d8762ca9ba32815910cc2be495da59a2.ppt
- Количество слайдов: 81
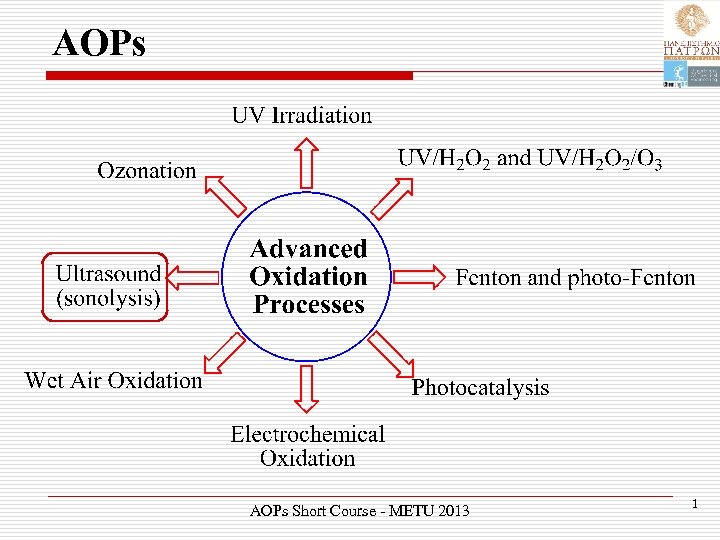 AOPs Short Course - METU 2013 1
AOPs Short Course - METU 2013 1
 Ultrasound processes Ø Research into the use of ultrasound in environmental protection has received a considerable amount of attention. Ø The majority of investigations focus on the harnessing of cavitational effects for the destruction of biological or chemical pollutants in water and wastewater. AOPs Short Course - METU 2013 2
Ultrasound processes Ø Research into the use of ultrasound in environmental protection has received a considerable amount of attention. Ø The majority of investigations focus on the harnessing of cavitational effects for the destruction of biological or chemical pollutants in water and wastewater. AOPs Short Course - METU 2013 2
 Acoustic cavitation Ø The chemical effects of ultrasound irradiation are the result of the phenomenon of ultrasonically induced acoustic cavitation. Ø Use of power ultrasound in the range 20 -100 k. Hz. AOPs Short Course - METU 2013 3
Acoustic cavitation Ø The chemical effects of ultrasound irradiation are the result of the phenomenon of ultrasonically induced acoustic cavitation. Ø Use of power ultrasound in the range 20 -100 k. Hz. AOPs Short Course - METU 2013 3
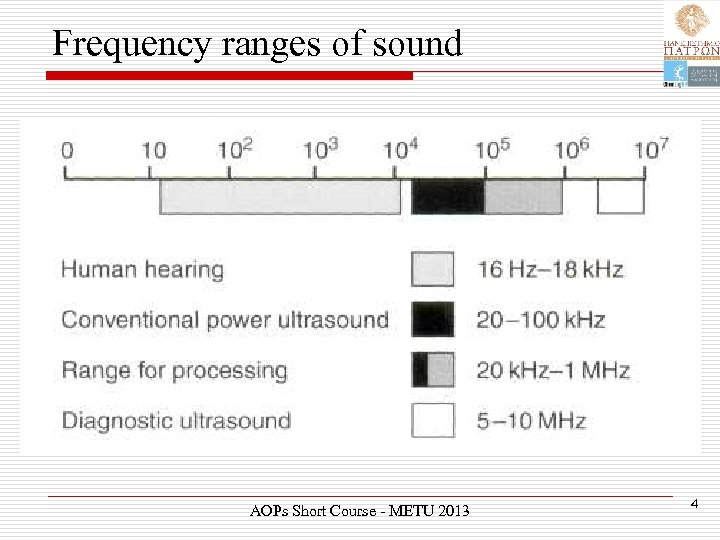 Frequency ranges of sound AOPs Short Course - METU 2013 4
Frequency ranges of sound AOPs Short Course - METU 2013 4
 Acoustic cavitation Ø Power ultrasound enhances chemical and physical changes in a liquid medium through the generation and subsequent destruction of cavitation bubbles. Ø Like any sound wave, ultrasound is propagated via a series of compression and rarefaction waves induced in the molecules of the medium through which it passes. AOPs Short Course - METU 2013 5
Acoustic cavitation Ø Power ultrasound enhances chemical and physical changes in a liquid medium through the generation and subsequent destruction of cavitation bubbles. Ø Like any sound wave, ultrasound is propagated via a series of compression and rarefaction waves induced in the molecules of the medium through which it passes. AOPs Short Course - METU 2013 5
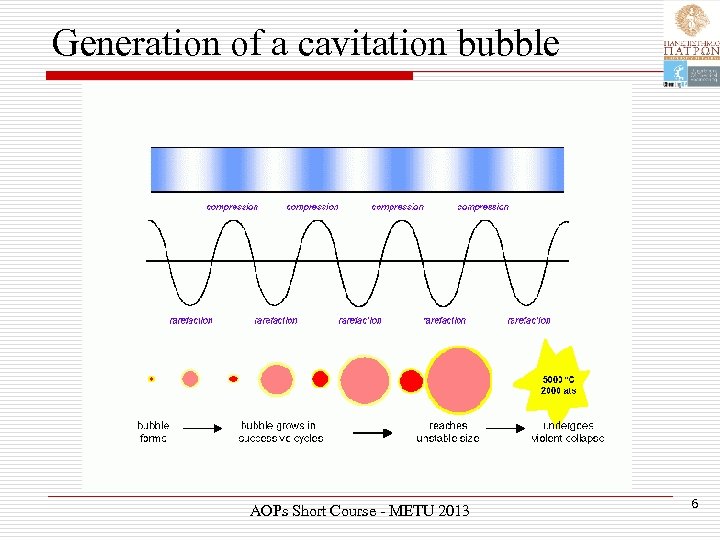 Generation of a cavitation bubble AOPs Short Course - METU 2013 6
Generation of a cavitation bubble AOPs Short Course - METU 2013 6
 Cavitation bubbles Ø At sufficiently high power the rarefaction cycle may exceed the attractive forces of the molecules of the liquid and cavitation bubbles will form. Ø Such bubbles grow by a process known as rectified diffusion, that is small amounts of vapour (or gas) from the medium enters the bubble during its expansion phase and is not fully expelled during compression. AOPs Short Course - METU 2013 7
Cavitation bubbles Ø At sufficiently high power the rarefaction cycle may exceed the attractive forces of the molecules of the liquid and cavitation bubbles will form. Ø Such bubbles grow by a process known as rectified diffusion, that is small amounts of vapour (or gas) from the medium enters the bubble during its expansion phase and is not fully expelled during compression. AOPs Short Course - METU 2013 7
 Cavitation bubbles collapse Ø The bubbles grow over the period of a few cycles to an equilibrium size for the particular frequency applied. Ø It is the fate of these bubbles when they collapse in succeeding compression cycles that generates the energy for chemical and mechanical effects. AOPs Short Course - METU 2013 8
Cavitation bubbles collapse Ø The bubbles grow over the period of a few cycles to an equilibrium size for the particular frequency applied. Ø It is the fate of these bubbles when they collapse in succeeding compression cycles that generates the energy for chemical and mechanical effects. AOPs Short Course - METU 2013 8
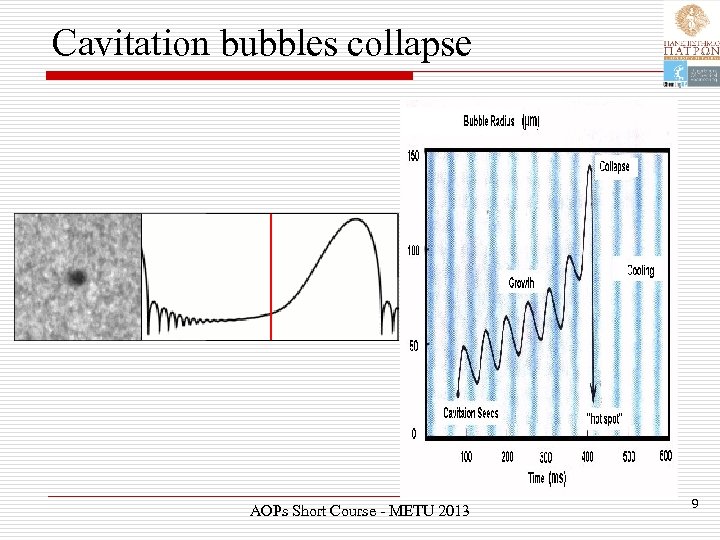 Cavitation bubbles collapse AOPs Short Course - METU 2013 9
Cavitation bubbles collapse AOPs Short Course - METU 2013 9
 “Hotspots” Ø Cavitation bubble collapse is a remarkable phenomenon induced throughout the liquid by the power of sound. Ø In aqueous systems at an ultrasonic frequency of 20 k. Hz each cavitation bubble collapse acts as a localized hotspot generating temperatures of about 4000 K and pressures in excess of 1000 atmospheres. AOPs Short Course - METU 2013 10
“Hotspots” Ø Cavitation bubble collapse is a remarkable phenomenon induced throughout the liquid by the power of sound. Ø In aqueous systems at an ultrasonic frequency of 20 k. Hz each cavitation bubble collapse acts as a localized hotspot generating temperatures of about 4000 K and pressures in excess of 1000 atmospheres. AOPs Short Course - METU 2013 10
