b97ad6531010c9824f52109b4dd47497.ppt
- Количество слайдов: 55
 Applications of Muon Spin Spectroscopy in Chemistry Paul Percival TRIUMF and Department of Chemistry, Simon Fraser University
Applications of Muon Spin Spectroscopy in Chemistry Paul Percival TRIUMF and Department of Chemistry, Simon Fraser University
 µSR in Chemistry ― Overview q Is there any demand? q Is chemistry any different to physics? q Are the needs of chemists different from physicists? q Why do chemists prefer a continuous source? q Why do chemists prefer a pulsed source? q Why do chemists feel misunderstood/unwelcome? Paul Percival October 2012
µSR in Chemistry ― Overview q Is there any demand? q Is chemistry any different to physics? q Are the needs of chemists different from physicists? q Why do chemists prefer a continuous source? q Why do chemists prefer a pulsed source? q Why do chemists feel misunderstood/unwelcome? Paul Percival October 2012
 µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø the muon as part of the problem q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø the muon as part of the problem q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
 µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø the muon as part of the problem q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø the muon as part of the problem q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
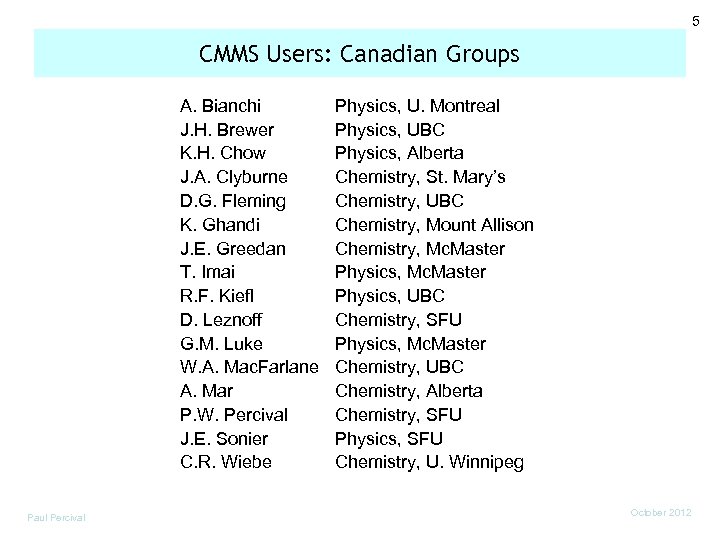 5 CMMS Users: Canadian Groups A. Bianchi J. H. Brewer K. H. Chow J. A. Clyburne D. G. Fleming K. Ghandi J. E. Greedan T. Imai R. F. Kiefl D. Leznoff G. M. Luke W. A. Mac. Farlane A. Mar P. W. Percival J. E. Sonier C. R. Wiebe Paul Percival Physics, U. Montreal Physics, UBC Physics, Alberta Chemistry, St. Mary’s Chemistry, UBC Chemistry, Mount Allison Chemistry, Mc. Master Physics, UBC Chemistry, SFU Physics, Mc. Master Chemistry, UBC Chemistry, Alberta Chemistry, SFU Physics, SFU Chemistry, U. Winnipeg October 2012
5 CMMS Users: Canadian Groups A. Bianchi J. H. Brewer K. H. Chow J. A. Clyburne D. G. Fleming K. Ghandi J. E. Greedan T. Imai R. F. Kiefl D. Leznoff G. M. Luke W. A. Mac. Farlane A. Mar P. W. Percival J. E. Sonier C. R. Wiebe Paul Percival Physics, U. Montreal Physics, UBC Physics, Alberta Chemistry, St. Mary’s Chemistry, UBC Chemistry, Mount Allison Chemistry, Mc. Master Physics, UBC Chemistry, SFU Physics, Mc. Master Chemistry, UBC Chemistry, Alberta Chemistry, SFU Physics, SFU Chemistry, U. Winnipeg October 2012
 6 Canadian Major Users completely reliant on CMMS A. Bianchi J. H. Brewer K. H. Chow J. A. Clyburne D. G. Fleming K. Ghandi J. E. Greedan T. Imai R. F. Kiefl D. Leznoff G. M. Luke W. A. Mac. Farlane A. Mar P. W. Percival J. E. Sonier C. R. Wiebe Paul Percival Physics, U. Montreal Physics, UBC Physics, Alberta Chemistry, St. Mary’s Chemistry, UBC Chemistry, Mount Allison Chemistry, Mc. Master Physics, UBC Chemistry, SFU Physics, Mc. Master Chemistry, UBC Chemistry, Alberta Chemistry, SFU Physics, SFU Chemistry, U. Winnipeg October 2012
6 Canadian Major Users completely reliant on CMMS A. Bianchi J. H. Brewer K. H. Chow J. A. Clyburne D. G. Fleming K. Ghandi J. E. Greedan T. Imai R. F. Kiefl D. Leznoff G. M. Luke W. A. Mac. Farlane A. Mar P. W. Percival J. E. Sonier C. R. Wiebe Paul Percival Physics, U. Montreal Physics, UBC Physics, Alberta Chemistry, St. Mary’s Chemistry, UBC Chemistry, Mount Allison Chemistry, Mc. Master Physics, UBC Chemistry, SFU Physics, Mc. Master Chemistry, UBC Chemistry, Alberta Chemistry, SFU Physics, SFU Chemistry, U. Winnipeg October 2012
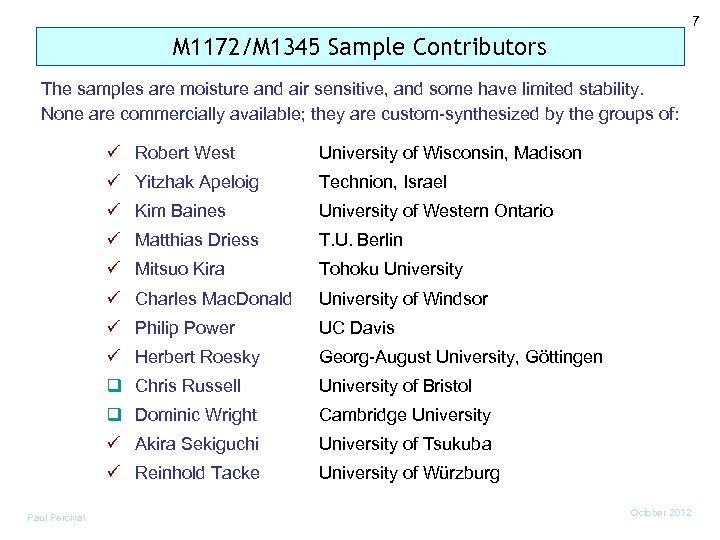 7 M 1172/M 1345 Sample Contributors The samples are moisture and air sensitive, and some have limited stability. None are commercially available; they are custom-synthesized by the groups of: ü Robert West ü Yitzhak Apeloig Technion, Israel ü Kim Baines University of Western Ontario ü Matthias Driess T. U. Berlin ü Mitsuo Kira Tohoku University ü Charles Mac. Donald University of Windsor ü Philip Power UC Davis ü Herbert Roesky Georg-August University, Göttingen q Chris Russell University of Bristol q Dominic Wright Cambridge University ü Akira Sekiguchi University of Tsukuba ü Reinhold Tacke Paul Percival University of Wisconsin, Madison University of Würzburg October 2012
