a1a46c504b972a563c24897b29ed82d4.ppt
- Количество слайдов: 41

Application of Westgard multi rules in medical laboratories Dr. Meliyanthi Gunatillaka Consultant Chemical Pathologist National Hospital of Srilanka Colombo

Components of Quality Assurance § Internal Quality Control (IQC) Steps taken by all health care professionals in their day to day activities to ensure generation of reliable laboratory results. (Prospective activity) Pre-analytical phase Analytical phase Post-analytical phase 3/19/2018 2

Components of Quality Assurance cont… § External Quality Assessment (EQA) Organized Inter-laboratory comparison Tool to assess IQC to improve performance Performed by an independent agency Retrospective and periodic 3/19/2018 3
Control of analytical phase • Validated analytical methods • Calibration of analytical procedure using traceability established calibration material • Quality control material in the assay Monitor the performance by Levy Jennings charts Application of Westgard multi rules • Equipment & Reagents 3/19/2018 4

• • Measurement Procedure • Is used to determine the value of a quantity • This estimate contains a measurement error, which is the difference between the obtained value and the true value of the measurand. • Measurement error has 2 components: Random error & systematic error 3/19/2018 5
Measurement Accuracy Is the extent of the agreement between the result of a measurement and the true value. ( True value of an analyte is obtained by using a reference method and CRM with stated uncertainties and traceability. )( Practically not possible) (Approximations are obtained using QC material) Accuracy encompasses the concepts of precision and trueness ( Earlier it was only the trueness) 3/19/2018 6
• ACCURACY • PRECISION Is the extent of agreement between independent results of repeated measurements • Imprecision : Expressed as SD or Coefficient of. Variation CV mmol/L or CV % • TRUNESS Is the extent of agreement between the mean value of repetitions and the true value of the measured • Expressed as a % of difference between the mean value of multiple repetitions and the true value. 3/19/2018 7

Random error Is an unpredictable analytical variation which influence each measurement differently in either a positive or negative direction and to a different extent in magnitude. 3/19/2018 8

• Possible causes of imprecision are • Wrong pipetting technique • Variable reaction timing and temperature of measurement procedures • Instrument instability 3/19/2018 9

Systematic error (bias) Constant systematic bias denotes a constant difference between the true value and the observed value regardless of the concentration level. Proportional systematic bias denotes a difference between the true value and the observed value, which changes proportionally as the concentration level changes. 3/19/2018 10
Possible causes of systematic errors Errors in the assigned value to the calibrator Deterioration of calibration material Incorrect sample or reagent volume pipetted Incorrect reaction timing or temperature Incorrect instrument setting (wavelength) Calculation errors Presence of interferents in samples(affect the individual samples) 3/19/2018 11

• Calculations • Mean If the distribution of results is a normal Gaussian curve the mean is the total score of all the measurements divided by the number of measurements. If not, remove the outliers (values with > 3 SD) and recalculate the mean. 3/19/2018 12
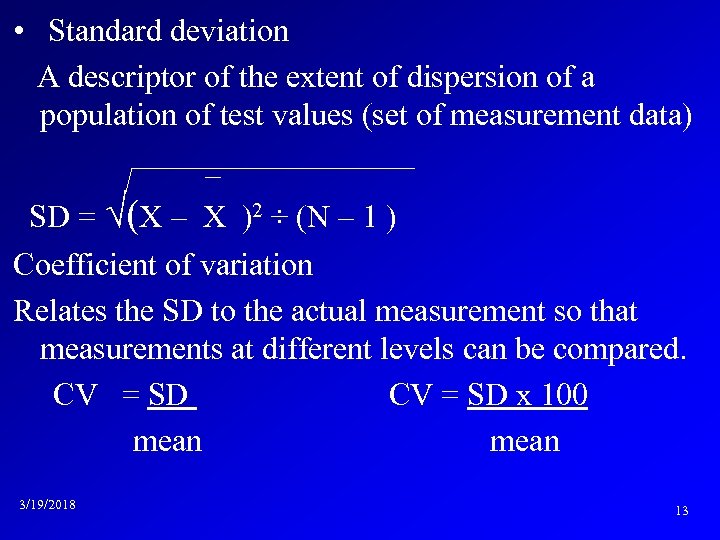
• Standard deviation A descriptor of the extent of dispersion of a population of test values (set of measurement data) _ SD = √(X – X )2 ÷ (N – 1 ) Coefficient of variation Relates the SD to the actual measurement so that measurements at different levels can be compared. CV = SD x 100 mean 3/19/2018 13