 Essential components Ø ü ü Ø Two essential components: a liquid medium a source of high-energy vibrations. The liquid medium is necessary because sonochemistry is driven by acoustic cavitation and this can only occur in liquids. Ø The source of the vibrational energy is the transducer. AOPs Short Course - METU 2013 11
Essential components Ø ü ü Ø Two essential components: a liquid medium a source of high-energy vibrations. The liquid medium is necessary because sonochemistry is driven by acoustic cavitation and this can only occur in liquids. Ø The source of the vibrational energy is the transducer. AOPs Short Course - METU 2013 11
 Equipment Ø The two most common pieces of equipment that are used for the generation of acoustic cavitation in the laboratory are: ü the ultrasonic bath ü the probe system (more powerful) AOPs Short Course - METU 2013 12
Equipment Ø The two most common pieces of equipment that are used for the generation of acoustic cavitation in the laboratory are: ü the ultrasonic bath ü the probe system (more powerful) AOPs Short Course - METU 2013 12
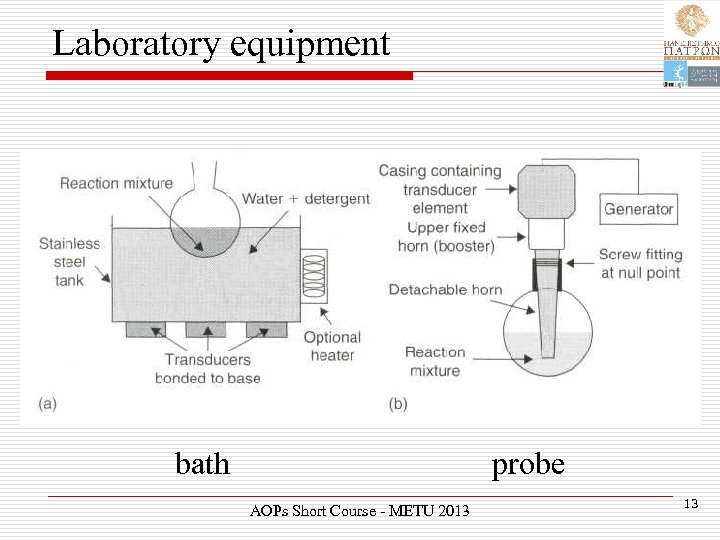 Laboratory equipment probe bath AOPs Short Course - METU 2013 13
Laboratory equipment probe bath AOPs Short Course - METU 2013 13
 Effects of cavitation bubbles Ø The cavitation bubble has a variety of effects within the liquid medium depending upon the type of system in which it is generated. These systems can be broadly divided into ü homogeneous liquid ü heterogeneous solid/liquid ü heterogeneous liquid/liquid AOPs Short Course - METU 2013 14
Effects of cavitation bubbles Ø The cavitation bubble has a variety of effects within the liquid medium depending upon the type of system in which it is generated. These systems can be broadly divided into ü homogeneous liquid ü heterogeneous solid/liquid ü heterogeneous liquid/liquid AOPs Short Course - METU 2013 14
 Homogeneous liquid-phase reactions Ø There are three major zones in which cavitation collapse can influence such systems: Ø In the bulk liquid immediately surrounding the bubble (shear forces). Ø In the bubble itself (extreme conditions of temperature and pressure on collapse lead to chemical effects). AOPs Short Course - METU 2013 15
Homogeneous liquid-phase reactions Ø There are three major zones in which cavitation collapse can influence such systems: Ø In the bulk liquid immediately surrounding the bubble (shear forces). Ø In the bubble itself (extreme conditions of temperature and pressure on collapse lead to chemical effects). AOPs Short Course - METU 2013 15
 Ø Interface of the bubble and bulk liquid (chemical activity occurs). Ø It is where surfactant species might accumulate and also the region where radicals formed within the bubble begin to react with species in the bulk medium. AOPs Short Course - METU 2013 16
Ø Interface of the bubble and bulk liquid (chemical activity occurs). Ø It is where surfactant species might accumulate and also the region where radicals formed within the bubble begin to react with species in the bulk medium. AOPs Short Course - METU 2013 16
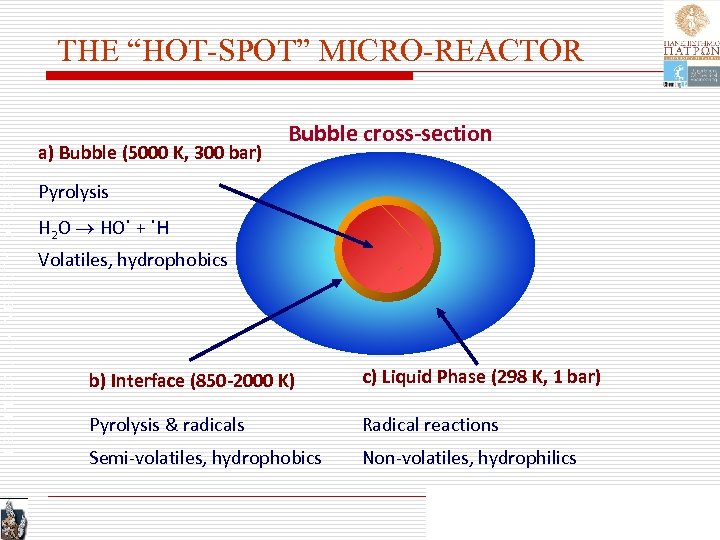 TECHNICAL UNIVERSITY OF CRETE THE “HOT-SPOT” MICRO-REACTOR a) Bubble (5000 K, 300 bar) Pyrolysis Bubble cross-section . . H 2 O HO + H Volatiles, hydrophobics b) Interface (850 -2000 K) c) Liquid Phase (298 K, 1 bar) Pyrolysis & radicals Radical reactions Semi-volatiles, hydrophobics Non-volatiles, hydrophilics
TECHNICAL UNIVERSITY OF CRETE THE “HOT-SPOT” MICRO-REACTOR a) Bubble (5000 K, 300 bar) Pyrolysis Bubble cross-section . . H 2 O HO + H Volatiles, hydrophobics b) Interface (850 -2000 K) c) Liquid Phase (298 K, 1 bar) Pyrolysis & radicals Radical reactions Semi-volatiles, hydrophobics Non-volatiles, hydrophilics
 Chemical reactions Ø When water is sonicated the extreme conditions generated on collapse of the cavitation bubbles are sufficient to cause rupture of the O—H bond itself. Ø This results in the formation of radical species and the production of oxygen gas and hydrogen peroxide. AOPs Short Course - METU 2013 18
Chemical reactions Ø When water is sonicated the extreme conditions generated on collapse of the cavitation bubbles are sufficient to cause rupture of the O—H bond itself. Ø This results in the formation of radical species and the production of oxygen gas and hydrogen peroxide. AOPs Short Course - METU 2013 18
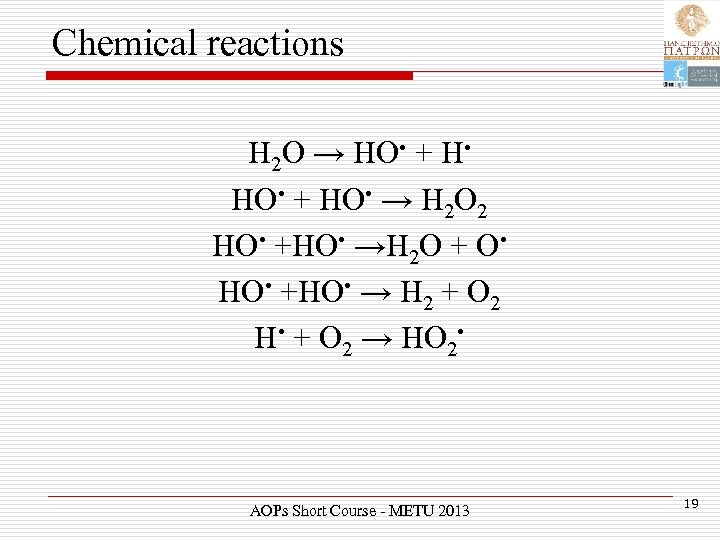 Chemical reactions H 2 O → HO • + H • HO • + HO • → H 2 O 2 HO • +HO • →H 2 O + O • HO • +HO • → H 2 + O 2 H • + O 2 → HO 2 • AOPs Short Course - METU 2013 19
Chemical reactions H 2 O → HO • + H • HO • + HO • → H 2 O 2 HO • +HO • →H 2 O + O • HO • +HO • → H 2 + O 2 H • + O 2 → HO 2 • AOPs Short Course - METU 2013 19