7 M 1172/M 1345 Sample Contributors The samples are moisture and air sensitive, and some have limited stability. None are commercially available; they are custom-synthesized by the groups of: ü Robert West ü Yitzhak Apeloig Technion, Israel ü Kim Baines University of Western Ontario ü Matthias Driess T. U. Berlin ü Mitsuo Kira Tohoku University ü Charles Mac. Donald University of Windsor ü Philip Power UC Davis ü Herbert Roesky Georg-August University, Göttingen q Chris Russell University of Bristol q Dominic Wright Cambridge University ü Akira Sekiguchi University of Tsukuba ü Reinhold Tacke Paul Percival University of Wisconsin, Madison University of Würzburg October 2012
 µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø Chemical applications focus on Muonium q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø Chemical applications focus on Muonium q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
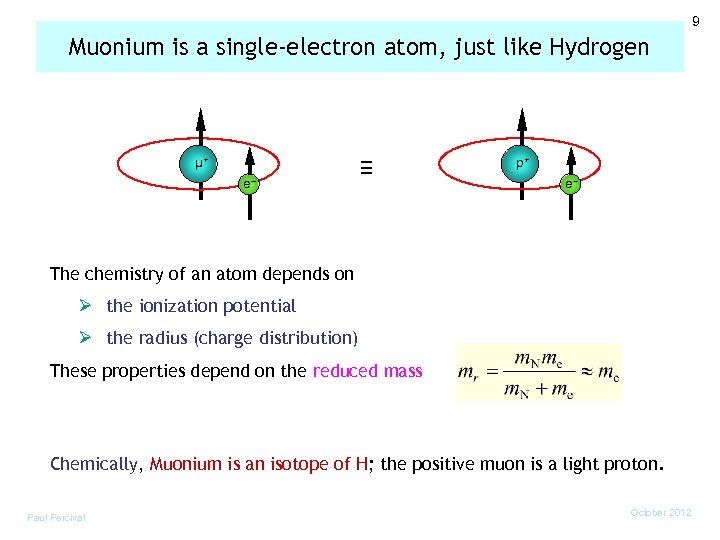 9 Muonium is a single-electron atom, just like Hydrogen µ+ e– ≡ p+ e– The chemistry of an atom depends on Ø the ionization potential Ø the radius (charge distribution) These properties depend on the reduced mass Chemically, Muonium is an isotope of H; the positive muon is a light proton. Paul Percival October 2012
9 Muonium is a single-electron atom, just like Hydrogen µ+ e– ≡ p+ e– The chemistry of an atom depends on Ø the ionization potential Ø the radius (charge distribution) These properties depend on the reduced mass Chemically, Muonium is an isotope of H; the positive muon is a light proton. Paul Percival October 2012
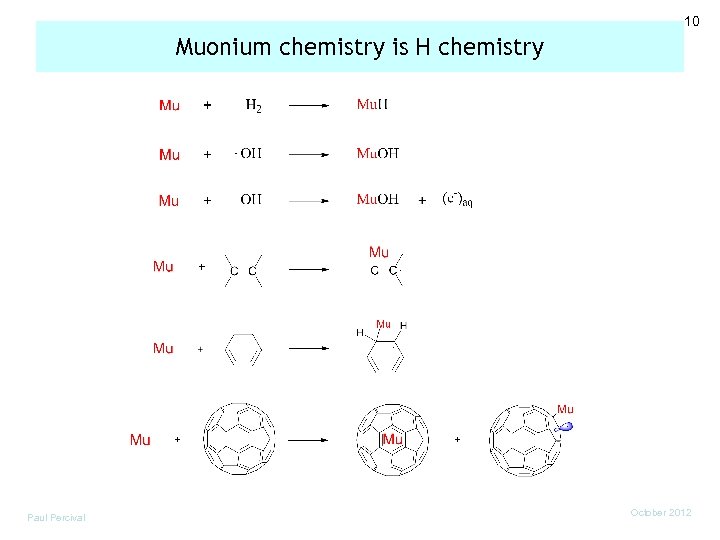 10 Muonium chemistry is H chemistry Paul Percival October 2012
10 Muonium chemistry is H chemistry Paul Percival October 2012
 11 Muonium Chemistry Research v How fast does it react? kinetic isotope effects; tunneling v How is the reaction affected by the environment? T, P, density v What is the chemical reaction? reactivity, mechanism v What products are formed? free radical chemistry v Structure of free radicals geometry, bonding v Dynamics of free radicals intramolecular motion, tumbling v Free radicals as probes soft matter v Muonium as a probe fullerenes, semiconductors v Muonium diffusion ice, semiconductors Paul Percival October 2012
11 Muonium Chemistry Research v How fast does it react? kinetic isotope effects; tunneling v How is the reaction affected by the environment? T, P, density v What is the chemical reaction? reactivity, mechanism v What products are formed? free radical chemistry v Structure of free radicals geometry, bonding v Dynamics of free radicals intramolecular motion, tumbling v Free radicals as probes soft matter v Muonium as a probe fullerenes, semiconductors v Muonium diffusion ice, semiconductors Paul Percival October 2012
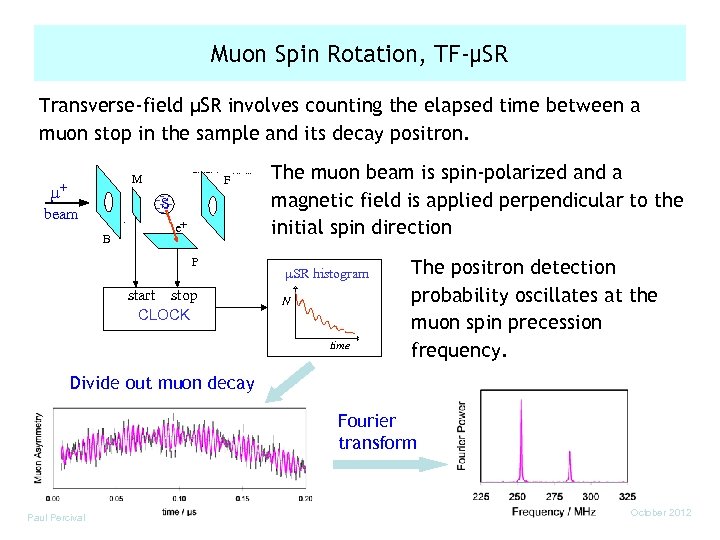 Muon Spin Rotation, TF-µSR Transverse-field µSR involves counting the elapsed time between a muon stop in the sample and its decay positron. M µ+ F S beam B e+ P start stop CLOCK The muon beam is spin-polarized and a magnetic field is applied perpendicular to the initial spin direction µSR histogram N time The positron detection probability oscillates at the muon spin precession frequency. Divide out muon decay Fourier transform Paul Percival October 2012
Muon Spin Rotation, TF-µSR Transverse-field µSR involves counting the elapsed time between a muon stop in the sample and its decay positron. M µ+ F S beam B e+ P start stop CLOCK The muon beam is spin-polarized and a magnetic field is applied perpendicular to the initial spin direction µSR histogram N time The positron detection probability oscillates at the muon spin precession frequency. Divide out muon decay Fourier transform Paul Percival October 2012
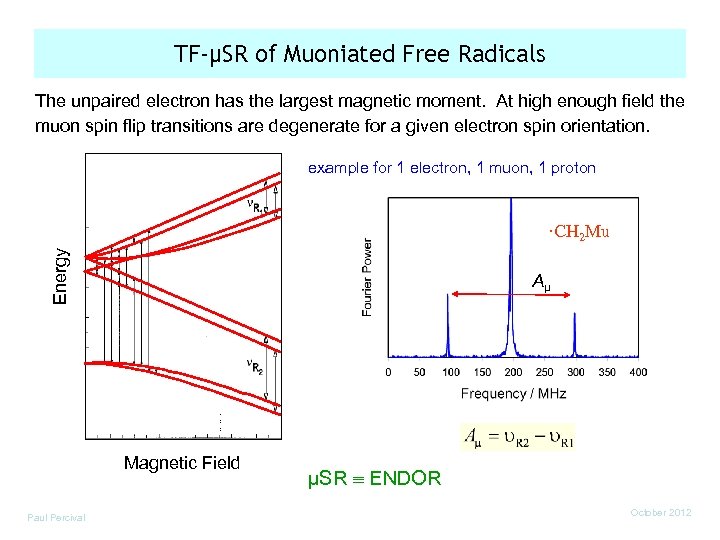 TF-µSR of Muoniated Free Radicals The unpaired electron has the largest magnetic moment. At high enough field the muon spin flip transitions are degenerate for a given electron spin orientation. example for 1 electron, 1 muon, 1 proton Energy ·CH 2 Mu Aµ Magnetic Field Paul Percival µSR ENDOR October 2012
TF-µSR of Muoniated Free Radicals The unpaired electron has the largest magnetic moment. At high enough field the muon spin flip transitions are degenerate for a given electron spin orientation. example for 1 electron, 1 muon, 1 proton Energy ·CH 2 Mu Aµ Magnetic Field Paul Percival µSR ENDOR October 2012
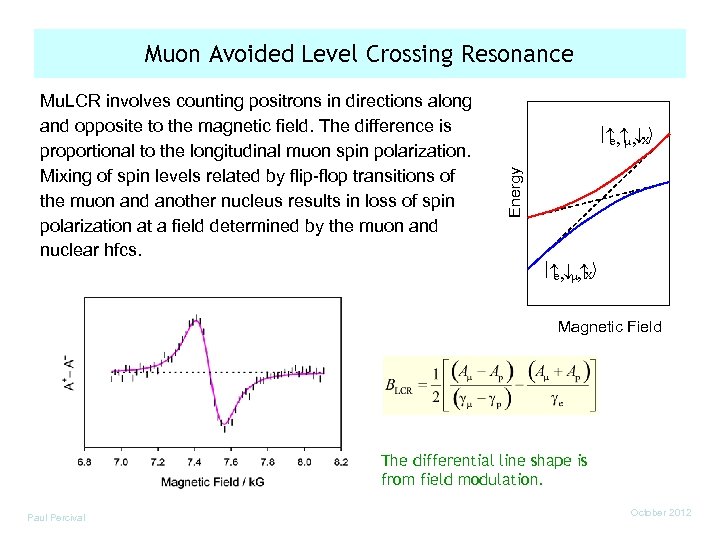 Muon Avoided Level Crossing Resonance , , ¯X e m Energy Mu. LCR involves counting positrons in directions along and opposite to the magnetic field. The difference is proportional to the longitudinal muon spin polarization. Mixing of spin levels related by flip-flop transitions of the muon and another nucleus results in loss of spin polarization at a field determined by the muon and nuclear hfcs. , ¯m, X e Magnetic Field The differential line shape is from field modulation. Paul Percival October 2012