Selection of Quality control material Control materials should be used as they were patients samples and each and every one of the steps and stages that make up measurement procedure should be followed Essential characteristics Homogenicity: No significant differences between the parts that make up a lot of the control material (dispensing errors, variability in lyophylization due to residual water and reconstitution errors) ( Check the procedure for reconstitution of QC) 3/19/2018 14

• Stability The component that is analysed must be stable over a sufficient period of time. Once recontituted the aliquoted vials should be stored in appropriate temperatures according the manufacturers instructions. Labels : date of reconstituition No freezing and thawing cycles. ( ALP increases if stored at 2 - 80 C) 3/19/2018 15

• Value of the control quantity • Value should be close to the clinical decision level or cut off levels • If various cut off values exist, a number of control material can be used with different concentrations • Physiological level : upper limit of reference range • Pathological ranges : low, medium & high • Commutability The QC material must respond to the changes or alterations in the measurement procedure in the same way as patients samples. 3/19/2018 16

• Types of QC material Defined matrix material : non biological or systhetic matrix in the form of buffers, stabilizing agents Biological matrix: Derived from biological fluids, blood, plasma, serum & urine Matrix varies from lot to lot Human origin : especially for immunoassays Animal origin: cheaper and less risk of infections 3/19/2018 17

• Quality Control materials with assigned values • Manufacturers assigned value Method & instrument specific value Details of the certified reference material used ( with UM & stated references) Range and standard deviation should be given Laboratory assigned value The mean value obtained following minimum of 20 repititions should fall within the 2 SD of the method & instrument specific assigned value of the manufacturer ( preferably within the UM) If not : peer review world wide values : local agent 3/19/2018 iif 18
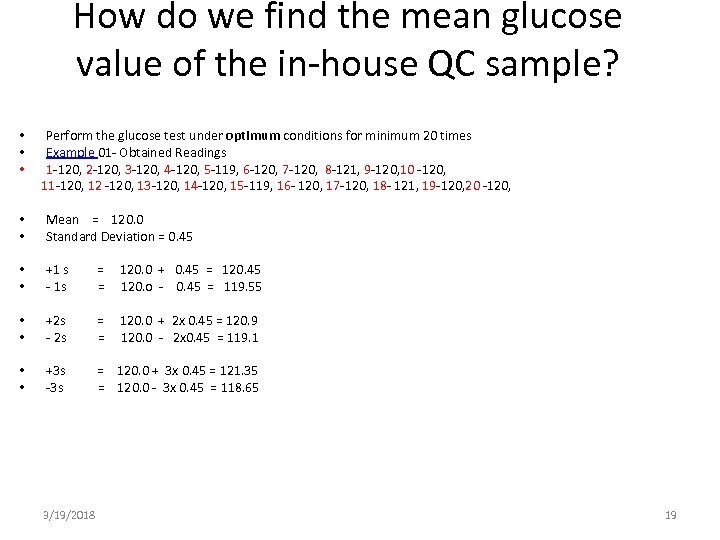
How do we find the mean glucose value of the in-house QC sample? • • • Perform the glucose test under optimum conditions for minimum 20 times Example 01 - Obtained Readings 1 -120, 2 -120, 3 -120, 4 -120, 5 -119, 6 -120, 7 -120, 8 -121, 9 -120, 10 -120, 11 -120, 12 -120, 13 -120, 14 -120, 15 -119, 16 - 120, 17 -120, 18 - 121, 19 -120, 20 -120, • • Mean = 120. 0 Standard Deviation = 0. 45 • • +1 s - 1 s = = 120. 0 + 0. 45 = 120. 45 120. o - 0. 45 = 119. 55 • • +2 s - 2 s = = 120. 0 + 2 x 0. 45 = 120. 9 120. 0 - 2 x 0. 45 = 119. 1 • • +3 s -3 s = 120. 0 + 3 x 0. 45 = 121. 35 = 120. 0 - 3 x 0. 45 = 118. 65 3/19/2018 19
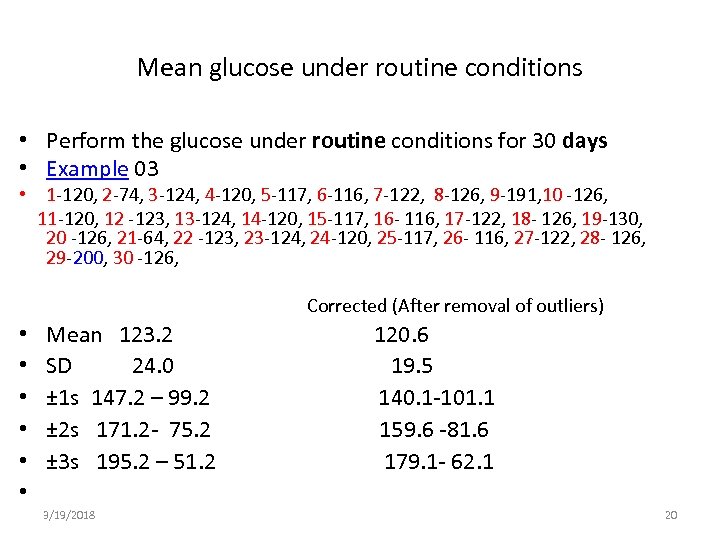
Mean glucose under routine conditions • Perform the glucose under routine conditions for 30 days • Example 03 • 1 -120, 2 -74, 3 -124, 4 -120, 5 -117, 6 -116, 7 -122, 8 -126, 9 -191, 10 -126, 11 -120, 12 -123, 13 -124, 14 -120, 15 -117, 16 - 116, 17 -122, 18 - 126, 19 -130, 20 -126, 21 -64, 22 -123, 23 -124, 24 -120, 25 -117, 26 - 116, 27 -122, 28 - 126, 29 -200, 30 -126, Corrected (After removal of outliers) • • • Mean 123. 2 SD 24. 0 ± 1 s 147. 2 – 99. 2 ± 2 s 171. 2 - 75. 2 ± 3 s 195. 2 – 51. 2 3/19/2018 120. 6 19. 5 140. 1 -101. 1 159. 6 -81. 6 179. 1 - 62. 1 20