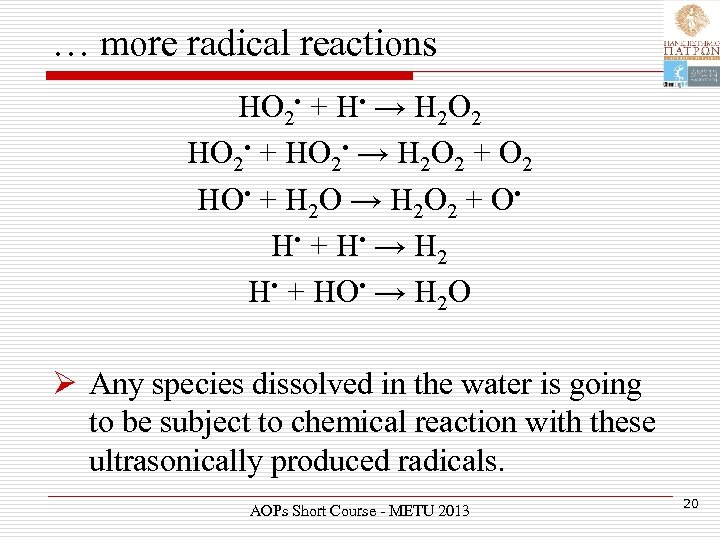 … more radical reactions HO 2 • + H • → H 2 O 2 HO 2 • + HO 2 • → H 2 O 2 + O 2 HO • + H 2 O → H 2 O 2 + O • H • + H • → H 2 H • + HO • → H 2 O Ø Any species dissolved in the water is going to be subject to chemical reaction with these ultrasonically produced radicals. AOPs Short Course - METU 2013 20
… more radical reactions HO 2 • + H • → H 2 O 2 HO 2 • + HO 2 • → H 2 O 2 + O 2 HO • + H 2 O → H 2 O 2 + O • H • + H • → H 2 H • + HO • → H 2 O Ø Any species dissolved in the water is going to be subject to chemical reaction with these ultrasonically produced radicals. AOPs Short Course - METU 2013 20
 Heterogeneous particle-liquid reactions Ø Acoustic cavitation can produce dramatic effects on particulate material or agglomerates in a liquid. Ø In this case the solid surface is not the continuous surface as above but is a small powder fragment suspended in the medium Ø Under these conditions cavitation can achieve both a reduction in particle size and efficient dispersion. AOPs Short Course - METU 2013 21
Heterogeneous particle-liquid reactions Ø Acoustic cavitation can produce dramatic effects on particulate material or agglomerates in a liquid. Ø In this case the solid surface is not the continuous surface as above but is a small powder fragment suspended in the medium Ø Under these conditions cavitation can achieve both a reduction in particle size and efficient dispersion. AOPs Short Course - METU 2013 21
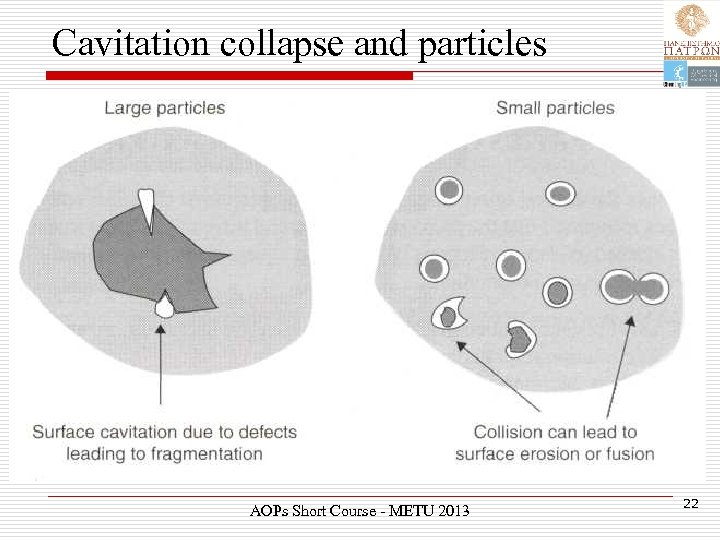 Cavitation collapse and particles AOPs Short Course - METU 2013 22
Cavitation collapse and particles AOPs Short Course - METU 2013 22
 Cavitation and particles Ø Surface imperfections or trapped gas can act as the nuclei for cavitation bubble formation on the surface of a particle. Ø Subsequent surface collapse can then lead to shock waves which break the particle apart. AOPs Short Course - METU 2013 23
Cavitation and particles Ø Surface imperfections or trapped gas can act as the nuclei for cavitation bubble formation on the surface of a particle. Ø Subsequent surface collapse can then lead to shock waves which break the particle apart. AOPs Short Course - METU 2013 23
 Cavitation and particles Ø Cavitation bubble collapse in the liquid phase near to a particle can force it into rapid motion. Ø Under these circumstances the general dispersive effect is accompanied by interparticle collisions that can also lead to erosion, surface cleaning and wetting of the particles and particle size reduction. AOPs Short Course - METU 2013 24
Cavitation and particles Ø Cavitation bubble collapse in the liquid phase near to a particle can force it into rapid motion. Ø Under these circumstances the general dispersive effect is accompanied by interparticle collisions that can also lead to erosion, surface cleaning and wetting of the particles and particle size reduction. AOPs Short Course - METU 2013 24
 Case studies Ø Ultrasound alone: degradation of aqueous solutions of emerging environmental contaminants. Ø Combined application of ultrasound with: ü ozone ü Ti. O 2 photocatalysis (surface cleaning, particle size reduction, increased mass transfer to the catalyst surface) ü electrochemical oxidation AOPs Short Course - METU 2013 25
Case studies Ø Ultrasound alone: degradation of aqueous solutions of emerging environmental contaminants. Ø Combined application of ultrasound with: ü ozone ü Ti. O 2 photocatalysis (surface cleaning, particle size reduction, increased mass transfer to the catalyst surface) ü electrochemical oxidation AOPs Short Course - METU 2013 25
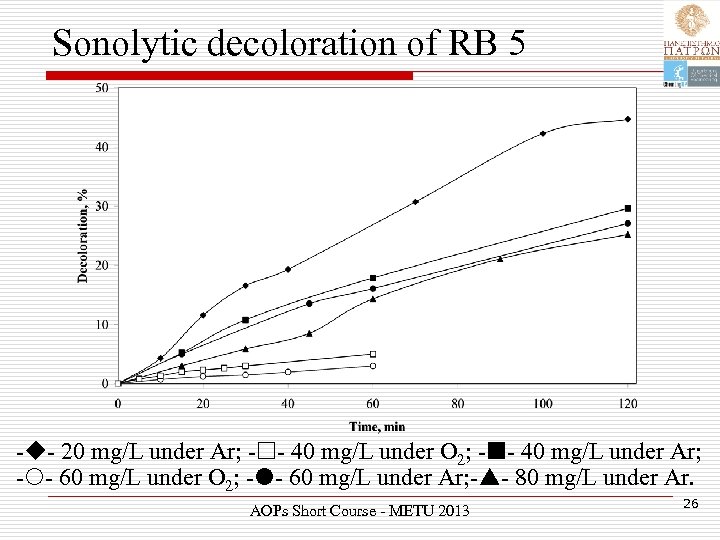 Sonolytic decoloration of RB 5 - - 20 mg/L under Ar; - - 40 mg/L under O 2; - - 40 mg/L under Ar; - - 60 mg/L under O 2; - - 60 mg/L under Ar; - - 80 mg/L under Ar. AOPs Short Course - METU 2013 26
Sonolytic decoloration of RB 5 - - 20 mg/L under Ar; - - 40 mg/L under O 2; - - 40 mg/L under Ar; - - 60 mg/L under O 2; - - 60 mg/L under Ar; - - 80 mg/L under Ar. AOPs Short Course - METU 2013 26
 Sonolytic decoloration of RB 5 Ø Sonochemical decoloration proceeded relatively slow. Ø RB 5 is a non-volatile and highly soluble compound. Ø Reactions inside or in the vicinity of the bubble are unlikely to occur. Ø Its degradation will be driven by hydroxyl radical-mediated secondary activity in the liquid bulk. AOPs Short Course - METU 2013 27
Sonolytic decoloration of RB 5 Ø Sonochemical decoloration proceeded relatively slow. Ø RB 5 is a non-volatile and highly soluble compound. Ø Reactions inside or in the vicinity of the bubble are unlikely to occur. Ø Its degradation will be driven by hydroxyl radical-mediated secondary activity in the liquid bulk. AOPs Short Course - METU 2013 27