Muon Avoided Level Crossing Resonance , , ¯X e m Energy Mu. LCR involves counting positrons in directions along and opposite to the magnetic field. The difference is proportional to the longitudinal muon spin polarization. Mixing of spin levels related by flip-flop transitions of the muon and another nucleus results in loss of spin polarization at a field determined by the muon and nuclear hfcs. , ¯m, X e Magnetic Field The differential line shape is from field modulation. Paul Percival October 2012
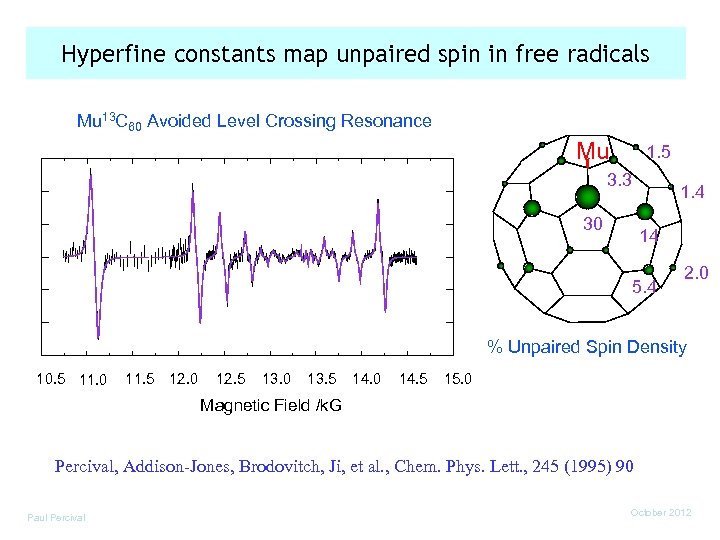 Hyperfine constants map unpaired spin in free radicals Mu 13 C 60 Avoided Level Crossing Resonance Mu 1. 5 3. 3 30 1. 4 14 5. 4 2. 0 % Unpaired Spin Density 10. 5 11. 0 11. 5 12. 0 12. 5 13. 0 13. 5 14. 0 14. 5 15. 0 Magnetic Field /k. G Percival, Addison-Jones, Brodovitch, Ji, et al. , Chem. Phys. Lett. , 245 (1995) 90 Paul Percival October 2012
Hyperfine constants map unpaired spin in free radicals Mu 13 C 60 Avoided Level Crossing Resonance Mu 1. 5 3. 3 30 1. 4 14 5. 4 2. 0 % Unpaired Spin Density 10. 5 11. 0 11. 5 12. 0 12. 5 13. 0 13. 5 14. 0 14. 5 15. 0 Magnetic Field /k. G Percival, Addison-Jones, Brodovitch, Ji, et al. , Chem. Phys. Lett. , 245 (1995) 90 Paul Percival October 2012
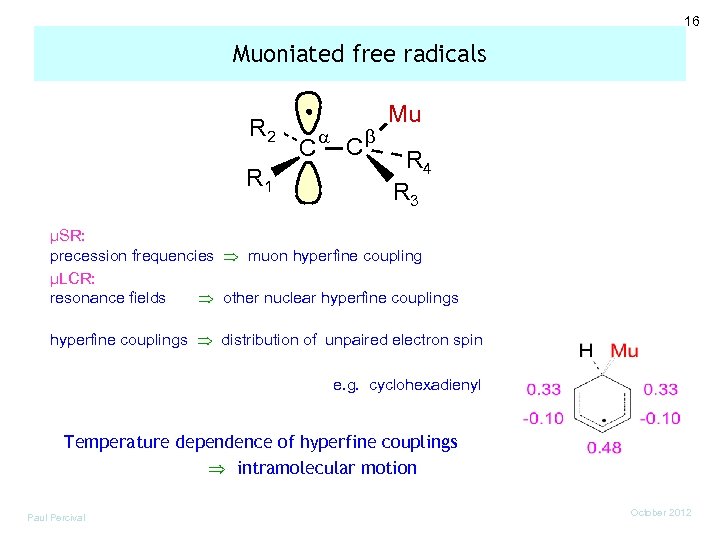 16 Muoniated free radicals R 2 R 1 C a C b Mu R 4 R 3 µSR: precession frequencies muon hyperfine coupling µLCR: resonance fields other nuclear hyperfine couplings distribution of unpaired electron spin e. g. cyclohexadienyl Temperature dependence of hyperfine couplings intramolecular motion Paul Percival October 2012
16 Muoniated free radicals R 2 R 1 C a C b Mu R 4 R 3 µSR: precession frequencies muon hyperfine coupling µLCR: resonance fields other nuclear hyperfine couplings distribution of unpaired electron spin e. g. cyclohexadienyl Temperature dependence of hyperfine couplings intramolecular motion Paul Percival October 2012
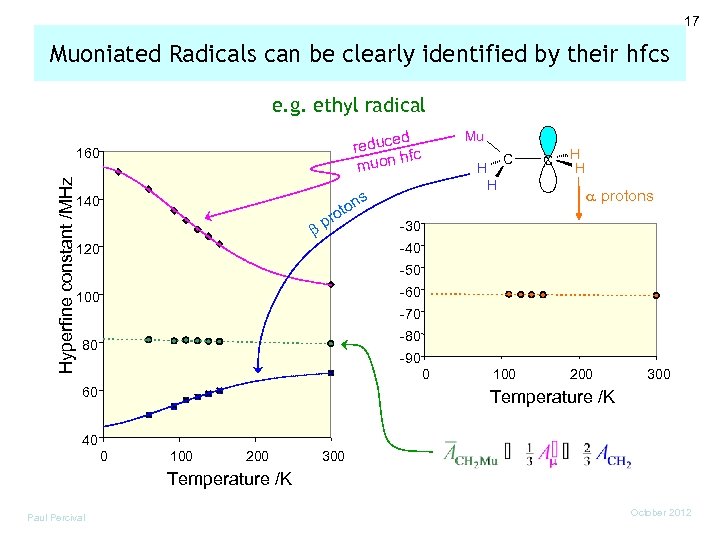 17 Muoniated Radicals can be clearly identified by their hfcs e. g. ethyl radical ed reduc fc h muon Hyperfine constant /MHz 160 s ton ro 140 bp Mu H H C C H H a protons -30 -40 120 -50 -60 100 -70 -80 80 -90 0 60 100 200 300 Temperature /K 40 0 100 200 300 Temperature /K Paul Percival October 2012
17 Muoniated Radicals can be clearly identified by their hfcs e. g. ethyl radical ed reduc fc h muon Hyperfine constant /MHz 160 s ton ro 140 bp Mu H H C C H H a protons -30 -40 120 -50 -60 100 -70 -80 80 -90 0 60 100 200 300 Temperature /K 40 0 100 200 300 Temperature /K Paul Percival October 2012
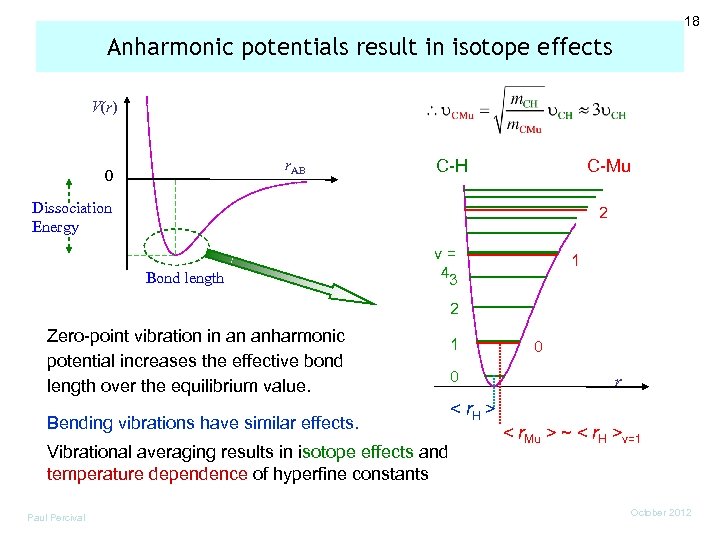 18 Anharmonic potentials result in isotope effects V(r) r. AB 0 C-H C-Mu Dissociation Energy 2 Bond length v= 43 1 2 Zero-point vibration in an anharmonic potential increases the effective bond length over the equilibrium value. Bending vibrations have similar effects. Vibrational averaging results in isotope effects and temperature dependence of hyperfine constants Paul Percival 1 0 0 r < r. H > < r. Mu > ~ < r. H >v=1 October 2012
18 Anharmonic potentials result in isotope effects V(r) r. AB 0 C-H C-Mu Dissociation Energy 2 Bond length v= 43 1 2 Zero-point vibration in an anharmonic potential increases the effective bond length over the equilibrium value. Bending vibrations have similar effects. Vibrational averaging results in isotope effects and temperature dependence of hyperfine constants Paul Percival 1 0 0 r < r. H > < r. Mu > ~ < r. H >v=1 October 2012
 µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø the muon as part of the problem q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø the muon as part of the problem q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
 20 Sample environments for “chemical” samples v Low pressure gases Mu kinetics, isolated free radicals Ø large volumes, large detectors ü surface muons v High pressure gases for slow Mu reactions Ø pressure vessel Ø decay muon channel v Liquids close to ambient conditions typically noxious and/or reactive ü surface muons v Superheated and/or pressurized liquids sc-CO 2, sc-H 2 O Ø pressure vessel Ø decay muon channel v Solids ice, fullerenes, polymers ü surface muons ü “standard” cryostats and ovens Paul Percival October 2012
