a08239e2a0ed08dda9e4cd7616a44903.ppt
- Количество слайдов: 23
 Application of IR Raman Spectroscopy • 3 IR regions • Structure and Functional Group • • • Absorption IR Reflection IR Photoacoustic IR IR Emission Micro 10 -1
Application of IR Raman Spectroscopy • 3 IR regions • Structure and Functional Group • • • Absorption IR Reflection IR Photoacoustic IR IR Emission Micro 10 -1
 Mid-IR • Mid-IR absorption § Samples à Placed in cell (salt) à Combined with oil § Need cell that does not absorb IR à KBr, Na. Cl * Tends to absorb water § Gases § Solutions à Solvent issues * Dissolution of cell 10 -2
Mid-IR • Mid-IR absorption § Samples à Placed in cell (salt) à Combined with oil § Need cell that does not absorb IR à KBr, Na. Cl * Tends to absorb water § Gases § Solutions à Solvent issues * Dissolution of cell 10 -2
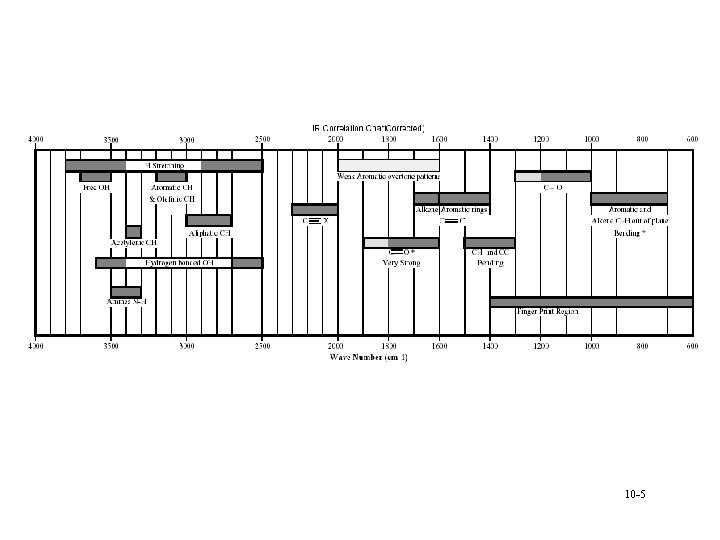
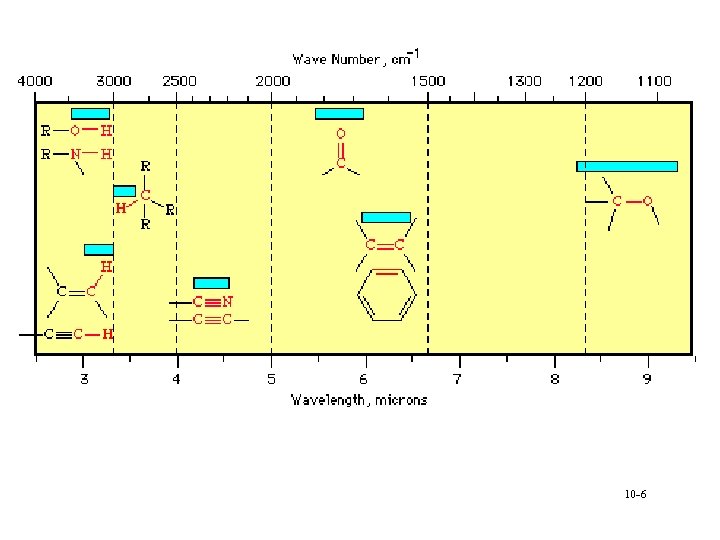
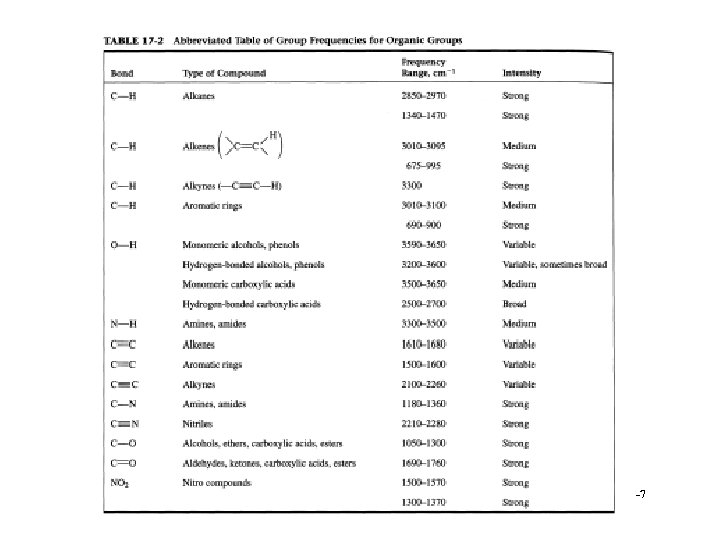
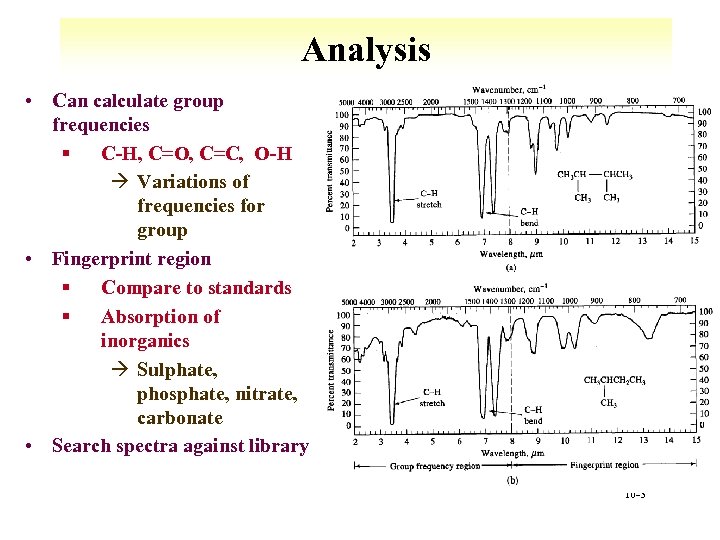 Analysis • Can calculate group frequencies § C-H, C=O, C=C, O-H à Variations of frequencies for group • Fingerprint region § Compare to standards § Absorption of inorganics à Sulphate, phosphate, nitrate, carbonate • Search spectra against library 10 -3
Analysis • Can calculate group frequencies § C-H, C=O, C=C, O-H à Variations of frequencies for group • Fingerprint region § Compare to standards § Absorption of inorganics à Sulphate, phosphate, nitrate, carbonate • Search spectra against library 10 -3