QC Charts and rules § Shewhart/Levey and Jennings chart Analyze the QC material by the analytical method to be controlled on at least 20 times under optimal conditions and calculate the mean, standard deviation and CV % (OCV) Remove the outliers Construct the control chart : Y axis-control value X axis-days 3/19/2018 21
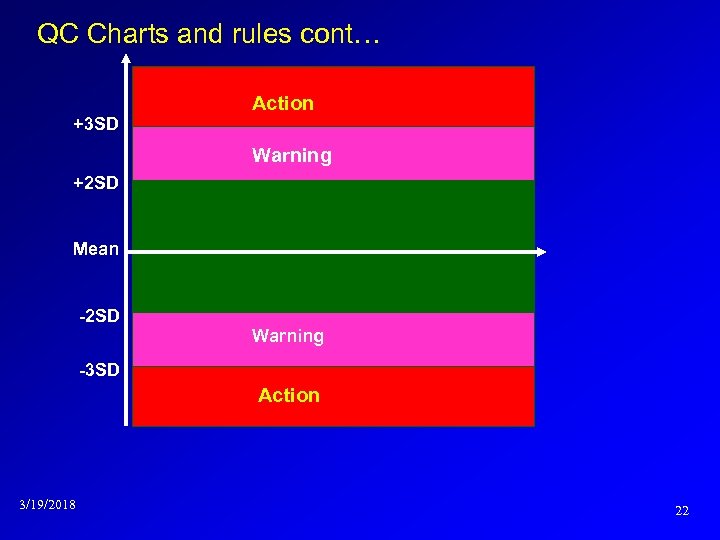
QC Charts and rules cont… +3 SD Action Warning +2 SD Mean -2 SD Warning -3 SD Action 3/19/2018 22
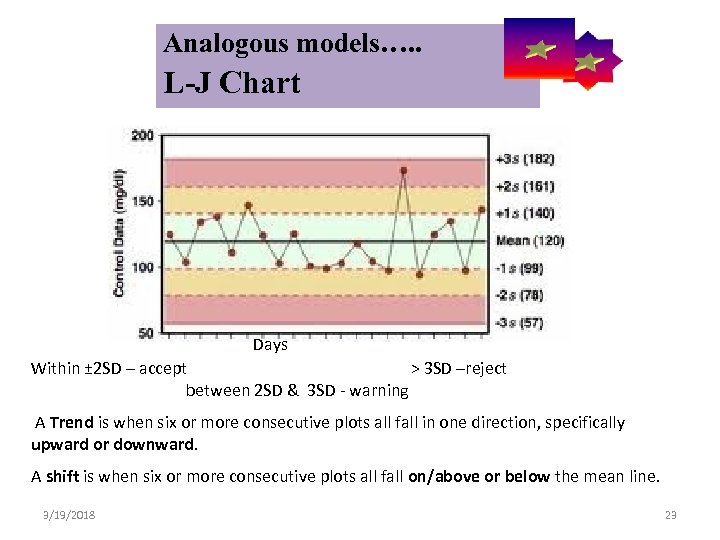
Analogous models…. . Control Symbolic Models Used in Internal Quality L-J Chart Days Within ± 2 SD – accept > 3 SD –reject between 2 SD & 3 SD - warning A Trend is when six or more consecutive plots all fall in one direction, specifically upward or downward. A shift is when six or more consecutive plots all fall on/above or below the mean line. 3/19/2018 23