20 Sample environments for “chemical” samples v Low pressure gases Mu kinetics, isolated free radicals Ø large volumes, large detectors ü surface muons v High pressure gases for slow Mu reactions Ø pressure vessel Ø decay muon channel v Liquids close to ambient conditions typically noxious and/or reactive ü surface muons v Superheated and/or pressurized liquids sc-CO 2, sc-H 2 O Ø pressure vessel Ø decay muon channel v Solids ice, fullerenes, polymers ü surface muons ü “standard” cryostats and ovens Paul Percival October 2012
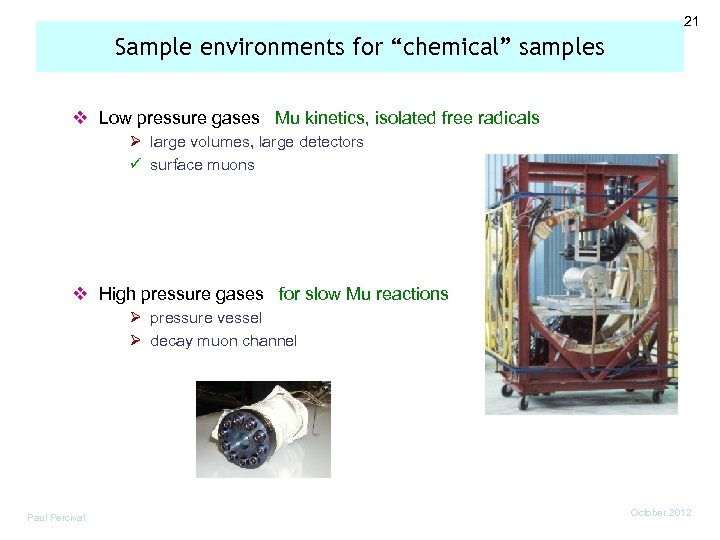 21 Sample environments for “chemical” samples v Low pressure gases Mu kinetics, isolated free radicals Ø large volumes, large detectors ü surface muons v High pressure gases for slow Mu reactions Ø pressure vessel Ø decay muon channel Paul Percival October 2012
21 Sample environments for “chemical” samples v Low pressure gases Mu kinetics, isolated free radicals Ø large volumes, large detectors ü surface muons v High pressure gases for slow Mu reactions Ø pressure vessel Ø decay muon channel Paul Percival October 2012
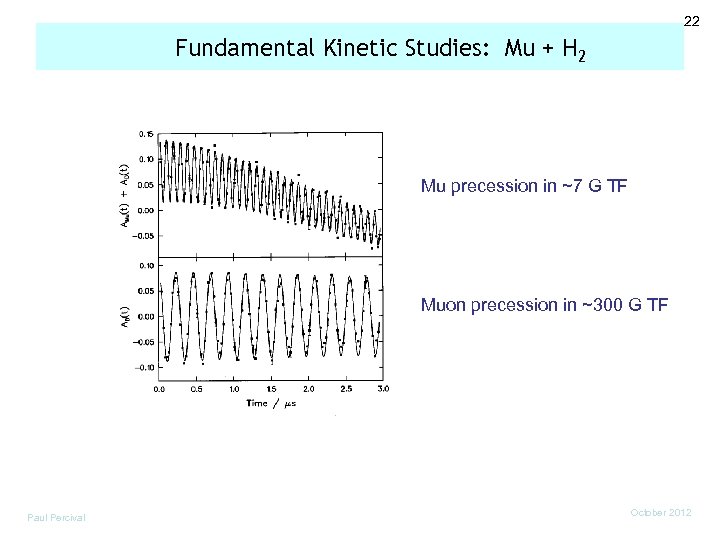 22 Fundamental Kinetic Studies: Mu + H 2 Mu precession in ~7 G TF Muon precession in ~300 G TF Paul Percival October 2012
22 Fundamental Kinetic Studies: Mu + H 2 Mu precession in ~7 G TF Muon precession in ~300 G TF Paul Percival October 2012
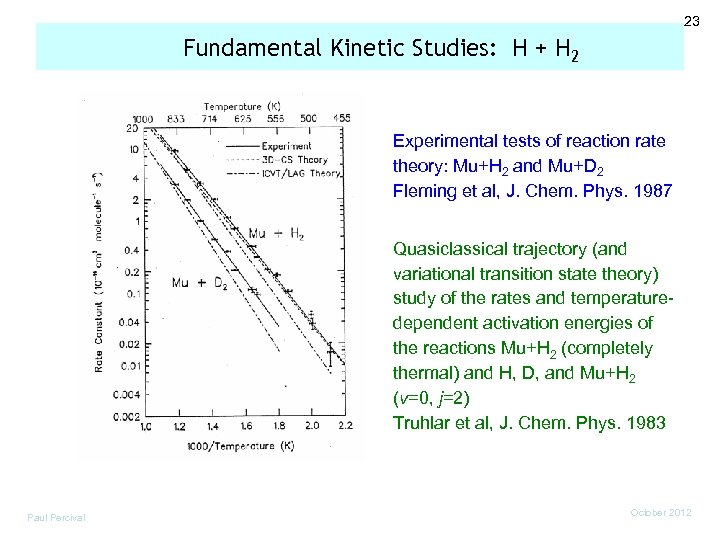 23 Fundamental Kinetic Studies: H + H 2 Experimental tests of reaction rate theory: Mu+H 2 and Mu+D 2 Fleming et al, J. Chem. Phys. 1987 Quasiclassical trajectory (and variational transition state theory) study of the rates and temperaturedependent activation energies of the reactions Mu+H 2 (completely thermal) and H, D, and Mu+H 2 (v=0, j=2) Truhlar et al, J. Chem. Phys. 1983 Paul Percival October 2012
23 Fundamental Kinetic Studies: H + H 2 Experimental tests of reaction rate theory: Mu+H 2 and Mu+D 2 Fleming et al, J. Chem. Phys. 1987 Quasiclassical trajectory (and variational transition state theory) study of the rates and temperaturedependent activation energies of the reactions Mu+H 2 (completely thermal) and H, D, and Mu+H 2 (v=0, j=2) Truhlar et al, J. Chem. Phys. 1983 Paul Percival October 2012
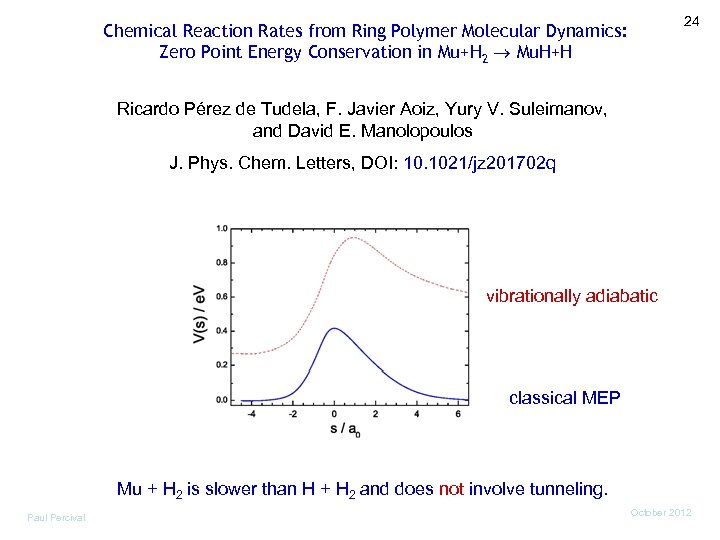 24 Chemical Reaction Rates from Ring Polymer Molecular Dynamics: Zero Point Energy Conservation in Mu+H 2 Mu. H+H Ricardo Pérez de Tudela, F. Javier Aoiz, Yury V. Suleimanov, and David E. Manolopoulos J. Phys. Chem. Letters, DOI: 10. 1021/jz 201702 q vibrationally adiabatic classical MEP Mu + H 2 is slower than H + H 2 and does not involve tunneling. Paul Percival October 2012
24 Chemical Reaction Rates from Ring Polymer Molecular Dynamics: Zero Point Energy Conservation in Mu+H 2 Mu. H+H Ricardo Pérez de Tudela, F. Javier Aoiz, Yury V. Suleimanov, and David E. Manolopoulos J. Phys. Chem. Letters, DOI: 10. 1021/jz 201702 q vibrationally adiabatic classical MEP Mu + H 2 is slower than H + H 2 and does not involve tunneling. Paul Percival October 2012
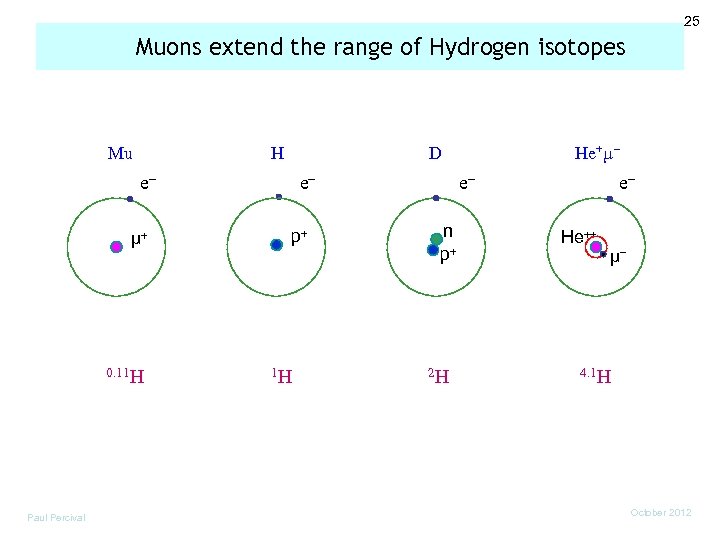 25 Muons extend the range of Hydrogen isotopes Mu H e µ 0. 11 H Paul Percival He+µ D e p 1 H e n p 2 H e He µ 4. 1 H October 2012
25 Muons extend the range of Hydrogen isotopes Mu H e µ 0. 11 H Paul Percival He+µ D e p 1 H e n p 2 H e He µ 4. 1 H October 2012
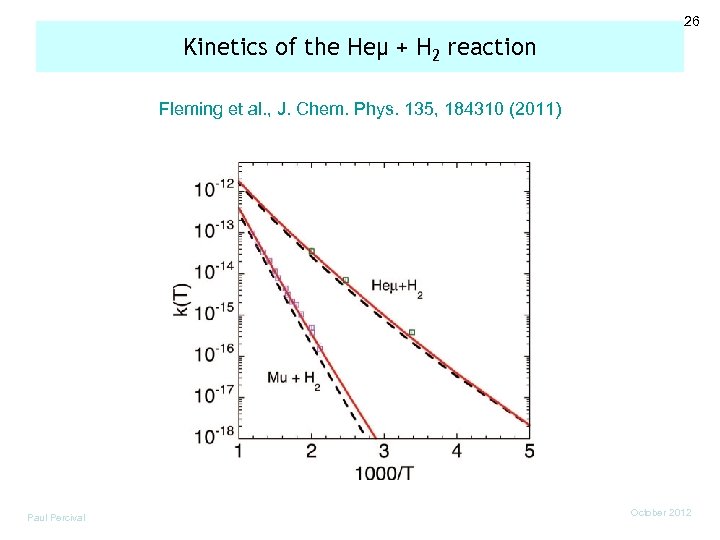 26 Kinetics of the Heμ + H 2 reaction Fleming et al. , J. Chem. Phys. 135, 184310 (2011) Paul Percival October 2012
26 Kinetics of the Heμ + H 2 reaction Fleming et al. , J. Chem. Phys. 135, 184310 (2011) Paul Percival October 2012
 27 Sample environments for “chemical” samples v Low pressure gases Mu kinetics, isolated free radicals Ø large volumes, large detectors ü surface muons v High pressure gases for slow Mu reactions Ø pressure vessel Ø decay muon channel v Liquids close to ambient conditions typically noxious and/or reactive ü surface muons Paul Percival October 2012
27 Sample environments for “chemical” samples v Low pressure gases Mu kinetics, isolated free radicals Ø large volumes, large detectors ü surface muons v High pressure gases for slow Mu reactions Ø pressure vessel Ø decay muon channel v Liquids close to ambient conditions typically noxious and/or reactive ü surface muons Paul Percival October 2012
