: //www. bruker. com/fileadmin/user_upload/8 -PDF-Docs/X-ray. Diffraction_Elemental. Analysis/XRD/Webinars/Bruker_AXS_Industrial_Minerals_Webinar_Slides. pdf
X-ray Diffraction. . . Why “Phase” Matters 1. The atomic (chemical) composition is obtained from XRF 2. The same atoms may arrange in different geometries or crystal structures the resulting natural materials are called minerals or phases 3. The crystal structure determines the physical properties of a material. Therefore, minerals with the same/similar chemistry may differ in: • Hardness • Colour or spectral absorption of light • Durability or wear resistance • Solubility • Cleavability, grindability • many more. . . 4. Knowing the phases has immediate financial impact: • The economic value of a material • Costs of mining, transport, processability • Properties of a final product http: //www. bruker. com/fileadmin/user_upload/8 -PDF-Docs/X-ray. Diffraction_Elemental. Analysis/XRD/Webinars/Bruker_AXS_Industrial_Minerals_Webinar_Slides. pdf
 What is X-ray Diffraction? The Mineral “Fingerprint” http: //www. bruker. com/fileadmin/user_upload/8 -PDF-Docs/X-ray. Diffraction_Elemental. Analysis/XRD/Webinars/Bruker_AXS_Industrial_Minerals_Webinar_Slides. pdf
What is X-ray Diffraction? The Mineral “Fingerprint” http: //www. bruker. com/fileadmin/user_upload/8 -PDF-Docs/X-ray. Diffraction_Elemental. Analysis/XRD/Webinars/Bruker_AXS_Industrial_Minerals_Webinar_Slides. pdf
 What is X-ray Diffraction and How Does It Work? Bragg’s equation: λ = 2 d sin Θ • The x-ray wavelength is of the same order as the distances in the crystal lattice • For each possible d-spacing (distance between parallel sets of planes with atoms in a crystal lattice), a peak is observed at a distinct angle 2Θ. • The atoms and their positions in the unit cell determine the peak intensities. http: //www. bruker. com/fileadmin/user_upload/8 -PDF-Docs/X-ray. Diffraction_Elemental. Analysis/XRD/Webinars/Bruker_AXS_Industrial_Minerals_Webinar_Slides. pdf
What is X-ray Diffraction and How Does It Work? Bragg’s equation: λ = 2 d sin Θ • The x-ray wavelength is of the same order as the distances in the crystal lattice • For each possible d-spacing (distance between parallel sets of planes with atoms in a crystal lattice), a peak is observed at a distinct angle 2Θ. • The atoms and their positions in the unit cell determine the peak intensities. http: //www. bruker. com/fileadmin/user_upload/8 -PDF-Docs/X-ray. Diffraction_Elemental. Analysis/XRD/Webinars/Bruker_AXS_Industrial_Minerals_Webinar_Slides. pdf
 What is X-ray Diffraction and How Does It Work? Bragg’s equation: nλ = 2 d sin Θ • The x-ray wavelength is of the same order as the distances in the crystal lattice • For each possible d-spacing (distance between parallel sets of planes with atoms in a crystal lattice), a peak is observed at a distinct angle 2Θ. • The atoms and their positions in the unit cell determine the peak intensities. http: //www. bruker. com/fileadmin/user_upload/8 -PDF-Docs/X-ray. Diffraction_Elemental. Analysis/XRD/Webinars/Bruker_AXS_Industrial_Minerals_Webinar_Slides. pdf
What is X-ray Diffraction and How Does It Work? Bragg’s equation: nλ = 2 d sin Θ • The x-ray wavelength is of the same order as the distances in the crystal lattice • For each possible d-spacing (distance between parallel sets of planes with atoms in a crystal lattice), a peak is observed at a distinct angle 2Θ. • The atoms and their positions in the unit cell determine the peak intensities. http: //www. bruker. com/fileadmin/user_upload/8 -PDF-Docs/X-ray. Diffraction_Elemental. Analysis/XRD/Webinars/Bruker_AXS_Industrial_Minerals_Webinar_Slides. pdf
 Bragg’s law and minerals identification Let λ is known value, then di-spacings for a crystal can be calculated from measured angles Θi:
Bragg’s law and minerals identification Let λ is known value, then di-spacings for a crystal can be calculated from measured angles Θi:
 Bragg’s law and XRD application Let d is known value, then wave lengths λi in incident X-ray spectrum can be calculated from measured angles Θi: d = Const
Bragg’s law and XRD application Let d is known value, then wave lengths λi in incident X-ray spectrum can be calculated from measured angles Θi: d = Const
 Bragg’s law and Wave dispersion spectroscopy Let d is known value, then wave lengths λi in incident X-ray spectrum can be calculated from measured angles Θi: d = Const WD spectrum of a geological material between 0. 69 and 0. 98 Å
Bragg’s law and Wave dispersion spectroscopy Let d is known value, then wave lengths λi in incident X-ray spectrum can be calculated from measured angles Θi: d = Const WD spectrum of a geological material between 0. 69 and 0. 98 Å
 XRF instruments: EDS and WDS Compact EDS instruments S 2 RANGER QUANTAX for microscopes WDS instruments S 8 LION S 2 PICOFOX TXRF S 1 TRACER turbo-SD S 8 TIGER
XRF instruments: EDS and WDS Compact EDS instruments S 2 RANGER QUANTAX for microscopes WDS instruments S 8 LION S 2 PICOFOX TXRF S 1 TRACER turbo-SD S 8 TIGER
 Multichannel XRF instruments
Multichannel XRF instruments
 Summary Practical Uses • XRF is used in the commercial production of metals, glasses, ceramics, and building materials • Utilized in research fields such as geochemistry!, archaeology, construction, forensic science, environmental studies, the petroleum industry, and mining • XRD is the most applied technique for routine mineral identification.
Summary Practical Uses • XRF is used in the commercial production of metals, glasses, ceramics, and building materials • Utilized in research fields such as geochemistry!, archaeology, construction, forensic science, environmental studies, the petroleum industry, and mining • XRD is the most applied technique for routine mineral identification.