QC Charts and rules cont… Introduce a control specimen daily to the analytical run, plot each value on the chart. After 20 days calculate the mean, SD and RCV, remove the outliers & recalculate the mean, SD and construct the chart, plot daily control value and follow the rules to accept/reject the run. 3/19/2018 24
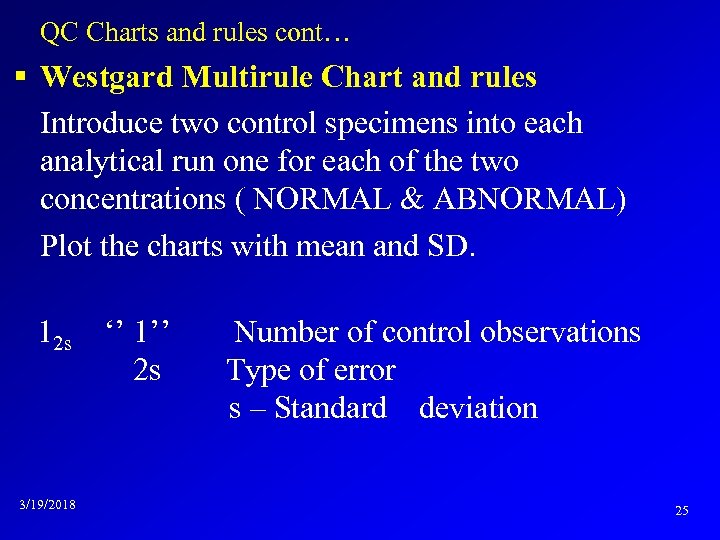
QC Charts and rules cont… § Westgard Multirule Chart and rules Introduce two control specimens into each analytical run one for each of the two concentrations ( NORMAL & ABNORMAL) Plot the charts with mean and SD. 12 s ‘’ 1’’ 2 s 3/19/2018 Number of control observations Type of error s – Standard deviation 25

QC Charts and rules cont… If both control results are within 2 SD from their target the batch is accepted If at least one control result is more than 2 SD from the target, the remaining rules are evaluated in turn, and the batch is rejected if any one rule is satisfied. If none is satisfied the batch is accepted. The situation should be investigated before the next batch is analyzed 3/19/2018 26