 Effect of gases Ø Improved decoloration was observed by replacing oxygen with argon. Ø Gas sparging enhances sonochemical activity as gases act as nucleation sites for cavitation. Ø There are three properties of gases that can affect sonochemical activity, namely: 1. The heat capacity ratio γ. AOPs Short Course - METU 2013 28
Effect of gases Ø Improved decoloration was observed by replacing oxygen with argon. Ø Gas sparging enhances sonochemical activity as gases act as nucleation sites for cavitation. Ø There are three properties of gases that can affect sonochemical activity, namely: 1. The heat capacity ratio γ. AOPs Short Course - METU 2013 28
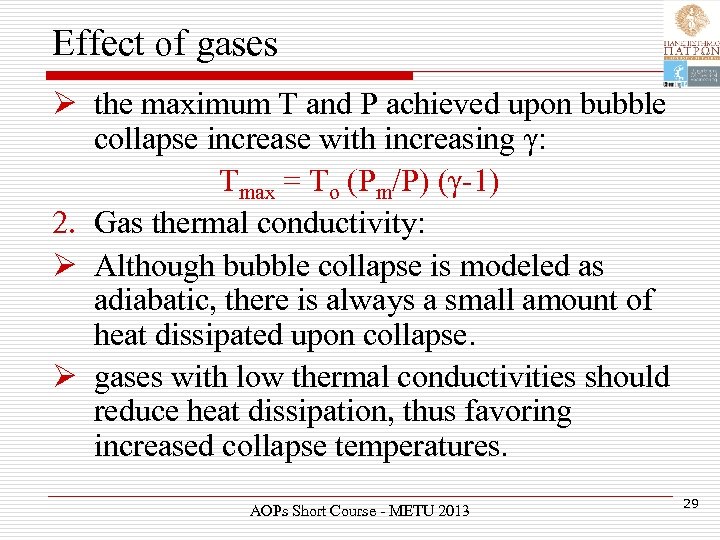 Effect of gases Ø the maximum T and P achieved upon bubble collapse increase with increasing γ: Tmax = To (Pm/P) (γ-1) 2. Gas thermal conductivity: Ø Although bubble collapse is modeled as adiabatic, there is always a small amount of heat dissipated upon collapse. Ø gases with low thermal conductivities should reduce heat dissipation, thus favoring increased collapse temperatures. AOPs Short Course - METU 2013 29
Effect of gases Ø the maximum T and P achieved upon bubble collapse increase with increasing γ: Tmax = To (Pm/P) (γ-1) 2. Gas thermal conductivity: Ø Although bubble collapse is modeled as adiabatic, there is always a small amount of heat dissipated upon collapse. Ø gases with low thermal conductivities should reduce heat dissipation, thus favoring increased collapse temperatures. AOPs Short Course - METU 2013 29
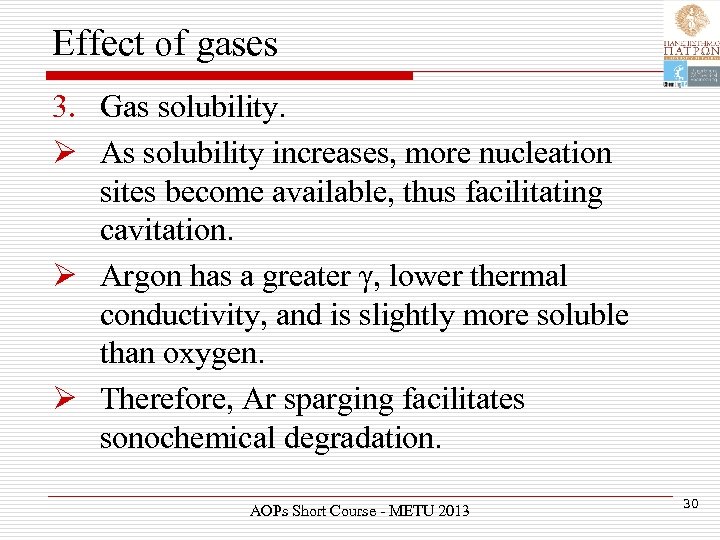 Effect of gases 3. Gas solubility. Ø As solubility increases, more nucleation sites become available, thus facilitating cavitation. Ø Argon has a greater γ, lower thermal conductivity, and is slightly more soluble than oxygen. Ø Therefore, Ar sparging facilitates sonochemical degradation. AOPs Short Course - METU 2013 30
Effect of gases 3. Gas solubility. Ø As solubility increases, more nucleation sites become available, thus facilitating cavitation. Ø Argon has a greater γ, lower thermal conductivity, and is slightly more soluble than oxygen. Ø Therefore, Ar sparging facilitates sonochemical degradation. AOPs Short Course - METU 2013 30
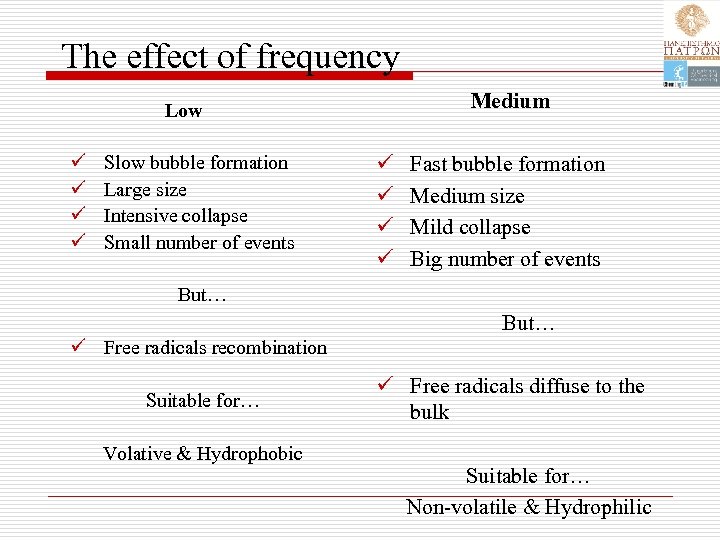 The effect of frequency Medium Low ü ü Slow bubble formation Large size Intensive collapse Small number of events ü ü Fast bubble formation Medium size Mild collapse Big number of events But… ü Free radicals recombination Suitable for… Volative & Hydrophobic ü Free radicals diffuse to the bulk Suitable for… Non-volatile & Hydrophilic
The effect of frequency Medium Low ü ü Slow bubble formation Large size Intensive collapse Small number of events ü ü Fast bubble formation Medium size Mild collapse Big number of events But… ü Free radicals recombination Suitable for… Volative & Hydrophobic ü Free radicals diffuse to the bulk Suitable for… Non-volatile & Hydrophilic
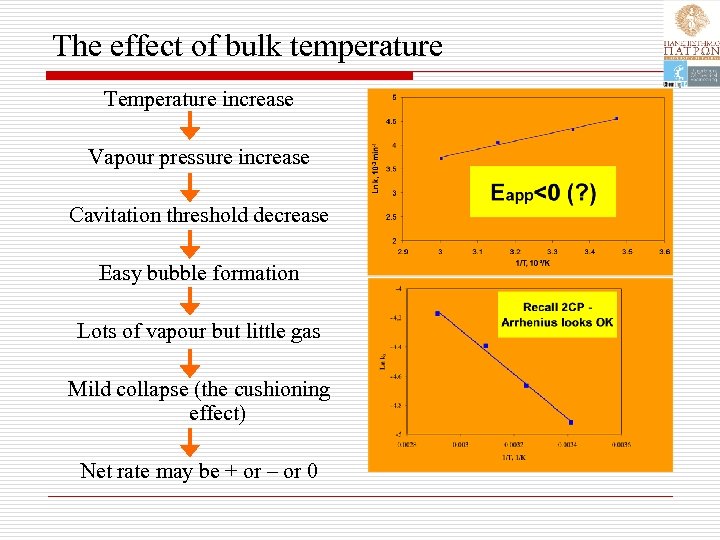 The effect of bulk temperature Temperature increase Vapour pressure increase Cavitation threshold decrease Easy bubble formation Lots of vapour but little gas Mild collapse (the cushioning effect) Net rate may be + or – or 0
The effect of bulk temperature Temperature increase Vapour pressure increase Cavitation threshold decrease Easy bubble formation Lots of vapour but little gas Mild collapse (the cushioning effect) Net rate may be + or – or 0
![Sono-photocatalytic degradation of RB 5 [Ti. O 2]=0. 25 g/L - - [RB 5]=40 Sono-photocatalytic degradation of RB 5 [Ti. O 2]=0. 25 g/L - - [RB 5]=40](https://present5.com/presentation/d8762ca9ba32815910cc2be495da59a2/image-33.jpg) Sono-photocatalytic degradation of RB 5 [Ti. O 2]=0. 25 g/L - - [RB 5]=40 mg/L; - - [RB 5]=60 mg/L. Open symbols=photocatalysis+sonolysis AOPs Short Course - METU 2013 33
Sono-photocatalytic degradation of RB 5 [Ti. O 2]=0. 25 g/L - - [RB 5]=40 mg/L; - - [RB 5]=60 mg/L. Open symbols=photocatalysis+sonolysis AOPs Short Course - METU 2013 33
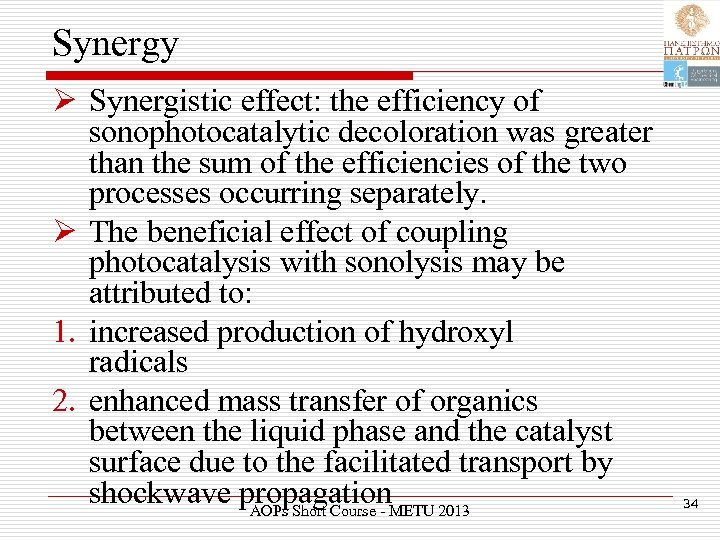 Synergy Ø Synergistic effect: the efficiency of sonophotocatalytic decoloration was greater than the sum of the efficiencies of the two processes occurring separately. Ø The beneficial effect of coupling photocatalysis with sonolysis may be attributed to: 1. increased production of hydroxyl radicals 2. enhanced mass transfer of organics between the liquid phase and the catalyst surface due to the facilitated transport by shockwave propagation. METU 2013 AOPs Short Course - 34