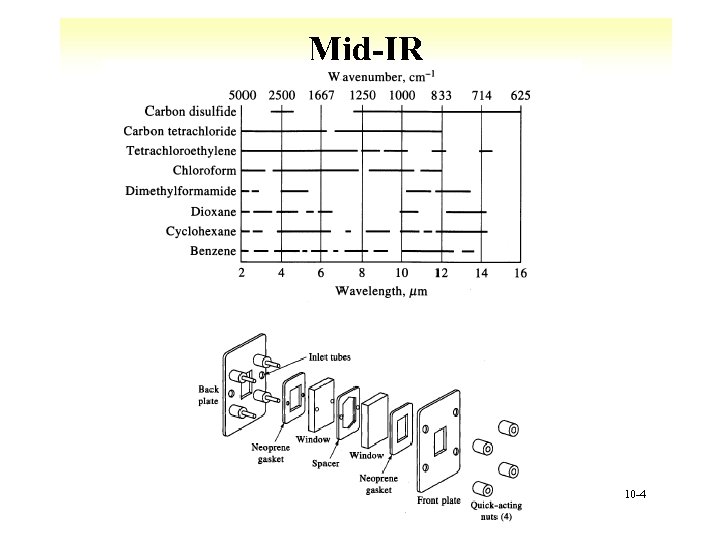 Mid-IR 10 -4
Mid-IR 10 -4
 10 -5
10 -5
 10 -6
10 -6
 10 -7
10 -7
 Interpretation • • • Alcohols and amines display strong broad O-H and N-H stretching bands in the region 3400 -3100 cm-1 § bands are broadened due to hydrogen bonding and a sharp 'non-bonded' peak can around 3400 cm-1. Alkene and alkyne C-H bonds display sharp stretching absorptions in the region 3100 -3000 cm-1 § bands are of medium intensity often obscured (i. e. , OH). Triple bond stretching absorptions occur in the region 2400 -2200 cm-1 § Nitriles are generally of medium intensity and are clearly defined § Alkynes absorb weakly unless they are highly asymmetric à symmetrical alkynes do not show absorption bands Carbonyl stretching bands occur in the region 1800 -1700 cm-1 § bands are generally very strong and broad § Carbonyl compounds (acyl halides, esters) are generally at higher wave number than simple ketones and aldehydes § amides are the lowest, absorbing in the region 1700 -1650 cm-1 Carbon-carbon double bond stretching occurs in the region around 1650 -1600 cm-1 § bands are generally sharp and of medium intensity § Aromatic compounds will display a series of sharp bands Carbon-oxygen single bonds display stretching bands in the region 1200 -1100 cm-1 § bands are generally strong and broad 10 -8