• Automatic chemistry analysers • Normal & abnormal QC : chemistry Daily & 50 th /100 th and at the end : day time night time : at the start • Low, medium & high QC: immunoassays Daily all 3 levels • Procedure : calibration of the method QC: all results within 2 SD : accept: run the samples IF one QC is out, check the violations of rules in other levels. Find the cause. ( calibration) Re run the QC : IF out again : new aliquot of QC All QC levels satisfactory – Run the samples 3/19/2018 27
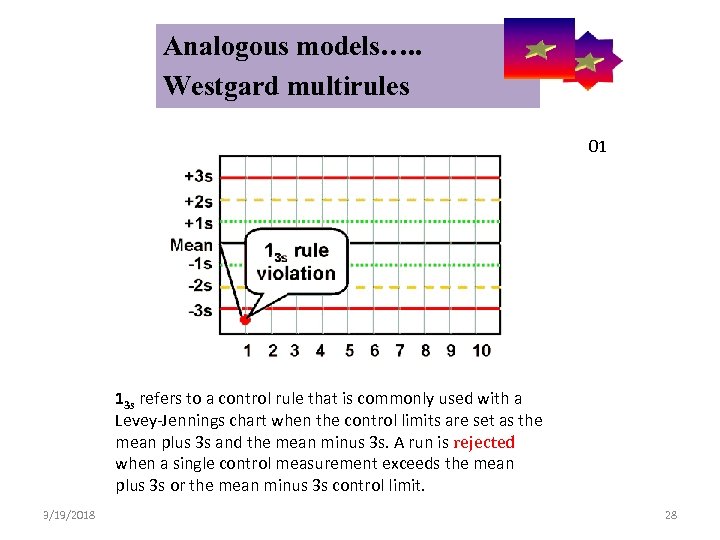
Analogous models…. . Control Symbolic Models Used in Internal Quality Westgard multirules 01 13 s refers to a control rule that is commonly used with a Levey-Jennings chart when the control limits are set as the mean plus 3 s and the mean minus 3 s. A run is rejected when a single control measurement exceeds the mean plus 3 s or the mean minus 3 s control limit. 3/19/2018 28
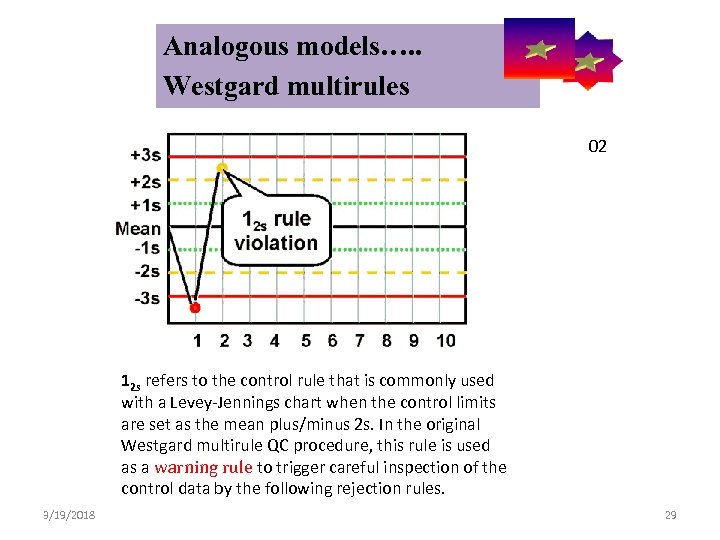