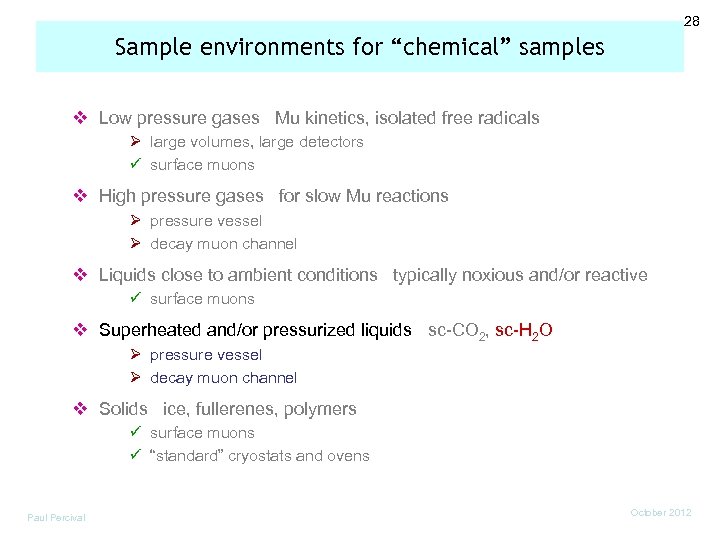 28 Sample environments for “chemical” samples v Low pressure gases Mu kinetics, isolated free radicals Ø large volumes, large detectors ü surface muons v High pressure gases for slow Mu reactions Ø pressure vessel Ø decay muon channel v Liquids close to ambient conditions typically noxious and/or reactive ü surface muons v Superheated and/or pressurized liquids sc-CO 2, sc-H 2 O Ø pressure vessel Ø decay muon channel v Solids ice, fullerenes, polymers ü surface muons ü “standard” cryostats and ovens Paul Percival October 2012
28 Sample environments for “chemical” samples v Low pressure gases Mu kinetics, isolated free radicals Ø large volumes, large detectors ü surface muons v High pressure gases for slow Mu reactions Ø pressure vessel Ø decay muon channel v Liquids close to ambient conditions typically noxious and/or reactive ü surface muons v Superheated and/or pressurized liquids sc-CO 2, sc-H 2 O Ø pressure vessel Ø decay muon channel v Solids ice, fullerenes, polymers ü surface muons ü “standard” cryostats and ovens Paul Percival October 2012
 29 Pressure cells for Hydrothermal Studies Paul Percival October 2012
29 Pressure cells for Hydrothermal Studies Paul Percival October 2012
 30 Supercritical Water Oxidation There are drastic changes in the physical properties of water close to and above the critical point (Tc = 374°C, Pc = 221 bar). This leads to unusual chemistry: v v organic compounds are miscible in SCW combustion of organic materials is possible SCWO facilities are being developed for the destruction of hazardous waste such as PCBs, sewage sludge, and chemical weapons. It has even been proposed for water recycling on long space flights. Many of these reactions involve free radical intermediates, but there is very little direct knowledge about these transient species under such extreme conditions. Paul Percival A Flame in Water! 30% methane in water at 2000 bar, 450ºC Schilling and Franck, Ber. Bunsenges. Phys. Chem. 92 (1988) 631. October 2012
30 Supercritical Water Oxidation There are drastic changes in the physical properties of water close to and above the critical point (Tc = 374°C, Pc = 221 bar). This leads to unusual chemistry: v v organic compounds are miscible in SCW combustion of organic materials is possible SCWO facilities are being developed for the destruction of hazardous waste such as PCBs, sewage sludge, and chemical weapons. It has even been proposed for water recycling on long space flights. Many of these reactions involve free radical intermediates, but there is very little direct knowledge about these transient species under such extreme conditions. Paul Percival A Flame in Water! 30% methane in water at 2000 bar, 450ºC Schilling and Franck, Ber. Bunsenges. Phys. Chem. 92 (1988) 631. October 2012
 31 Destruction of Chemical Weapons The Blue Grass Chemical Agent-Destruction Pilot Plant (BGCAPP) is a chemical weapons destruction facility under construction. The plant is being built to destroy the chemical weapons stockpile at the Blue Grass Army Depot (BGAD), near Richmond, Kentucky. The plant is dedicated to the destruction of 523 tons of nerve agents sarin (GB) and VX, and mustard agent, which constitute about two percent of the United States chemical weapons stockpile. Abstracted from Wikipedia. Further information at the General Atomics web site http: //www. ga. com/hazardous-waste-destruction/supercritical-water-oxidation/. . . Paul Percival October 2012
31 Destruction of Chemical Weapons The Blue Grass Chemical Agent-Destruction Pilot Plant (BGCAPP) is a chemical weapons destruction facility under construction. The plant is being built to destroy the chemical weapons stockpile at the Blue Grass Army Depot (BGAD), near Richmond, Kentucky. The plant is dedicated to the destruction of 523 tons of nerve agents sarin (GB) and VX, and mustard agent, which constitute about two percent of the United States chemical weapons stockpile. Abstracted from Wikipedia. Further information at the General Atomics web site http: //www. ga. com/hazardous-waste-destruction/supercritical-water-oxidation/. . . Paul Percival October 2012
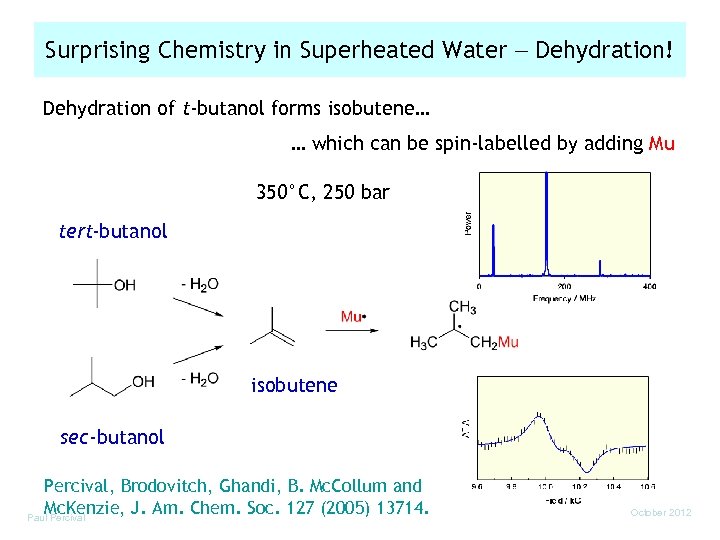 Surprising Chemistry in Superheated Water Dehydration! Dehydration of t-butanol forms isobutene… … which can be spin-labelled by adding Mu 350°C, 250 bar tert-butanol isobutene sec-butanol Percival, Brodovitch, Ghandi, B. Mc. Collum and Mc. Kenzie, J. Am. Chem. Soc. 127 (2005) 13714. Paul Percival October 2012
Surprising Chemistry in Superheated Water Dehydration! Dehydration of t-butanol forms isobutene… … which can be spin-labelled by adding Mu 350°C, 250 bar tert-butanol isobutene sec-butanol Percival, Brodovitch, Ghandi, B. Mc. Collum and Mc. Kenzie, J. Am. Chem. Soc. 127 (2005) 13714. Paul Percival October 2012