Interpretation • • • Alcohols and amines display strong broad O-H and N-H stretching bands in the region 3400 -3100 cm-1 § bands are broadened due to hydrogen bonding and a sharp 'non-bonded' peak can around 3400 cm-1. Alkene and alkyne C-H bonds display sharp stretching absorptions in the region 3100 -3000 cm-1 § bands are of medium intensity often obscured (i. e. , OH). Triple bond stretching absorptions occur in the region 2400 -2200 cm-1 § Nitriles are generally of medium intensity and are clearly defined § Alkynes absorb weakly unless they are highly asymmetric à symmetrical alkynes do not show absorption bands Carbonyl stretching bands occur in the region 1800 -1700 cm-1 § bands are generally very strong and broad § Carbonyl compounds (acyl halides, esters) are generally at higher wave number than simple ketones and aldehydes § amides are the lowest, absorbing in the region 1700 -1650 cm-1 Carbon-carbon double bond stretching occurs in the region around 1650 -1600 cm-1 § bands are generally sharp and of medium intensity § Aromatic compounds will display a series of sharp bands Carbon-oxygen single bonds display stretching bands in the region 1200 -1100 cm-1 § bands are generally strong and broad 10 -8
 Quantitative IR • Difficult to obtain reliable quantitative data based on IR § Deviations from Beer’s law à Narrow Bands and wide slit widths required * Require calibration sources § Complex spectra § Weak beam § Lack of reference cell à Need to normalize refraction * Take reference and sample with same cell 10 -9