Synergy Ø Synergistic effect: the efficiency of sonophotocatalytic decoloration was greater than the sum of the efficiencies of the two processes occurring separately. Ø The beneficial effect of coupling photocatalysis with sonolysis may be attributed to: 1. increased production of hydroxyl radicals 2. enhanced mass transfer of organics between the liquid phase and the catalyst surface due to the facilitated transport by shockwave propagation. METU 2013 AOPs Short Course - 34
 Synergy 3. increased catalytic activity due to ultrasound de-aggregating catalyst particles, thus increasing surface area and 4. cleaning and sweeping of the catalyst surface due to acoustic micro-streaming which allows more active catalyst sites to be available for reaction. AOPs Short Course - METU 2013 35
Synergy 3. increased catalytic activity due to ultrasound de-aggregating catalyst particles, thus increasing surface area and 4. cleaning and sweeping of the catalyst surface due to acoustic micro-streaming which allows more active catalyst sites to be available for reaction. AOPs Short Course - METU 2013 35
 Sonolytic degradation of Malachite Green AOPs Short Course - METU 2013 36
Sonolytic degradation of Malachite Green AOPs Short Course - METU 2013 36
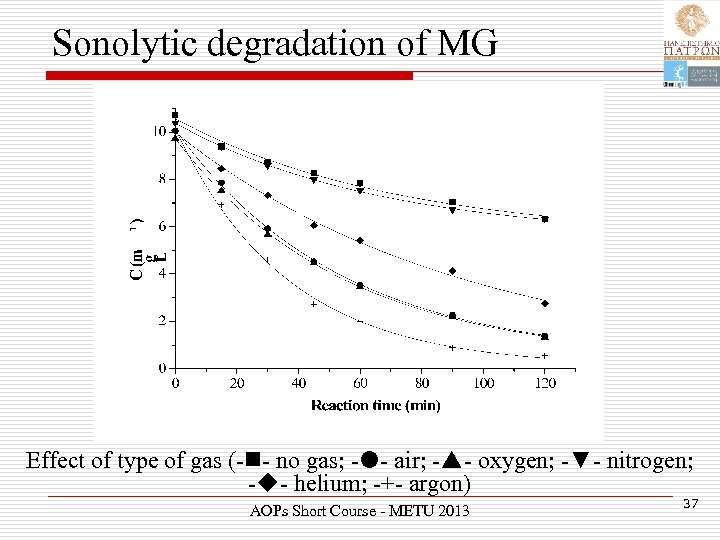 Sonolytic degradation of MG Effect of type of gas (- - no gas; - - air; - - oxygen; -▼- nitrogen; - - helium; -+- argon) AOPs Short Course - METU 2013 37
Sonolytic degradation of MG Effect of type of gas (- - no gas; - - air; - - oxygen; -▼- nitrogen; - - helium; -+- argon) AOPs Short Course - METU 2013 37
 Effect of gases Ø Reactivity: Ar>O 2 air>He>N 2 no gas Ø Ar has the greater γ and solubility in water as well as the lower thermal conductivity Ø He has high thermal conductivity and is also slightly soluble in water. Ø N 2 is less soluble in water than O 2 and may act as HO • scavenger. Ø Increased degradation with O 2 or air may also be due to the enhanced formation of ROS. AOPs Short Course - METU 2013 38
Effect of gases Ø Reactivity: Ar>O 2 air>He>N 2 no gas Ø Ar has the greater γ and solubility in water as well as the lower thermal conductivity Ø He has high thermal conductivity and is also slightly soluble in water. Ø N 2 is less soluble in water than O 2 and may act as HO • scavenger. Ø Increased degradation with O 2 or air may also be due to the enhanced formation of ROS. AOPs Short Course - METU 2013 38
 Phenolic compounds AOPs Short Course - METU 2013 39
Phenolic compounds AOPs Short Course - METU 2013 39
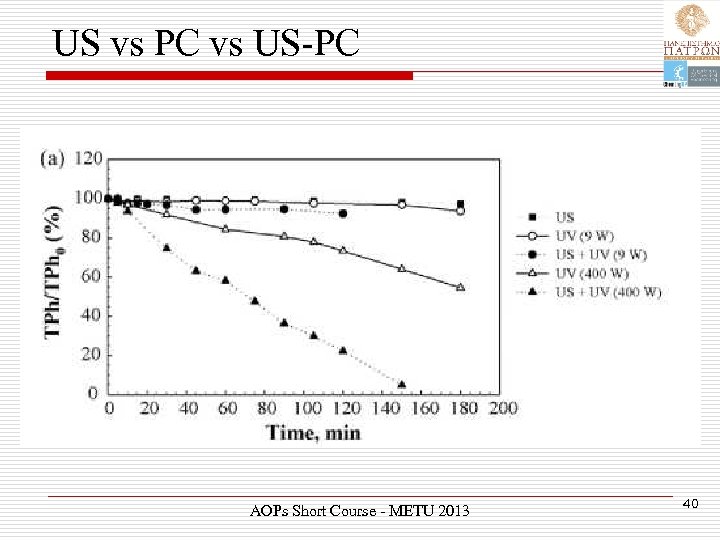 US vs PC vs US-PC AOPs Short Course - METU 2013 40
US vs PC vs US-PC AOPs Short Course - METU 2013 40
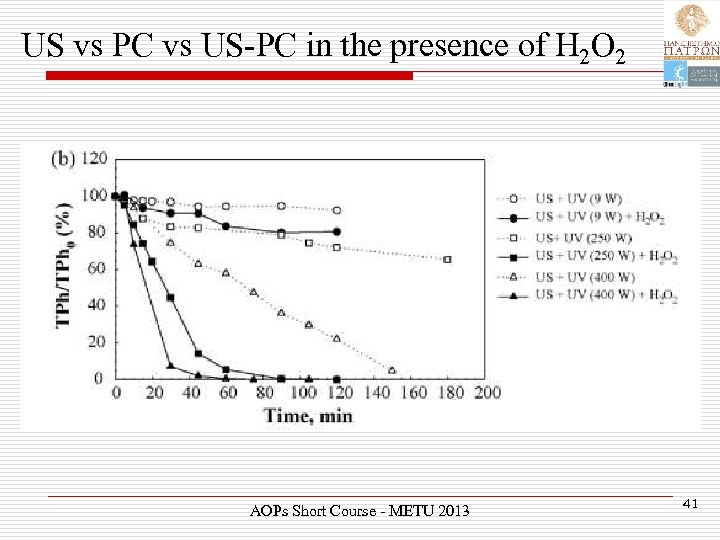 US vs PC vs US-PC in the presence of H 2 O 2 AOPs Short Course - METU 2013 41
US vs PC vs US-PC in the presence of H 2 O 2 AOPs Short Course - METU 2013 41
 … and the winner is Ø Coupling sonolysis with photocatalysis led to considerably increased degradation rates. Ø Addition of hydrogen peroxide enhanced markedly the rate of sonophotocatalytic degradation. Ø … sono-photo in the presence of H 2 O 2 AOPs Short Course - METU 2013 42
… and the winner is Ø Coupling sonolysis with photocatalysis led to considerably increased degradation rates. Ø Addition of hydrogen peroxide enhanced markedly the rate of sonophotocatalytic degradation. Ø … sono-photo in the presence of H 2 O 2 AOPs Short Course - METU 2013 42
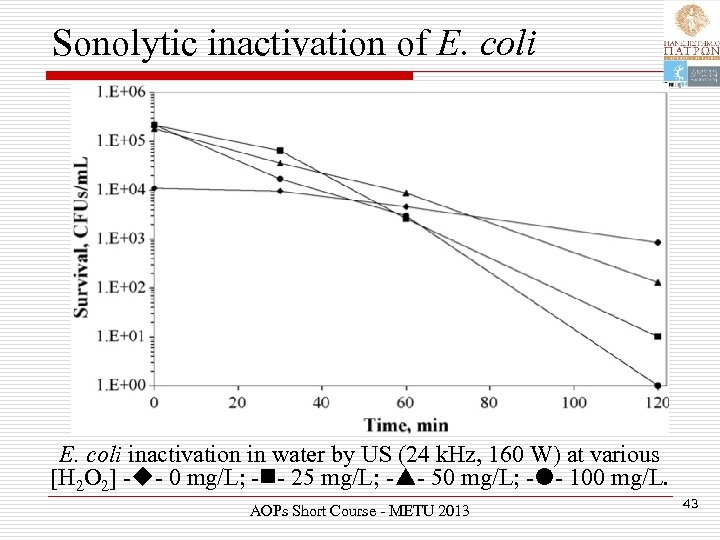 Sonolytic inactivation of E. coli inactivation in water by US (24 k. Hz, 160 W) at various [H 2 O 2] - - 0 mg/L; - - 25 mg/L; - - 50 mg/L; - - 100 mg/L. AOPs Short Course - METU 2013 43
Sonolytic inactivation of E. coli inactivation in water by US (24 k. Hz, 160 W) at various [H 2 O 2] - - 0 mg/L; - - 25 mg/L; - - 50 mg/L; - - 100 mg/L. AOPs Short Course - METU 2013 43
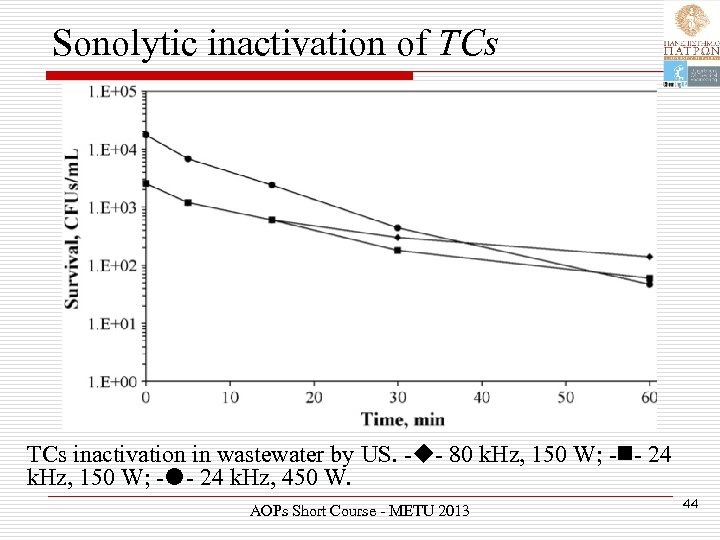 Sonolytic inactivation of TCs inactivation in wastewater by US. - - 80 k. Hz, 150 W; - - 24 k. Hz, 450 W. AOPs Short Course - METU 2013 44
Sonolytic inactivation of TCs inactivation in wastewater by US. - - 80 k. Hz, 150 W; - - 24 k. Hz, 450 W. AOPs Short Course - METU 2013 44
 AOPs Short Course - METU 2013 45
AOPs Short Course - METU 2013 45
 Electrochemical oxidation Ø Study on electrochemical oxidation for wastewater treatment goes back to the 19 th century, when electrochemical decomposition of cyanide was investigated. Ø Extensive investigation of this technology commenced since the late 1970 s. AOPs Short Course - METU 2013 46
Electrochemical oxidation Ø Study on electrochemical oxidation for wastewater treatment goes back to the 19 th century, when electrochemical decomposition of cyanide was investigated. Ø Extensive investigation of this technology commenced since the late 1970 s. AOPs Short Course - METU 2013 46