Analogous models…. . Control Symbolic Models Used in Internal Quality Westgard multirules 02 12 s refers to the control rule that is commonly used with a Levey-Jennings chart when the control limits are set as the mean plus/minus 2 s. In the original Westgard multirule QC procedure, this rule is used as a warning rule to trigger careful inspection of the control data by the following rejection rules. 3/19/2018 29
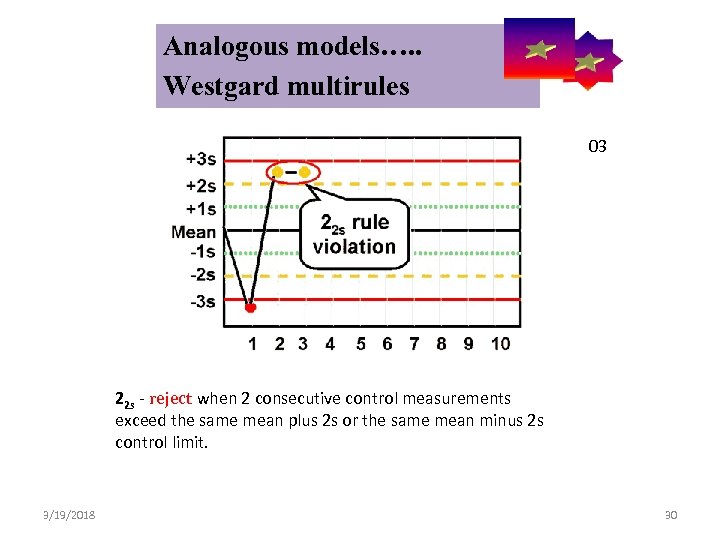
Analogous models…. . Control Symbolic Models Used in Internal Quality Westgard multirules 03 22 s - reject when 2 consecutive control measurements exceed the same mean plus 2 s or the same mean minus 2 s control limit. 3/19/2018 30
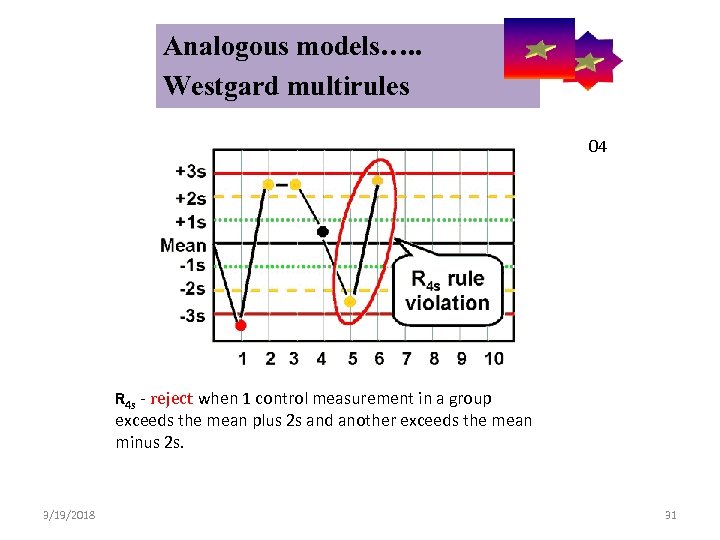
Analogous models…. . Control Symbolic Models Used in Internal Quality Westgard multirules 04 R 4 s - reject when 1 control measurement in a group exceeds the mean plus 2 s and another exceeds the mean minus 2 s. 3/19/2018 31
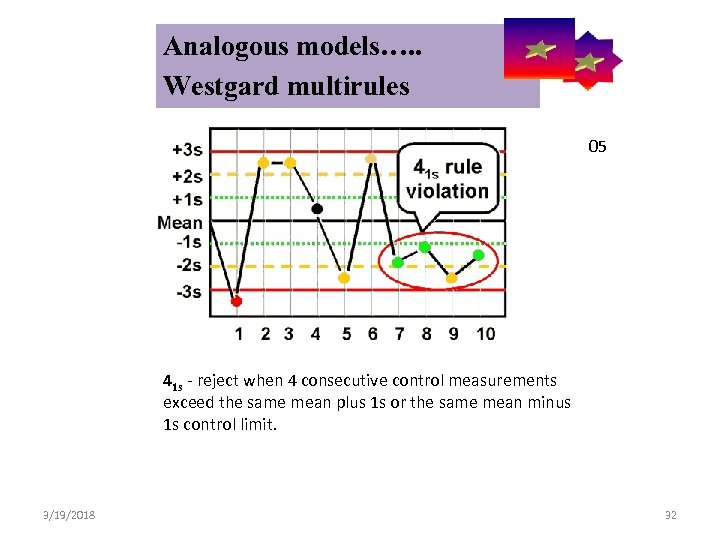
Analogous models…. . Control Symbolic Models Used in Internal Quality Westgard multirules 05 41 s - reject when 4 consecutive control measurements exceed the same mean plus 1 s or the same mean minus 1 s control limit. 3/19/2018 32