Quantitative IR • Difficult to obtain reliable quantitative data based on IR § Deviations from Beer’s law à Narrow Bands and wide slit widths required * Require calibration sources § Complex spectra § Weak beam § Lack of reference cell à Need to normalize refraction * Take reference and sample with same cell 10 -9
 Other methods • Reflectance IR § Measurement of absorbance from reflected IR à Surface measurement • Photoacoustic IR § can use tunable laser • Near IR § 700 nm to 2500 nm à Quantitative analysis of samples * CH, NH, and OH Ø Low absorption • Emission IR 10 -10
Other methods • Reflectance IR § Measurement of absorbance from reflected IR à Surface measurement • Photoacoustic IR § can use tunable laser • Near IR § 700 nm to 2500 nm à Quantitative analysis of samples * CH, NH, and OH Ø Low absorption • Emission IR 10 -10
 Raman Spectroscopy • Scattering of light § Fraction of scattered light in the visible differs from incident beam à Difference based on molecular structure * Based on quantized vibrational changes * Difference between incident and scattered light is in mid-IR region § No water interference à Can examine aqueous samples § Quartz or glass cells can be used § Competition with fluorescence 10 -11
Raman Spectroscopy • Scattering of light § Fraction of scattered light in the visible differs from incident beam à Difference based on molecular structure * Based on quantized vibrational changes * Difference between incident and scattered light is in mid-IR region § No water interference à Can examine aqueous samples § Quartz or glass cells can be used § Competition with fluorescence 10 -11