 Electrochemical oxidation Ø During the last two decades, research works have been focused on: ü the efficiency in oxidizing various pollutants on different electrodes ü improvement of the electrocatalytic activity and electrochemical stability of electrode materials ü investigation of factors affecting the process performance ü exploration of the mechanisms and kinetics of pollutant degradation. AOPs Short Course - METU 2013 47
Electrochemical oxidation Ø During the last two decades, research works have been focused on: ü the efficiency in oxidizing various pollutants on different electrodes ü improvement of the electrocatalytic activity and electrochemical stability of electrode materials ü investigation of factors affecting the process performance ü exploration of the mechanisms and kinetics of pollutant degradation. AOPs Short Course - METU 2013 47
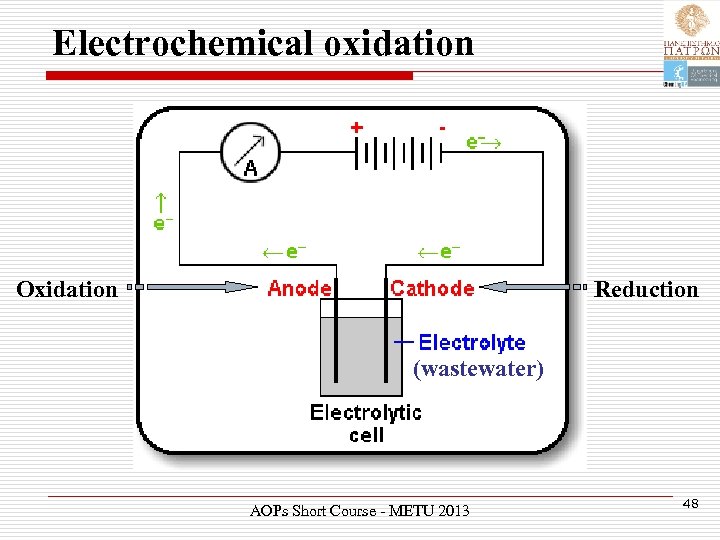 Electrochemical oxidation Oxidation Reduction (wastewater) AOPs Short Course - METU 2013 48
Electrochemical oxidation Oxidation Reduction (wastewater) AOPs Short Course - METU 2013 48
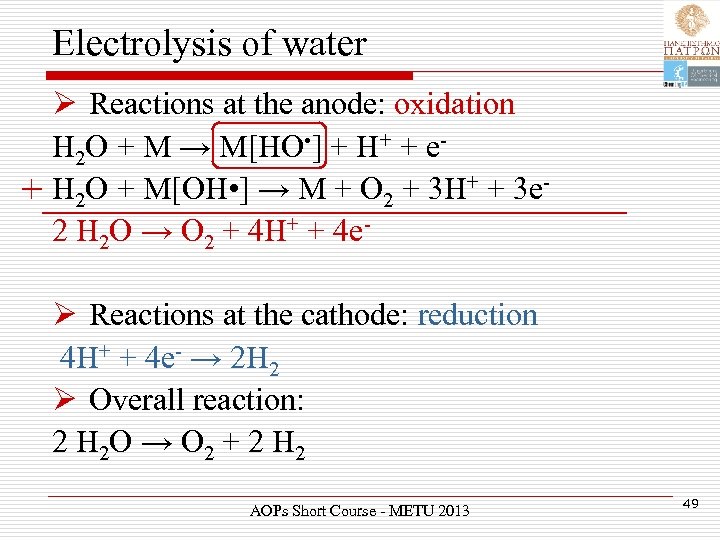 Electrolysis of water Ø Reactions at the anode: oxidation H 2 O + M → M[HO • ] + H+ + e+ H 2 O + M[OH • ] → M + O 2 + 3 H+ + 3 e 2 H 2 O → O 2 + 4 H+ + 4 eØ Reactions at the cathode: reduction 4 H+ + 4 e- → 2 H 2 Ø Overall reaction: 2 H 2 O → O 2 + 2 H 2 AOPs Short Course - METU 2013 49
Electrolysis of water Ø Reactions at the anode: oxidation H 2 O + M → M[HO • ] + H+ + e+ H 2 O + M[OH • ] → M + O 2 + 3 H+ + 3 e 2 H 2 O → O 2 + 4 H+ + 4 eØ Reactions at the cathode: reduction 4 H+ + 4 e- → 2 H 2 Ø Overall reaction: 2 H 2 O → O 2 + 2 H 2 AOPs Short Course - METU 2013 49
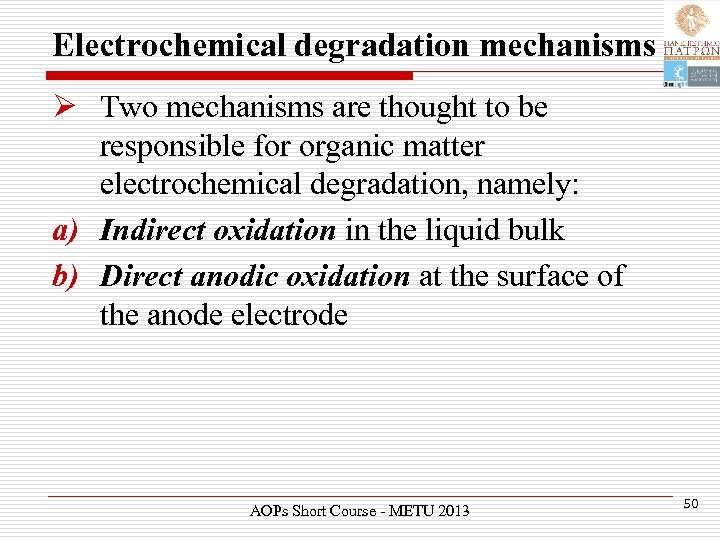 Electrochemical degradation mechanisms Ø Two mechanisms are thought to be responsible for organic matter electrochemical degradation, namely: a) Indirect oxidation in the liquid bulk b) Direct anodic oxidation at the surface of the anode electrode AOPs Short Course - METU 2013 50
Electrochemical degradation mechanisms Ø Two mechanisms are thought to be responsible for organic matter electrochemical degradation, namely: a) Indirect oxidation in the liquid bulk b) Direct anodic oxidation at the surface of the anode electrode AOPs Short Course - METU 2013 50
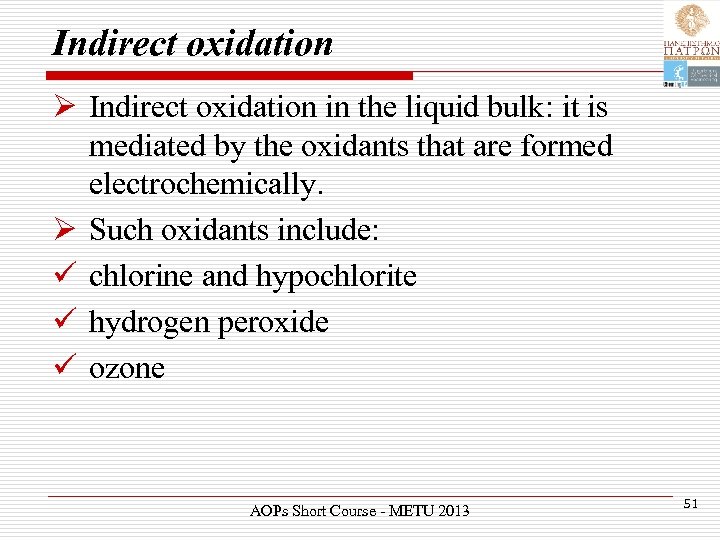 Indirect oxidation Ø Indirect oxidation in the liquid bulk: it is mediated by the oxidants that are formed electrochemically. Ø Such oxidants include: ü chlorine and hypochlorite ü hydrogen peroxide ü ozone AOPs Short Course - METU 2013 51
Indirect oxidation Ø Indirect oxidation in the liquid bulk: it is mediated by the oxidants that are formed electrochemically. Ø Such oxidants include: ü chlorine and hypochlorite ü hydrogen peroxide ü ozone AOPs Short Course - METU 2013 51
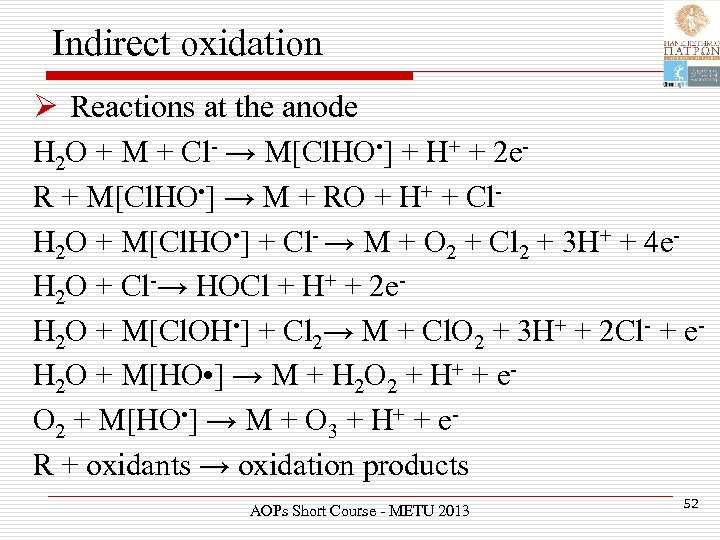 Indirect oxidation Ø Reactions at the anode H 2 O + M + Cl- → M[Cl. HO • ] + H+ + 2 e. R + M[Cl. HO • ] → M + RO + H+ + Cl. H 2 O + M[Cl. HO • ] + Cl- → M + O 2 + Cl 2 + 3 H+ + 4 e. H 2 O + Cl-→ HOCl + H+ + 2 e. H 2 O + M[Cl. OH • ] + Cl 2→ M + Cl. O 2 + 3 H+ + 2 Cl- + e. H 2 O + M[HO • ] → M + H 2 O 2 + H+ + e. O 2 + M[HO • ] → M + O 3 + H+ + e. R + oxidants → oxidation products AOPs Short Course - METU 2013 52
Indirect oxidation Ø Reactions at the anode H 2 O + M + Cl- → M[Cl. HO • ] + H+ + 2 e. R + M[Cl. HO • ] → M + RO + H+ + Cl. H 2 O + M[Cl. HO • ] + Cl- → M + O 2 + Cl 2 + 3 H+ + 4 e. H 2 O + Cl-→ HOCl + H+ + 2 e. H 2 O + M[Cl. OH • ] + Cl 2→ M + Cl. O 2 + 3 H+ + 2 Cl- + e. H 2 O + M[HO • ] → M + H 2 O 2 + H+ + e. O 2 + M[HO • ] → M + O 3 + H+ + e. R + oxidants → oxidation products AOPs Short Course - METU 2013 52
 Direct anodic oxidation Ø Electrochemical oxidation of pollutants can occur directly at the anode through the generation of physically adsorbed hydroxyl radicals, HO • , or chemisorbed “active oxygen’’ (oxygen in the oxide lattice, MOx+1). Ø The pollutants are adsorbed on the anode surface and destroyed by the anodic electron transfer reaction. AOPs Short Course - METU 2013 53
Direct anodic oxidation Ø Electrochemical oxidation of pollutants can occur directly at the anode through the generation of physically adsorbed hydroxyl radicals, HO • , or chemisorbed “active oxygen’’ (oxygen in the oxide lattice, MOx+1). Ø The pollutants are adsorbed on the anode surface and destroyed by the anodic electron transfer reaction. AOPs Short Course - METU 2013 53
 Mechanism of direct anodic oxidation Ø Anode electrodes have been classified as ‘‘active’’ and ‘‘non-active’’ anodes. Ø In both cases, the first reaction is the oxidation of water molecules leading to the formation of adsorbed hydroxyl radicals: H 2 O + M → M[HO • ] + H+ + e- AOPs Short Course - METU 2013 54