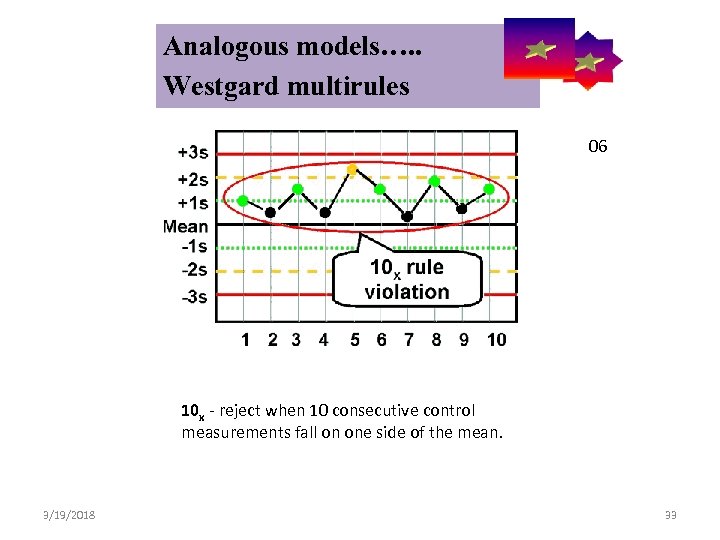
Analogous models…. . Control Symbolic Models Used in Internal Quality Westgard multirules 06 10 x - reject when 10 consecutive control measurements fall on one side of the mean. 3/19/2018 33
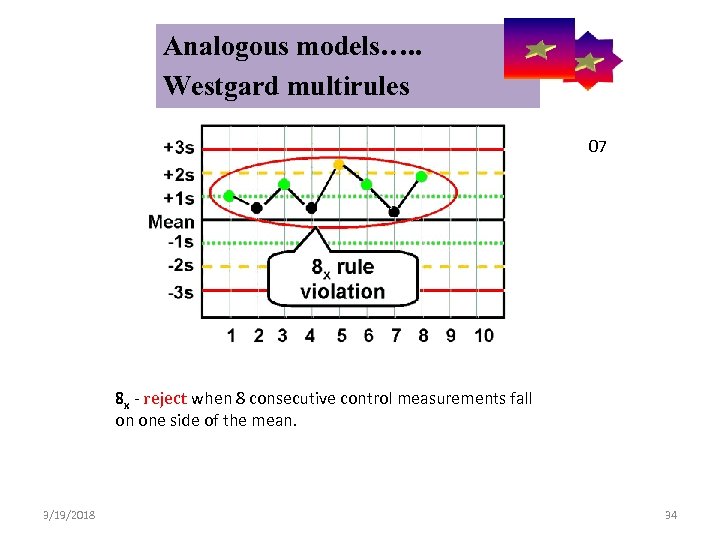
Analogous models…. . Control Symbolic Models Used in Internal Quality Westgard multirules 07 8 x - reject when 8 consecutive control measurements fall on one side of the mean. 3/19/2018 34
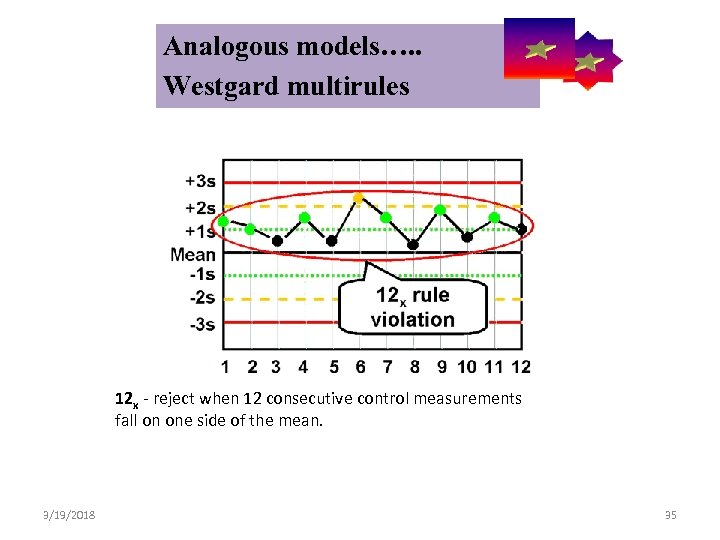
Analogous models…. . Control Symbolic Models Used in Internal Quality Westgard multirules 12 x - reject when 12 consecutive control measurements fall on one side of the mean. 3/19/2018 35
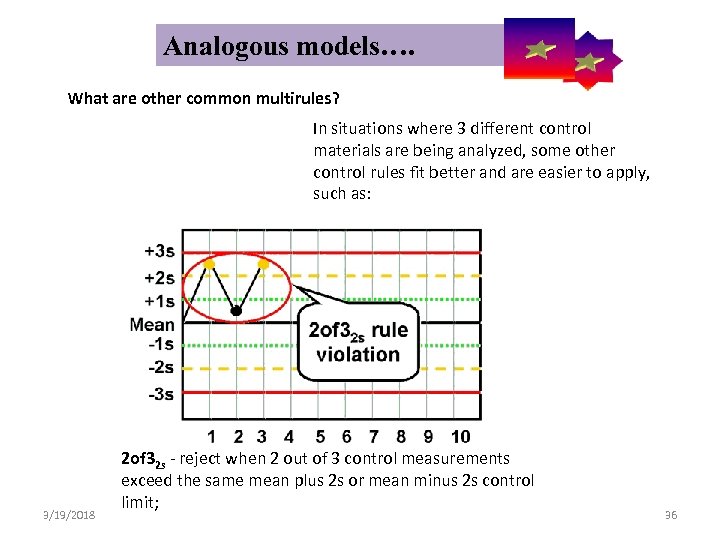
Analogous models…. Control Symbolic Models Used in Internal Quality What are other common multirules? 3/19/2018 In situations where 3 different control materials are being analyzed, some other control rules fit better and are easier to apply, such as: 2 of 32 s - reject when 2 out of 3 control measurements exceed the same mean plus 2 s or mean minus 2 s control limit; 36