 Raman Spectroscopy • Theory • Instrumentation • Application • Method § Excitation with UV or NIR § Measurement of scatter at 90 ° àMeasurement 1 E-5 of incident beam 10 -12
Raman Spectroscopy • Theory • Instrumentation • Application • Method § Excitation with UV or NIR § Measurement of scatter at 90 ° àMeasurement 1 E-5 of incident beam 10 -12
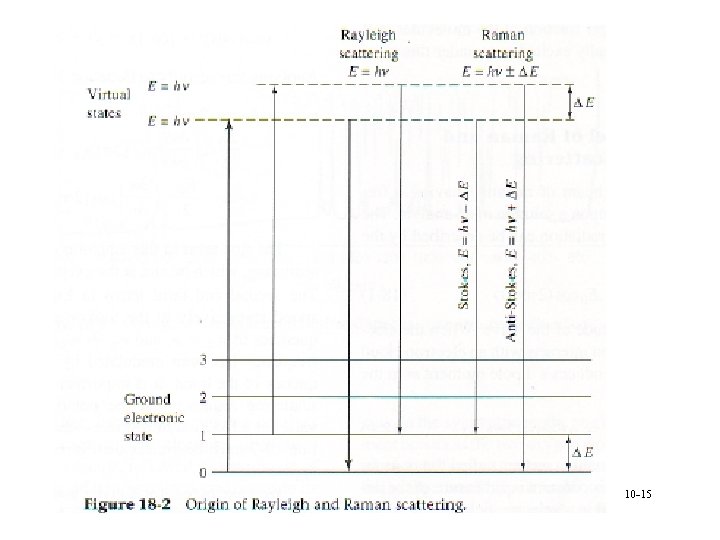
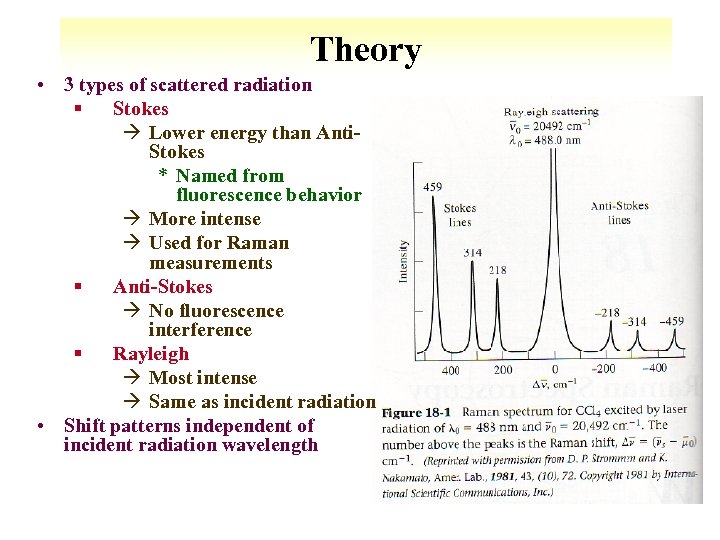 Theory • 3 types of scattered radiation § Stokes à Lower energy than Anti. Stokes * Named from fluorescence behavior à More intense à Used for Raman measurements § Anti-Stokes à No fluorescence interference § Rayleigh à Most intense à Same as incident radiation • Shift patterns independent of incident radiation wavelength 10 -13
Theory • 3 types of scattered radiation § Stokes à Lower energy than Anti. Stokes * Named from fluorescence behavior à More intense à Used for Raman measurements § Anti-Stokes à No fluorescence interference § Rayleigh à Most intense à Same as incident radiation • Shift patterns independent of incident radiation wavelength 10 -13
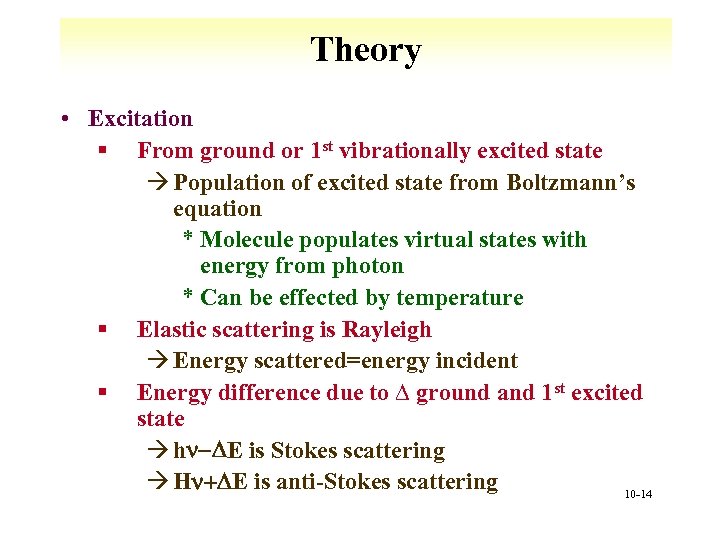 Theory • Excitation § From ground or 1 st vibrationally excited state à Population of excited state from Boltzmann’s equation * Molecule populates virtual states with energy from photon * Can be effected by temperature § Elastic scattering is Rayleigh à Energy scattered=energy incident § Energy difference due to ∆ ground and 1 st excited state à hn-DE is Stokes scattering à Hn+DE is anti-Stokes scattering 10 -14