Mechanism of direct anodic oxidation Ø Anode electrodes have been classified as ‘‘active’’ and ‘‘non-active’’ anodes. Ø In both cases, the first reaction is the oxidation of water molecules leading to the formation of adsorbed hydroxyl radicals: H 2 O + M → M[HO • ] + H+ + e- AOPs Short Course - METU 2013 54
 Active anodes (Ir. O 2) Ø With active electrodes there is a strong interaction between the electrode and the hydroxyl radical HO • . Ø Adsorbed hydroxyl radicals may interact with the anode, forming a so-called higher oxide MO. M[HO • ] → MO + H+ + e- AOPs Short Course - METU 2013 55
Active anodes (Ir. O 2) Ø With active electrodes there is a strong interaction between the electrode and the hydroxyl radical HO • . Ø Adsorbed hydroxyl radicals may interact with the anode, forming a so-called higher oxide MO. M[HO • ] → MO + H+ + e- AOPs Short Course - METU 2013 55
 Oxidation of organics in active anodes Ø The redox couple MO/M acts as a mediator in the oxidation of organics: MO + R → M + RO Ø This reaction is in competition with the side reaction of oxygen evolution, which is due to the chemical decomposition of the higher oxide: MO → M + ½ O 2 AOPs Short Course - METU 2013 56
Oxidation of organics in active anodes Ø The redox couple MO/M acts as a mediator in the oxidation of organics: MO + R → M + RO Ø This reaction is in competition with the side reaction of oxygen evolution, which is due to the chemical decomposition of the higher oxide: MO → M + ½ O 2 AOPs Short Course - METU 2013 56
 Non-active anodes (BDD) Ø In a non-active electrode, weak interactions exist between the hydroxyl radical and the electrode surface. Ø In this case, the oxidation of organics is mediated by hydroxyl radicals and may result in fully oxidized reaction products such as CO 2, H 2 O and inorganic ions. M[HO • ] + R → M + CO 2 + H 2 O + H+ + e. AOPs Short Course - METU 2013 57
Non-active anodes (BDD) Ø In a non-active electrode, weak interactions exist between the hydroxyl radical and the electrode surface. Ø In this case, the oxidation of organics is mediated by hydroxyl radicals and may result in fully oxidized reaction products such as CO 2, H 2 O and inorganic ions. M[HO • ] + R → M + CO 2 + H 2 O + H+ + e. AOPs Short Course - METU 2013 57
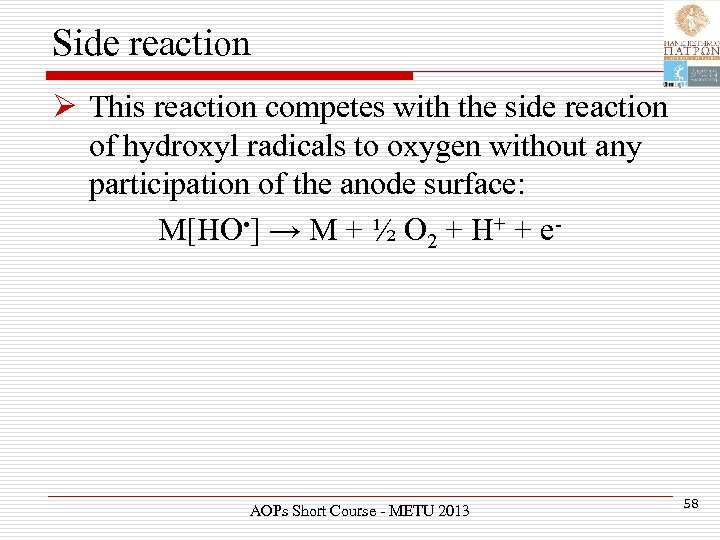 Side reaction Ø This reaction competes with the side reaction of hydroxyl radicals to oxygen without any participation of the anode surface: M[HO • ] → M + ½ O 2 + H+ + e- AOPs Short Course - METU 2013 58
Side reaction Ø This reaction competes with the side reaction of hydroxyl radicals to oxygen without any participation of the anode surface: M[HO • ] → M + ½ O 2 + H+ + e- AOPs Short Course - METU 2013 58
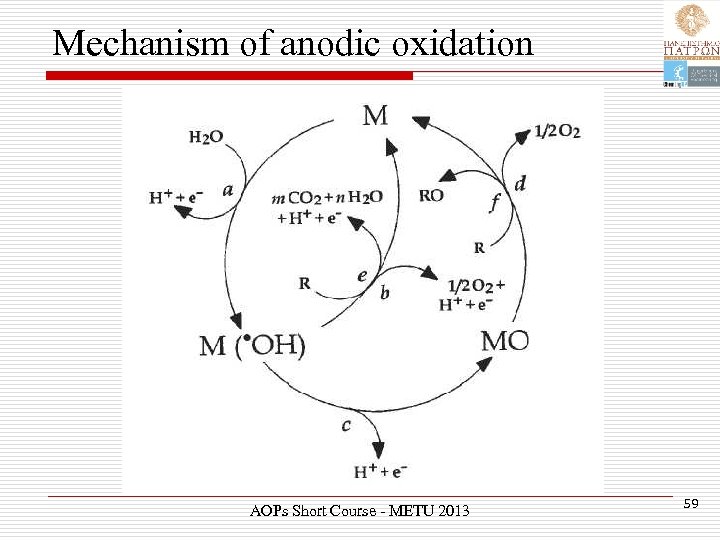 Mechanism of anodic oxidation AOPs Short Course - METU 2013 59
Mechanism of anodic oxidation AOPs Short Course - METU 2013 59
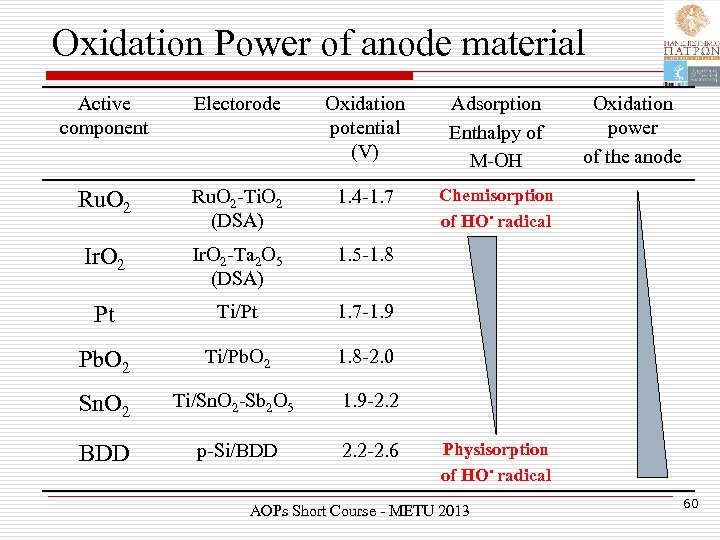 Oxidation Power of anode material Active component Electorode Oxidation potential (V) Adsorption Enthalpy of M-OH Ru. O 2 -Ti. O 2 (DSA) 1. 4 -1. 7 Chemisorption of HO • radical Ir. O 2 -Ta 2 O 5 (DSA) 1. 5 -1. 8 Pt Ti/Pt 1. 7 -1. 9 Pb. O 2 Ti/Pb. O 2 1. 8 -2. 0 Sn. O 2 Ti/Sn. O 2 -Sb 2 O 5 1. 9 -2. 2 BDD p-Si/BDD 2. 2 -2. 6 Oxidation power of the anode Physisorption of HO • radical AOPs Short Course - METU 2013 60
Oxidation Power of anode material Active component Electorode Oxidation potential (V) Adsorption Enthalpy of M-OH Ru. O 2 -Ti. O 2 (DSA) 1. 4 -1. 7 Chemisorption of HO • radical Ir. O 2 -Ta 2 O 5 (DSA) 1. 5 -1. 8 Pt Ti/Pt 1. 7 -1. 9 Pb. O 2 Ti/Pb. O 2 1. 8 -2. 0 Sn. O 2 Ti/Sn. O 2 -Sb 2 O 5 1. 9 -2. 2 BDD p-Si/BDD 2. 2 -2. 6 Oxidation power of the anode Physisorption of HO • radical AOPs Short Course - METU 2013 60
 Non-active anodes Ø A non-active anode does not participate in the anodic reaction and does not provide any catalytic active site for the adsorption of reactants and/or products from the aqueous medium. Ø In this case, the anode serves only as an inert substrate, which can act as a sink for the removal of electrons. Ø In principle, only outer-sphere reactions and water oxidation are possible with this kind of anode. AOPs Short Course - METU 2013 61
Non-active anodes Ø A non-active anode does not participate in the anodic reaction and does not provide any catalytic active site for the adsorption of reactants and/or products from the aqueous medium. Ø In this case, the anode serves only as an inert substrate, which can act as a sink for the removal of electrons. Ø In principle, only outer-sphere reactions and water oxidation are possible with this kind of anode. AOPs Short Course - METU 2013 61