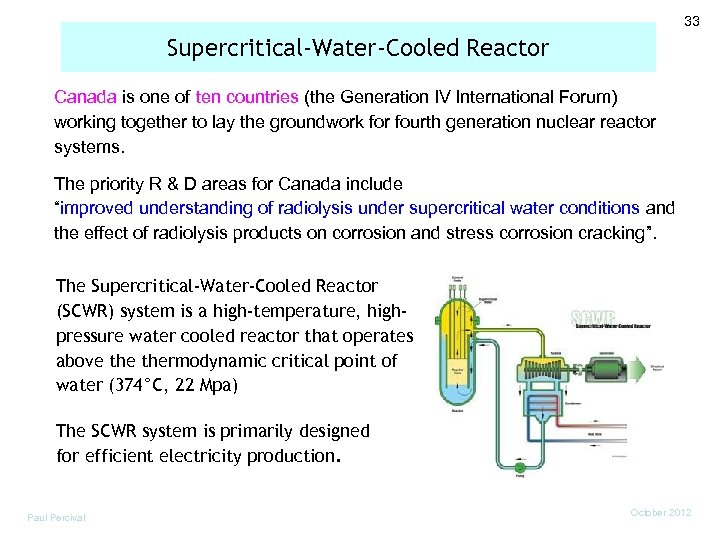 33 Supercritical-Water-Cooled Reactor Canada is one of ten countries (the Generation IV International Forum) working together to lay the groundwork for fourth generation nuclear reactor systems. The priority R & D areas for Canada include “improved understanding of radiolysis under supercritical water conditions and the effect of radiolysis products on corrosion and stress corrosion cracking”. The Supercritical-Water-Cooled Reactor (SCWR) system is a high-temperature, highpressure water cooled reactor that operates above thermodynamic critical point of water (374°C, 22 Mpa) The SCWR system is primarily designed for efficient electricity production. Paul Percival October 2012
33 Supercritical-Water-Cooled Reactor Canada is one of ten countries (the Generation IV International Forum) working together to lay the groundwork for fourth generation nuclear reactor systems. The priority R & D areas for Canada include “improved understanding of radiolysis under supercritical water conditions and the effect of radiolysis products on corrosion and stress corrosion cracking”. The Supercritical-Water-Cooled Reactor (SCWR) system is a high-temperature, highpressure water cooled reactor that operates above thermodynamic critical point of water (374°C, 22 Mpa) The SCWR system is primarily designed for efficient electricity production. Paul Percival October 2012
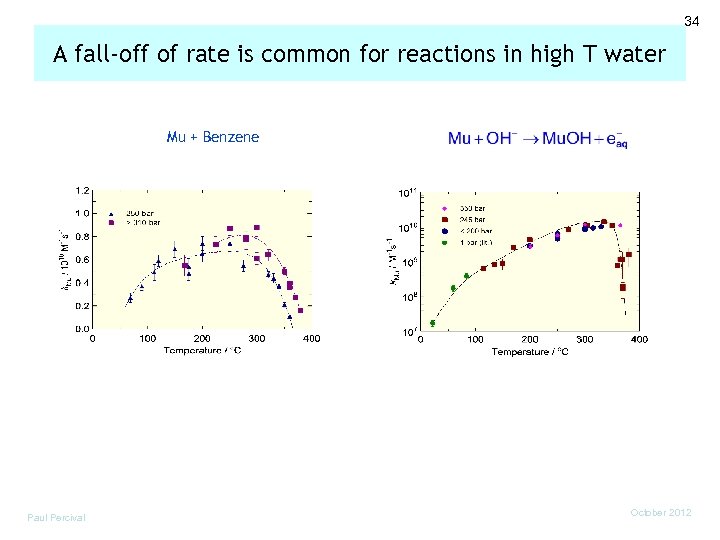 34 A fall-off of rate is common for reactions in high T water Mu + Benzene Paul Percival October 2012
34 A fall-off of rate is common for reactions in high T water Mu + Benzene Paul Percival October 2012
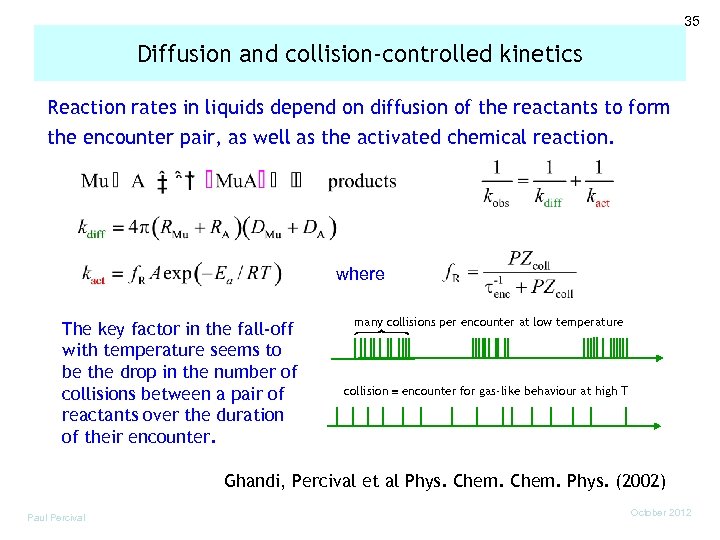 35 Diffusion and collision-controlled kinetics Reaction rates in liquids depend on diffusion of the reactants to form the encounter pair, as well as the activated chemical reaction. where The key factor in the fall-off with temperature seems to be the drop in the number of collisions between a pair of reactants over the duration of their encounter. many collisions per encounter at low temperature collision encounter for gas-like behaviour at high T Ghandi, Percival et al Phys. Chem. Phys. (2002) Paul Percival October 2012
35 Diffusion and collision-controlled kinetics Reaction rates in liquids depend on diffusion of the reactants to form the encounter pair, as well as the activated chemical reaction. where The key factor in the fall-off with temperature seems to be the drop in the number of collisions between a pair of reactants over the duration of their encounter. many collisions per encounter at low temperature collision encounter for gas-like behaviour at high T Ghandi, Percival et al Phys. Chem. Phys. (2002) Paul Percival October 2012
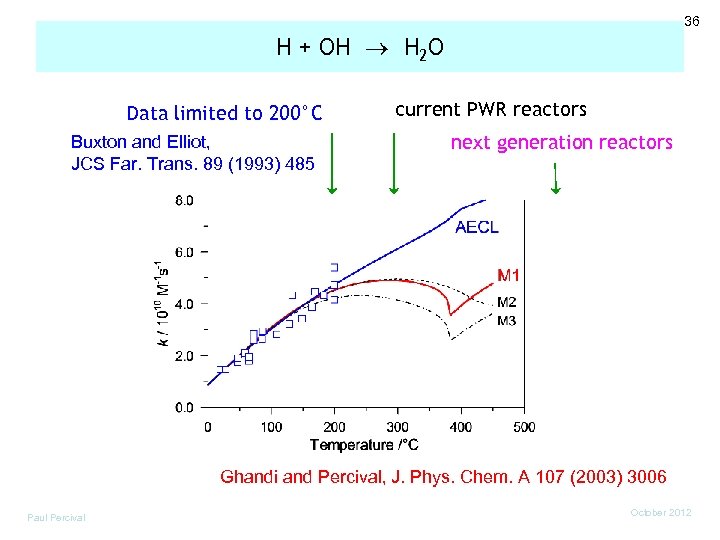 36 H + OH H 2 O Data limited to 200°C Buxton and Elliot, JCS Far. Trans. 89 (1993) 485 current PWR reactors next generation reactors Ghandi and Percival, J. Phys. Chem. A 107 (2003) 3006 Paul Percival October 2012
36 H + OH H 2 O Data limited to 200°C Buxton and Elliot, JCS Far. Trans. 89 (1993) 485 current PWR reactors next generation reactors Ghandi and Percival, J. Phys. Chem. A 107 (2003) 3006 Paul Percival October 2012
 µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø the muon as part of the problem q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø the muon as part of the problem q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
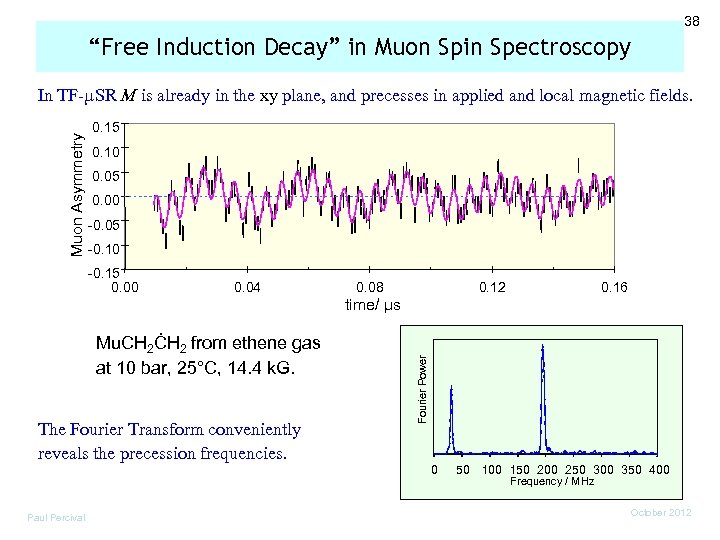 38 “Free Induction Decay” in Muon Spin Spectroscopy Muon Asymmetry In TF-µSR M is already in the xy plane, and precesses in applied and local magnetic fields. 0. 15 0. 10 0. 05 0. 00 -0. 05 -0. 10 -0. 15 0. 00 0. 04 0. 08 0. 12 0. 16 Mu. CH 2ĊH 2 from ethene gas at 10 bar, 25°C, 14. 4 k. G. The Fourier Transform conveniently reveals the precession frequencies. Paul Percival Fourier Power time/ µs 0 50 100 150 200 250 300 350 400 Frequency / MHz October 2012
38 “Free Induction Decay” in Muon Spin Spectroscopy Muon Asymmetry In TF-µSR M is already in the xy plane, and precesses in applied and local magnetic fields. 0. 15 0. 10 0. 05 0. 00 -0. 05 -0. 10 -0. 15 0. 00 0. 04 0. 08 0. 12 0. 16 Mu. CH 2ĊH 2 from ethene gas at 10 bar, 25°C, 14. 4 k. G. The Fourier Transform conveniently reveals the precession frequencies. Paul Percival Fourier Power time/ µs 0 50 100 150 200 250 300 350 400 Frequency / MHz October 2012