Theory • Excitation § From ground or 1 st vibrationally excited state à Population of excited state from Boltzmann’s equation * Molecule populates virtual states with energy from photon * Can be effected by temperature § Elastic scattering is Rayleigh à Energy scattered=energy incident § Energy difference due to ∆ ground and 1 st excited state à hn-DE is Stokes scattering à Hn+DE is anti-Stokes scattering 10 -14
 10 -15
10 -15
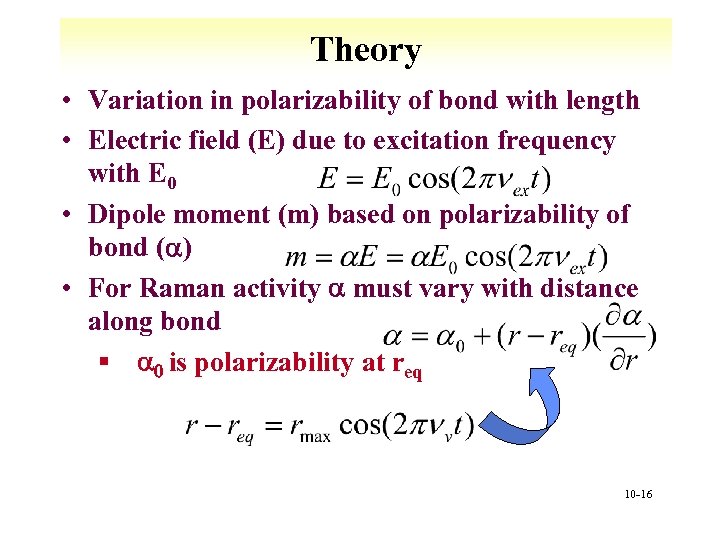 Theory • Variation in polarizability of bond with length • Electric field (E) due to excitation frequency with E 0 • Dipole moment (m) based on polarizability of bond (a) • For Raman activity a must vary with distance along bond § a 0 is polarizability at req 10 -16
Theory • Variation in polarizability of bond with length • Electric field (E) due to excitation frequency with E 0 • Dipole moment (m) based on polarizability of bond (a) • For Raman activity a must vary with distance along bond § a 0 is polarizability at req 10 -16
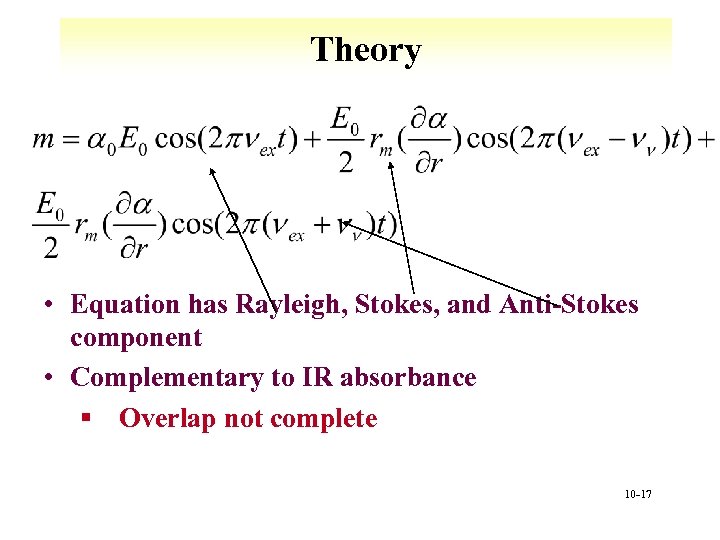 Theory • Equation has Rayleigh, Stokes, and Anti-Stokes component • Complementary to IR absorbance § Overlap not complete 10 -17
Theory • Equation has Rayleigh, Stokes, and Anti-Stokes component • Complementary to IR absorbance § Overlap not complete 10 -17
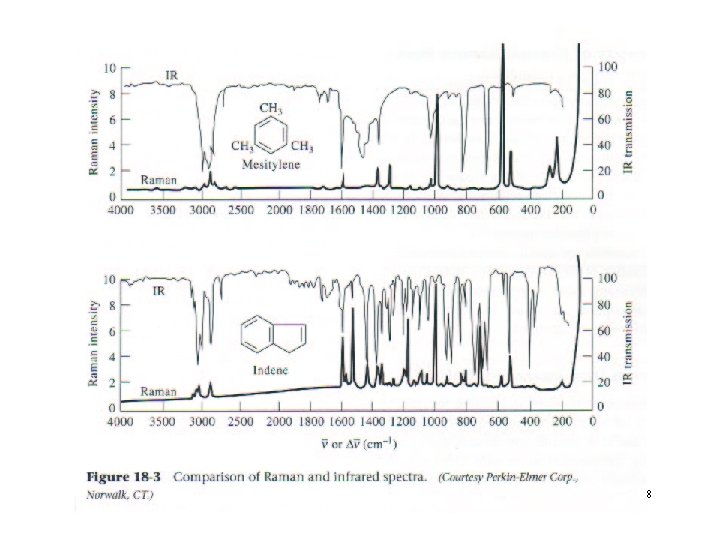 10 -18