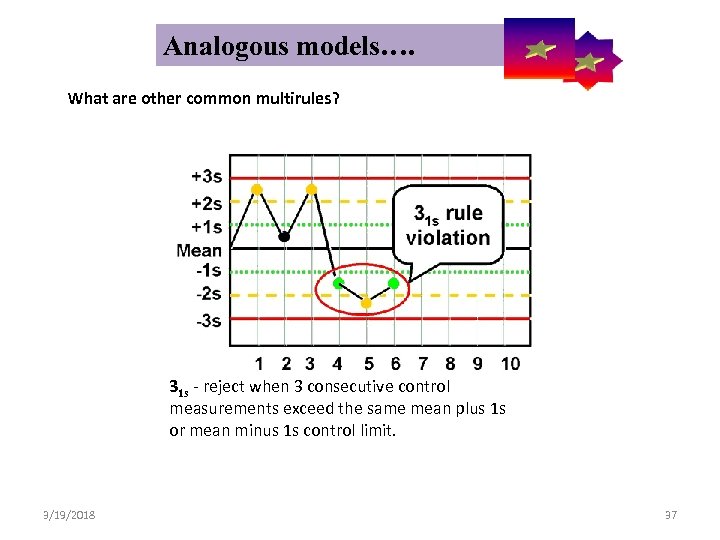
Analogous models…. Control Symbolic Models Used in Internal Quality What are other common multirules? 31 s - reject when 3 consecutive control measurements exceed the same mean plus 1 s or mean minus 1 s control limit. 3/19/2018 37
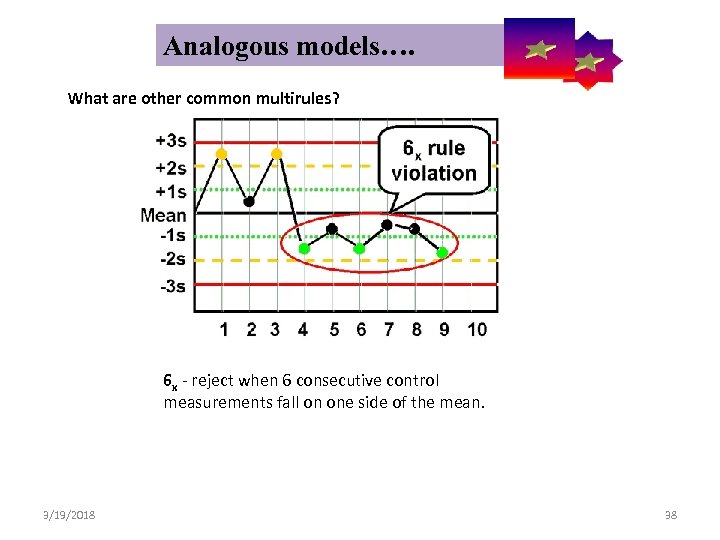
Analogous models…. Control Symbolic Models Used in Internal Quality What are other common multirules? 6 x - reject when 6 consecutive control measurements fall on one side of the mean. 3/19/2018 38
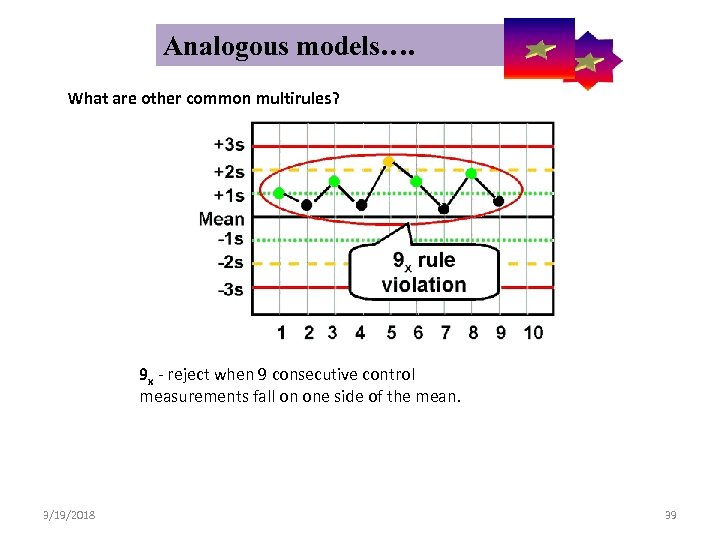
Analogous models…. Control Symbolic Models Used in Internal Quality What are other common multirules? 9 x - reject when 9 consecutive control measurements fall on one side of the mean. 3/19/2018 39
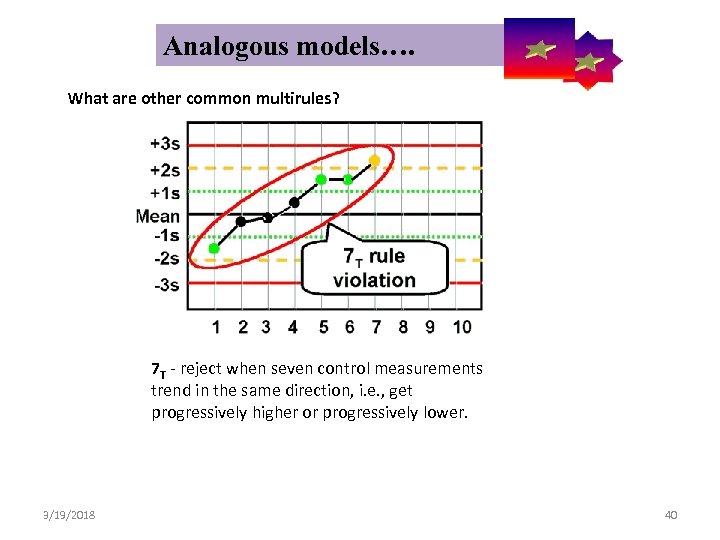
Analogous models…. Control Symbolic Models Used in Internal Quality What are other common multirules? 7 T - reject when seven control measurements trend in the same direction, i. e. , get progressively higher or progressively lower. 3/19/2018 40

Thank you 3/19/2018 41
a1a46c504b972a563c24897b29ed82d4.ppt