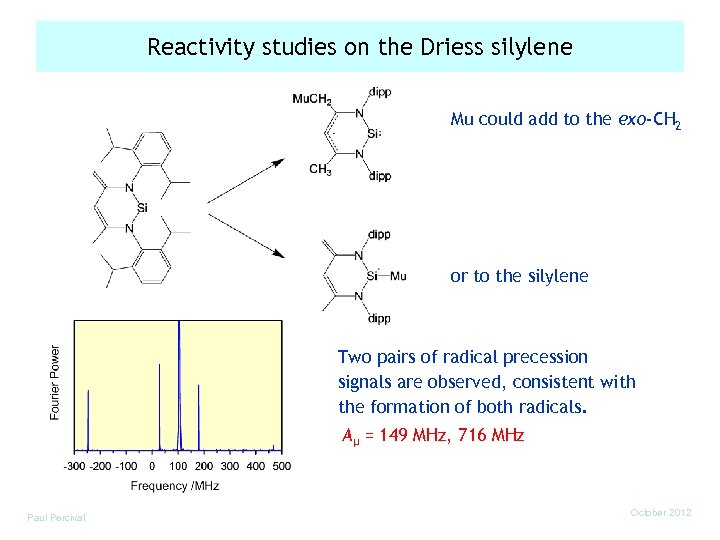 Reactivity studies on the Driess silylene Mu could add to the exo-CH 2 or to the silylene Two pairs of radical precession signals are observed, consistent with the formation of both radicals. Aµ = 149 MHz, 716 MHz Paul Percival October 2012
Reactivity studies on the Driess silylene Mu could add to the exo-CH 2 or to the silylene Two pairs of radical precession signals are observed, consistent with the formation of both radicals. Aµ = 149 MHz, 716 MHz Paul Percival October 2012
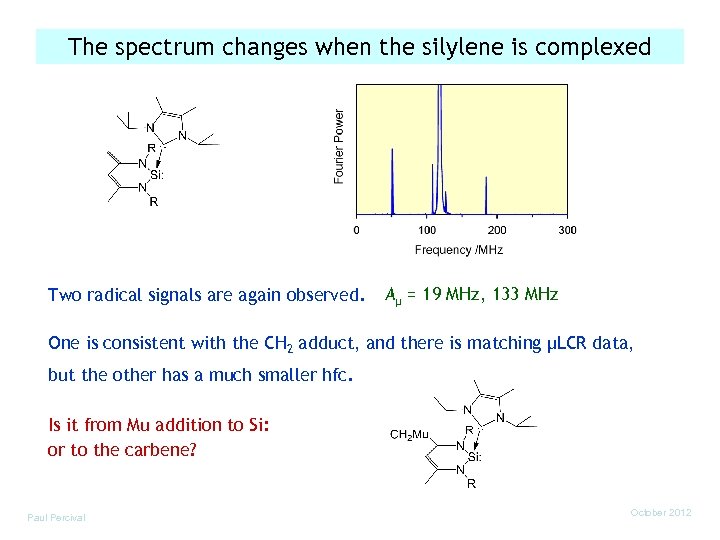 The spectrum changes when the silylene is complexed Two radical signals are again observed. Aµ = 19 MHz, 133 MHz One is consistent with the CH 2 adduct, and there is matching µLCR data, but the other has a much smaller hfc. Is it from Mu addition to Si: or to the carbene? Paul Percival October 2012
The spectrum changes when the silylene is complexed Two radical signals are again observed. Aµ = 19 MHz, 133 MHz One is consistent with the CH 2 adduct, and there is matching µLCR data, but the other has a much smaller hfc. Is it from Mu addition to Si: or to the carbene? Paul Percival October 2012
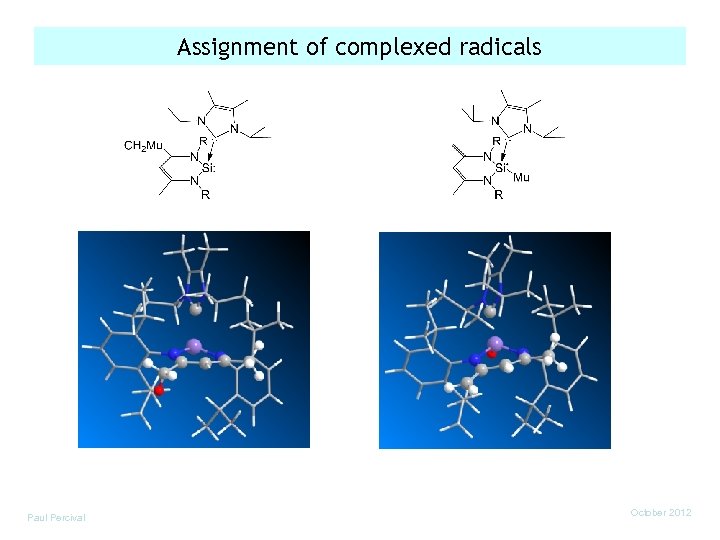 Assignment of complexed radicals Paul Percival October 2012
Assignment of complexed radicals Paul Percival October 2012
 µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø the muon as part of the problem q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation: RF or light q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø the muon as part of the problem q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation: RF or light q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
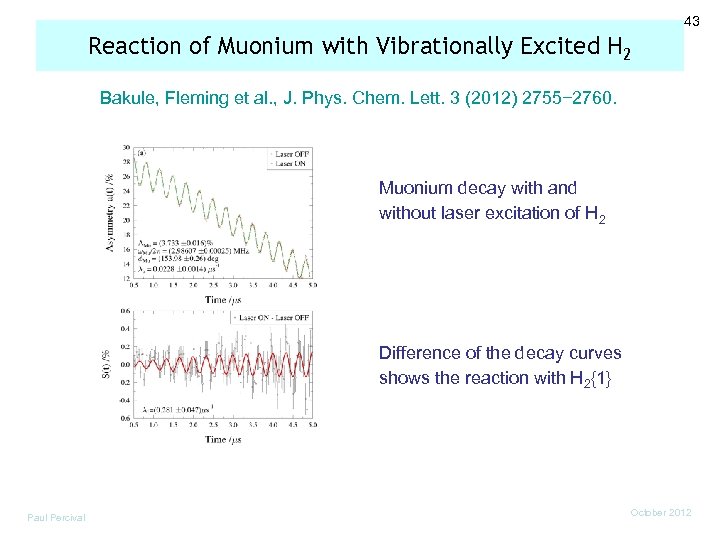 43 Reaction of Muonium with Vibrationally Excited H 2 Bakule, Fleming et al. , J. Phys. Chem. Lett. 3 (2012) 2755− 2760. Muonium decay with and without laser excitation of H 2 Difference of the decay curves shows the reaction with H 2{1} Paul Percival October 2012
43 Reaction of Muonium with Vibrationally Excited H 2 Bakule, Fleming et al. , J. Phys. Chem. Lett. 3 (2012) 2755− 2760. Muonium decay with and without laser excitation of H 2 Difference of the decay curves shows the reaction with H 2{1} Paul Percival October 2012
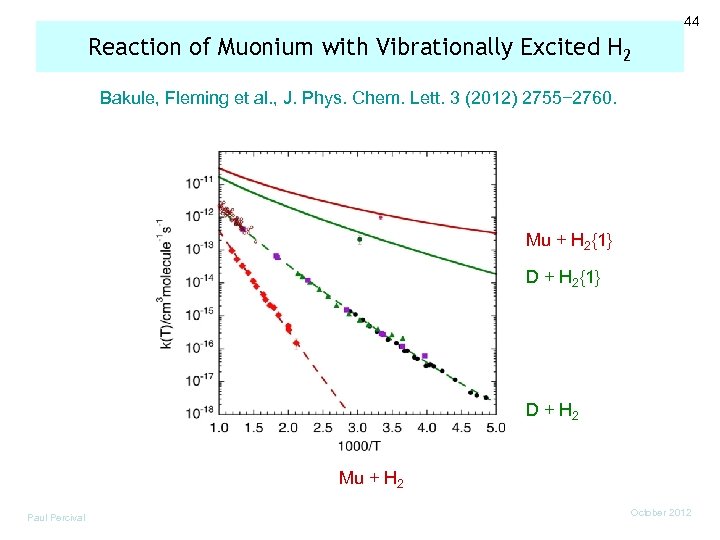 44 Reaction of Muonium with Vibrationally Excited H 2 Bakule, Fleming et al. , J. Phys. Chem. Lett. 3 (2012) 2755− 2760. Mu + H 2{1} D + H 2 Mu + H 2 Paul Percival October 2012
44 Reaction of Muonium with Vibrationally Excited H 2 Bakule, Fleming et al. , J. Phys. Chem. Lett. 3 (2012) 2755− 2760. Mu + H 2{1} D + H 2 Mu + H 2 Paul Percival October 2012
 µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø the muon as part of the problem q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
µSR in Chemistry ― Overview q Is there any demand? Ø experts versus users q Is chemistry any different to physics? Ø the muon as part of the problem q Are the needs of chemists different from physicists? Ø sample environment q Why do chemists prefer a continuous source? Ø high frequency precession q Why do chemists prefer a pulsed source? Ø coherent excitation q Why do chemists feel misunderstood/unwelcome? Ø cultural issues Paul Percival October 2012
 46 Challenges Facing a Future for CMMS as a User Facility What keeps potential Users away? v High Activation Barrier A new group needs 3 or 4 “experts” to mount an independent program v Approval Procedures designed for long-running subatomic physics experiments v High Level of Expertise Required User-friendly controls and procedures require stable set-ups. v Insufficient Support Personnel TRIUMF CMMS has far fewer support personnel than PSI and ISIS. v Attitude The “casual” User is a stranger in a strange land! Paul Percival October 2012
46 Challenges Facing a Future for CMMS as a User Facility What keeps potential Users away? v High Activation Barrier A new group needs 3 or 4 “experts” to mount an independent program v Approval Procedures designed for long-running subatomic physics experiments v High Level of Expertise Required User-friendly controls and procedures require stable set-ups. v Insufficient Support Personnel TRIUMF CMMS has far fewer support personnel than PSI and ISIS. v Attitude The “casual” User is a stranger in a strange land! Paul Percival October 2012
 END Paul E 945: Mu Percival radicals from carbenes and carbene analogues October 2012 Paul Percival, June 2007
END Paul E 945: Mu Percival radicals from carbenes and carbene analogues October 2012 Paul Percival, June 2007