10 -18
 Instrumentation • Laser source § Ar (488 nm, 514. 5 nm) § Kr (530. 9 nm, 647. 1 nm) § He/Ne (623 nm) § Diode (782 nm or 830 nm) § Nd/YAG (1064 nm) § Tunable lasers àIntensity proportional to n 4 * Consider energy and chemical effect of absorbing energy 10 -19
Instrumentation • Laser source § Ar (488 nm, 514. 5 nm) § Kr (530. 9 nm, 647. 1 nm) § He/Ne (623 nm) § Diode (782 nm or 830 nm) § Nd/YAG (1064 nm) § Tunable lasers àIntensity proportional to n 4 * Consider energy and chemical effect of absorbing energy 10 -19
 Instrumentation • Sample holder § Glass § Laser focusing allows small sample size § Liquid and solid samples can be examined § Use of fiber optics 10 -20
Instrumentation • Sample holder § Glass § Laser focusing allows small sample size § Liquid and solid samples can be examined § Use of fiber optics 10 -20
 Applications • Laser microprobes § Use of laser permits small sampling area • Resonance Raman § Use electronic absorption peak § Low concentrations can be examined à Lifetimes on 10 fs • Surface enhanced Raman § Increase of sensitivity by 1000 to 1 E 6 10 -21
Applications • Laser microprobes § Use of laser permits small sampling area • Resonance Raman § Use electronic absorption peak § Low concentrations can be examined à Lifetimes on 10 fs • Surface enhanced Raman § Increase of sensitivity by 1000 to 1 E 6 10 -21
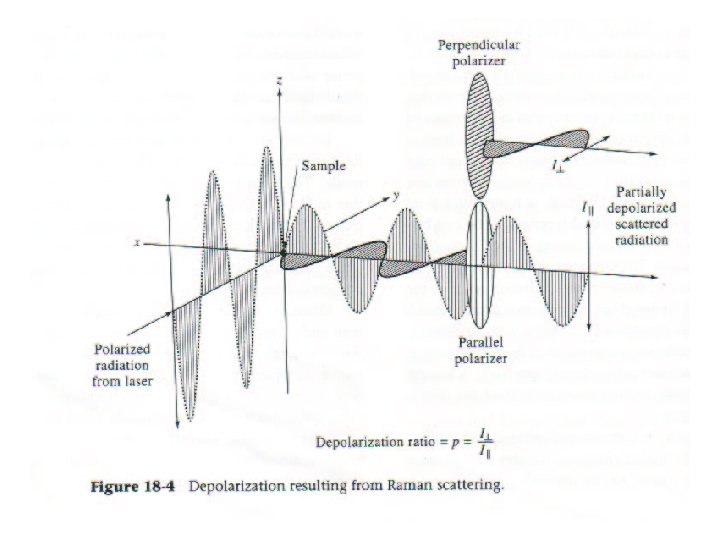 10 -22
10 -22
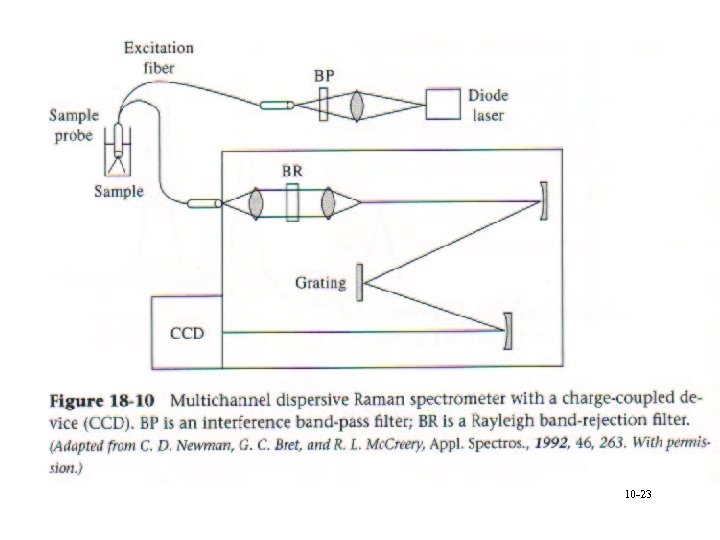 10 -23
10 -23