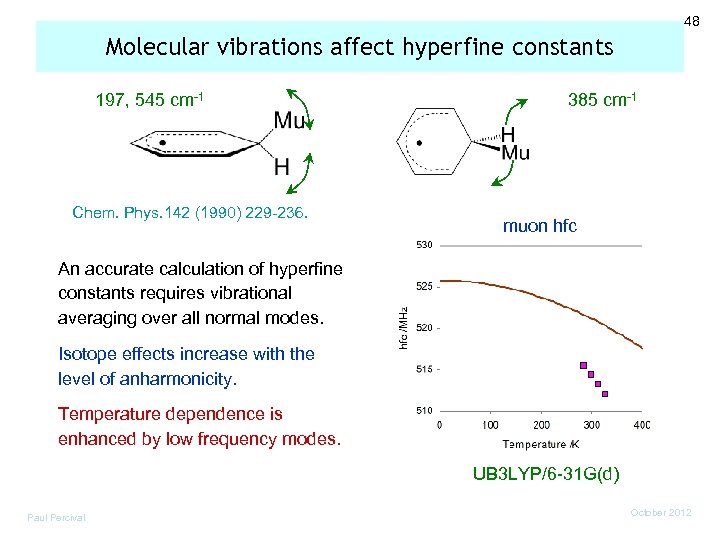 48 Molecular vibrations affect hyperfine constants 197, 545 cm-1 Chem. Phys. 142 (1990) 229 -236. 385 cm-1 muon hfc An accurate calculation of hyperfine constants requires vibrational averaging over all normal modes. Isotope effects increase with the level of anharmonicity. Temperature dependence is enhanced by low frequency modes. UB 3 LYP/6 -31 G(d) Paul Percival October 2012
48 Molecular vibrations affect hyperfine constants 197, 545 cm-1 Chem. Phys. 142 (1990) 229 -236. 385 cm-1 muon hfc An accurate calculation of hyperfine constants requires vibrational averaging over all normal modes. Isotope effects increase with the level of anharmonicity. Temperature dependence is enhanced by low frequency modes. UB 3 LYP/6 -31 G(d) Paul Percival October 2012
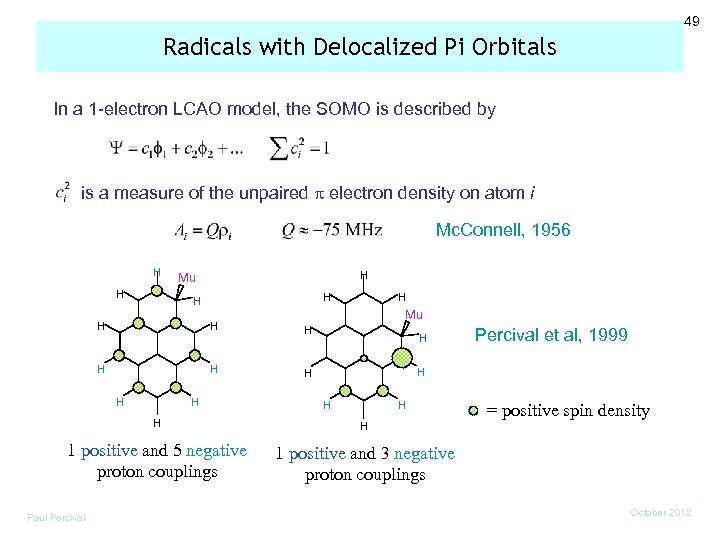 49 Radicals with Delocalized Pi Orbitals In a 1 -electron LCAO model, the SOMO is described by is a measure of the unpaired electron density on atom i Mc. Connell, 1956 H H H Mu H H H H H Percival et al, 1999 H H H 1 positive and 5 negative proton couplings = positive spin density 1 positive and 3 negative proton couplings Paul Percival October 2012
49 Radicals with Delocalized Pi Orbitals In a 1 -electron LCAO model, the SOMO is described by is a measure of the unpaired electron density on atom i Mc. Connell, 1956 H H H Mu H H H H H Percival et al, 1999 H H H 1 positive and 5 negative proton couplings = positive spin density 1 positive and 3 negative proton couplings Paul Percival October 2012
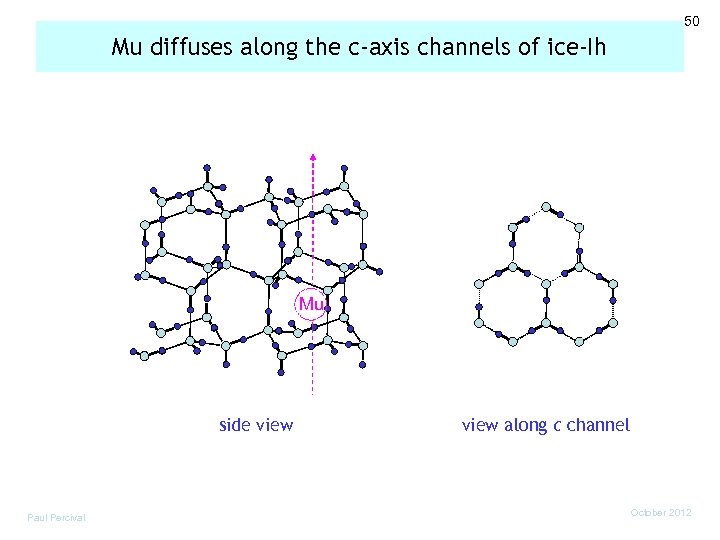 50 Mu diffuses along the c-axis channels of ice-Ih Mu side view Paul Percival view along c channel October 2012
50 Mu diffuses along the c-axis channels of ice-Ih Mu side view Paul Percival view along c channel October 2012
 Endohedral Muonium Mu@C 60 Muonium in a universe of its own Paul Percival October 2012
Endohedral Muonium Mu@C 60 Muonium in a universe of its own Paul Percival October 2012
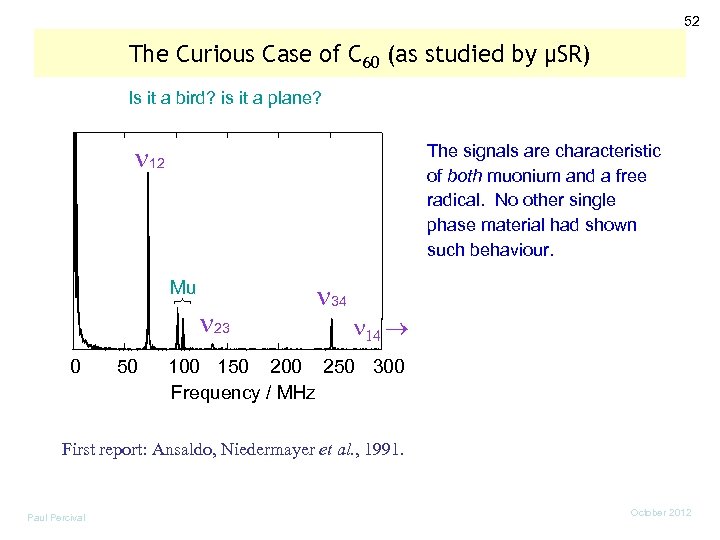 52 The Curious Case of C 60 (as studied by µSR) Is it a bird? is it a plane? 12 The signals are characteristic of both muonium and a free radical. No other single phase material had shown such behaviour. Mu 0 50 23 34 100 150 200 250 300 Frequency / MHz First report: Ansaldo, Niedermayer et al. , 1991. Paul Percival October 2012
52 The Curious Case of C 60 (as studied by µSR) Is it a bird? is it a plane? 12 The signals are characteristic of both muonium and a free radical. No other single phase material had shown such behaviour. Mu 0 50 23 34 100 150 200 250 300 Frequency / MHz First report: Ansaldo, Niedermayer et al. , 1991. Paul Percival October 2012
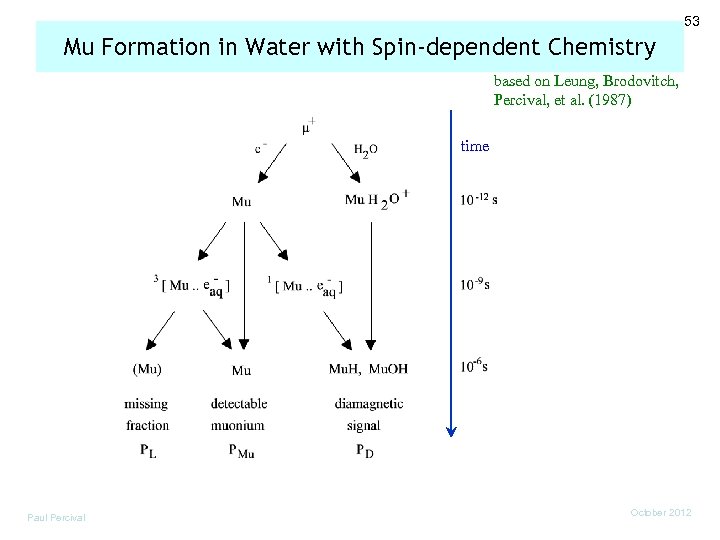 53 Mu Formation in Water with Spin-dependent Chemistry based on Leung, Brodovitch, Percival, et al. (1987) time Paul Percival October 2012
53 Mu Formation in Water with Spin-dependent Chemistry based on Leung, Brodovitch, Percival, et al. (1987) time Paul Percival October 2012
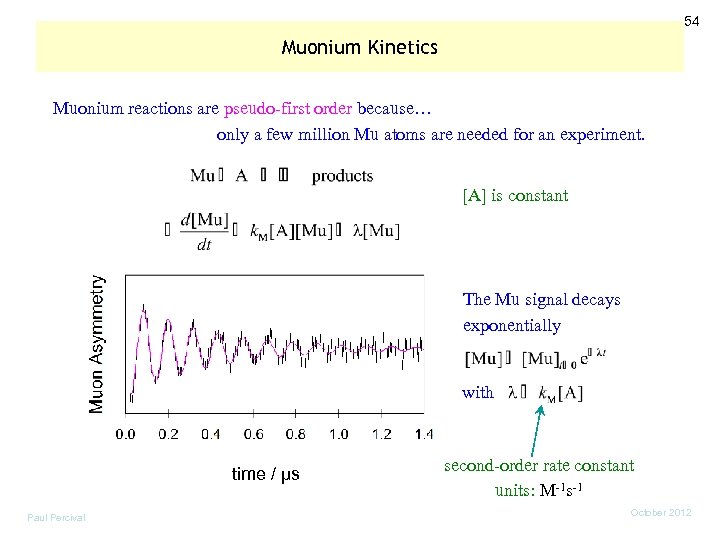 54 Muonium Kinetics Muonium reactions are pseudo-first order because… only a few million Mu atoms are needed for an experiment. [A] is constant The Mu signal decays exponentially with time / µs Paul Percival second-order rate constant units: M-1 s-1 October 2012
54 Muonium Kinetics Muonium reactions are pseudo-first order because… only a few million Mu atoms are needed for an experiment. [A] is constant The Mu signal decays exponentially with time / µs Paul Percival second-order rate constant units: M-1 s-1 October 2012
![55 The Ergodic Principle Complaint: What does [Mu] mean when we only have one 55 The Ergodic Principle Complaint: What does [Mu] mean when we only have one](https://present5.com/presentation/b97ad6531010c9824f52109b4dd47497/image-55.jpg) 55 The Ergodic Principle Complaint: What does [Mu] mean when we only have one Mu atom at a time? Answer: It doesn’t matter if the atoms are present at the same time or spread over an interval. The average of a parameter over time and the average over the statistical ensemble are the same Mu survival probability e–lt 37% t = 1/l Paul Percival time October 2012
55 The Ergodic Principle Complaint: What does [Mu] mean when we only have one Mu atom at a time? Answer: It doesn’t matter if the atoms are present at the same time or spread over an interval. The average of a parameter over time and the average over the statistical ensemble are the same Mu survival probability e–lt 37% t = 1/l Paul Percival time October 2012