![Non-active anodes Ø The weaker the M[HO • ] interaction, the higher the anode Non-active anodes Ø The weaker the M[HO • ] interaction, the higher the anode](https://present5.com/presentation/d8762ca9ba32815910cc2be495da59a2/image-62.jpg) Non-active anodes Ø The weaker the M[HO • ] interaction, the higher the anode reactivity for organics oxidation (fast chemical reaction). Ø Boron-doped diamond electrodes (BDD) are typical non-active electrodes, characterized by high stability and acceptable conductivity. AOPs Short Course - METU 2013 62
Non-active anodes Ø The weaker the M[HO • ] interaction, the higher the anode reactivity for organics oxidation (fast chemical reaction). Ø Boron-doped diamond electrodes (BDD) are typical non-active electrodes, characterized by high stability and acceptable conductivity. AOPs Short Course - METU 2013 62
 Boron doped diamond AOPs Short Course - METU 2013 63
Boron doped diamond AOPs Short Course - METU 2013 63
 Dia. Cell®-Adamanto technologies AOPs Short Course - METU 2013 64
Dia. Cell®-Adamanto technologies AOPs Short Course - METU 2013 64
 Dia. Cell AOPs Short Course - METU 2013 65
Dia. Cell AOPs Short Course - METU 2013 65
 Condiacell from Condias AOPs Short Course - METU 2013 66
Condiacell from Condias AOPs Short Course - METU 2013 66
 Pilot plant unit AOPs Short Course - METU 2013 67
Pilot plant unit AOPs Short Course - METU 2013 67
 Case studies Ø Electro-chemical oxidation of dye-house wastewater over a titanium–tantalum– platinum–iridium anode Ø Electrochemical oxidation of table olive oils processing wastewater over boron doped diamond anode AOPs Short Course - METU 2013 68
Case studies Ø Electro-chemical oxidation of dye-house wastewater over a titanium–tantalum– platinum–iridium anode Ø Electrochemical oxidation of table olive oils processing wastewater over boron doped diamond anode AOPs Short Course - METU 2013 68
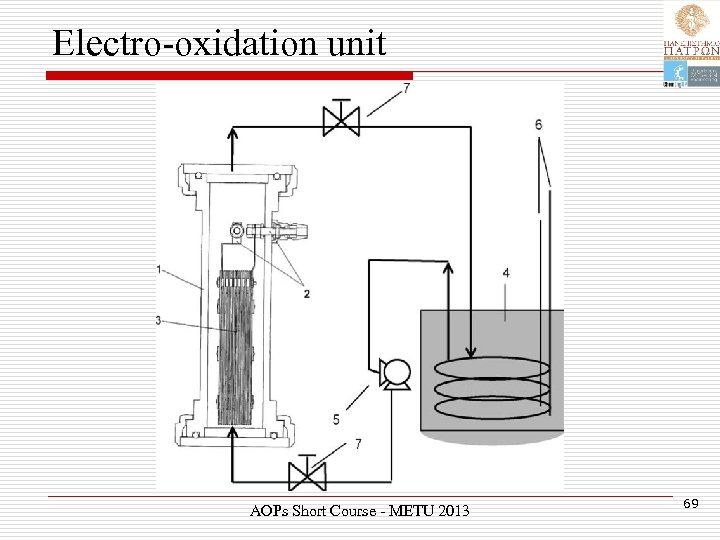 Electro-oxidation unit AOPs Short Course - METU 2013 69
Electro-oxidation unit AOPs Short Course - METU 2013 69
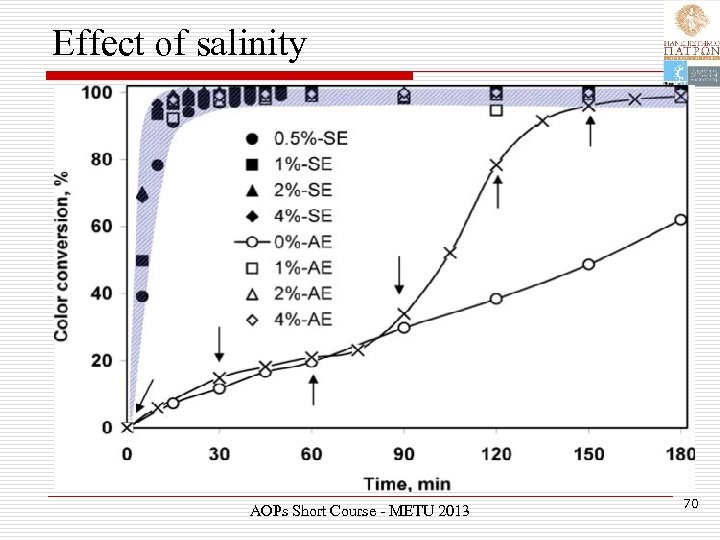 Effect of salinity AOPs Short Course - METU 2013 70
Effect of salinity AOPs Short Course - METU 2013 70
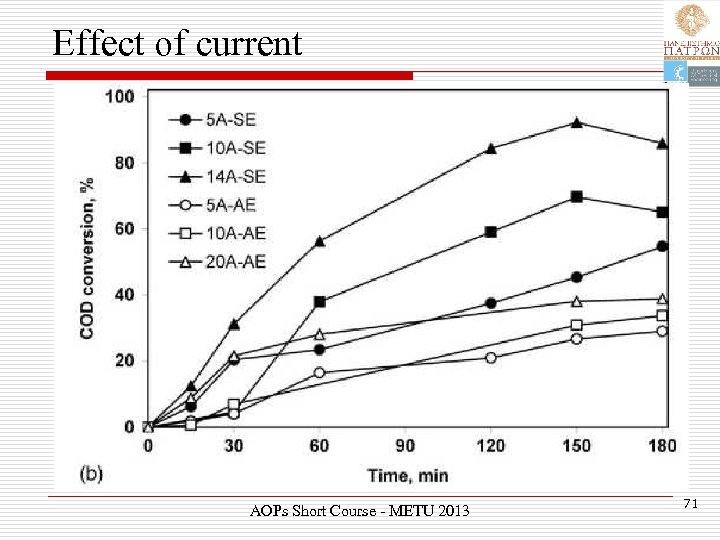 Effect of current AOPs Short Course - METU 2013 71
Effect of current AOPs Short Course - METU 2013 71
 Pilot plant unit AOPs Short Course - METU 2013 72
Pilot plant unit AOPs Short Course - METU 2013 72
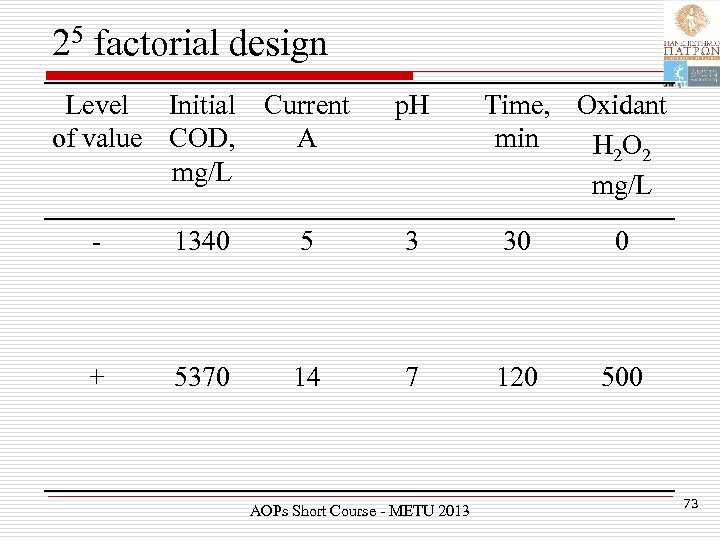 25 factorial design Level Initial of value COD, mg/L Current A p. H Time, Oxidant min H 2 O 2 mg/L - 1340 5 3 30 0 + 5370 14 7 120 500 AOPs Short Course - METU 2013 73
25 factorial design Level Initial of value COD, mg/L Current A p. H Time, Oxidant min H 2 O 2 mg/L - 1340 5 3 30 0 + 5370 14 7 120 500 AOPs Short Course - METU 2013 73
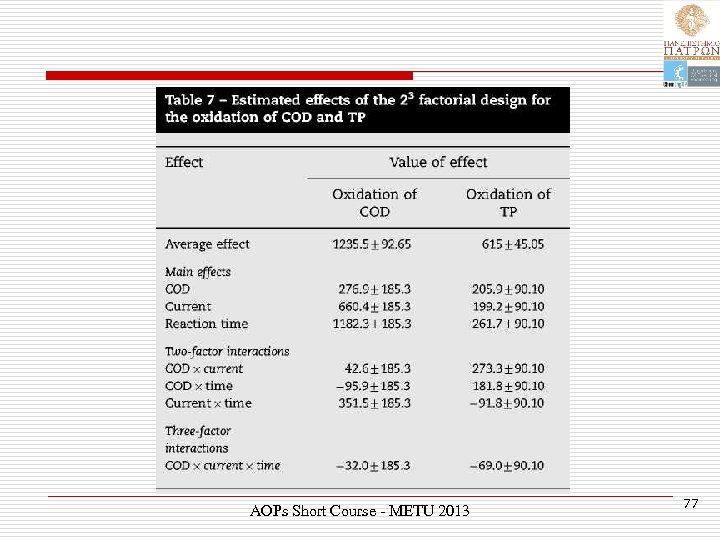
 Response factors Ø Depended variable or response Y 1: COD oxidized, mg/L Ø Depended variable or response Y 2: TP oxidized, mg/L AOPs Short Course - METU 2013 74
Response factors Ø Depended variable or response Y 1: COD oxidized, mg/L Ø Depended variable or response Y 2: TP oxidized, mg/L AOPs Short Course - METU 2013 74
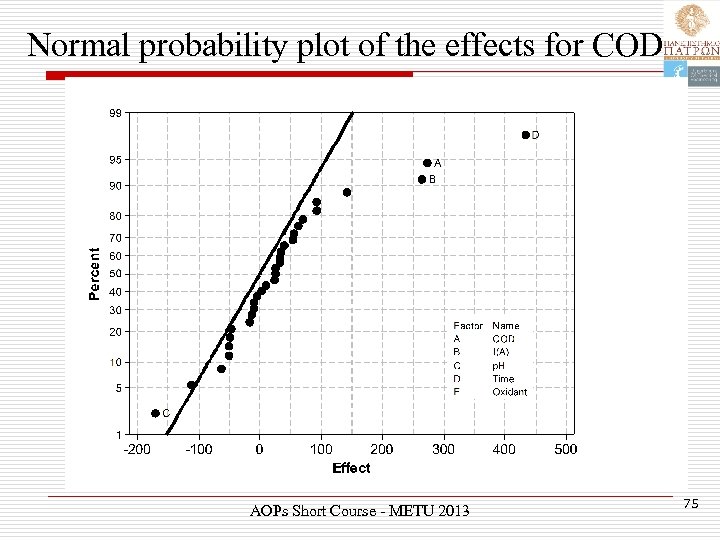 Normal probability plot of the effects for COD AOPs Short Course - METU 2013 75
Normal probability plot of the effects for COD AOPs Short Course - METU 2013 75
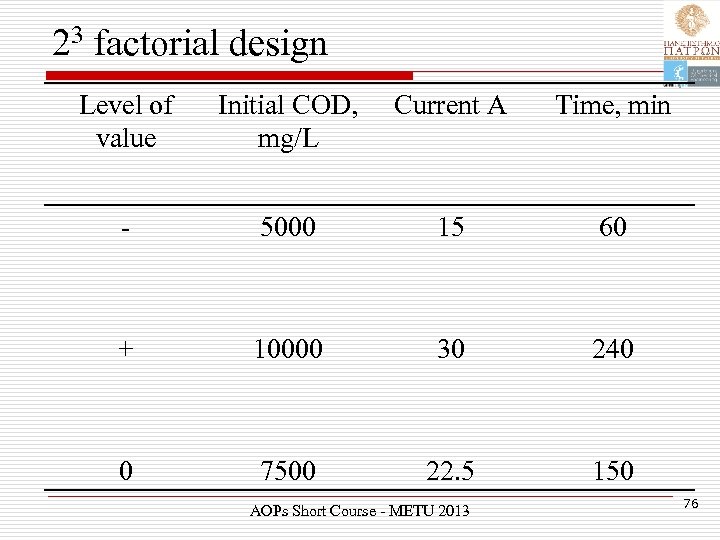 23 factorial design Level of value Initial COD, mg/L Current A Time, min - 5000 15 60 + 10000 30 240 0 7500 22. 5 150 AOPs Short Course - METU 2013 76
23 factorial design Level of value Initial COD, mg/L Current A Time, min - 5000 15 60 + 10000 30 240 0 7500 22. 5 150 AOPs Short Course - METU 2013 76
 AOPs Short Course - METU 2013 77
AOPs Short Course - METU 2013 77
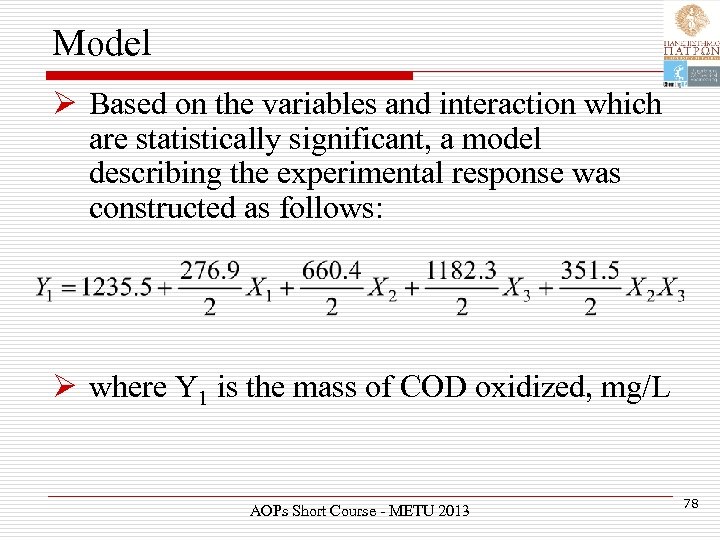 Model Ø Based on the variables and interaction which are statistically significant, a model describing the experimental response was constructed as follows: Ø where Y 1 is the mass of COD oxidized, mg/L AOPs Short Course - METU 2013 78
Model Ø Based on the variables and interaction which are statistically significant, a model describing the experimental response was constructed as follows: Ø where Y 1 is the mass of COD oxidized, mg/L AOPs Short Course - METU 2013 78
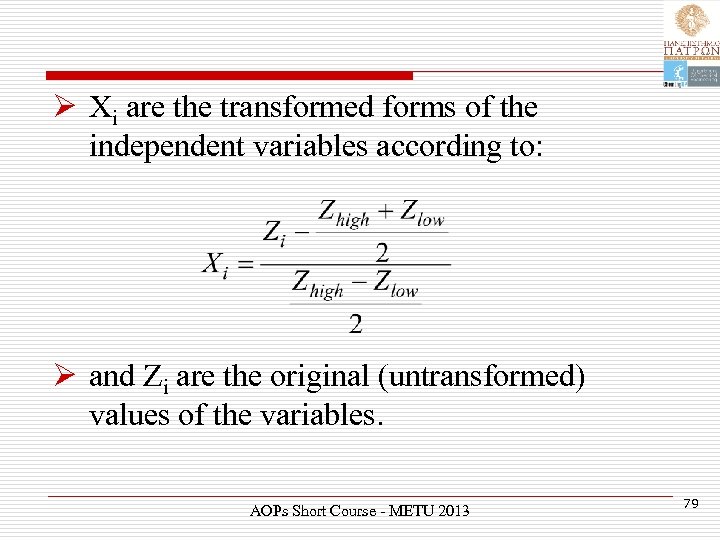 Ø Xi are the transformed forms of the independent variables according to: Ø and Zi are the original (untransformed) values of the variables. AOPs Short Course - METU 2013 79
Ø Xi are the transformed forms of the independent variables according to: Ø and Zi are the original (untransformed) values of the variables. AOPs Short Course - METU 2013 79
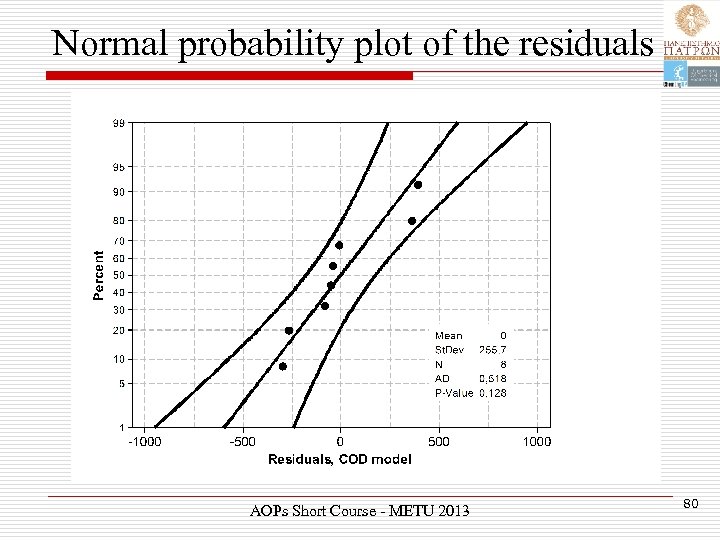 Normal probability plot of the residuals AOPs Short Course - METU 2013 80
Normal probability plot of the residuals AOPs Short Course - METU 2013 80
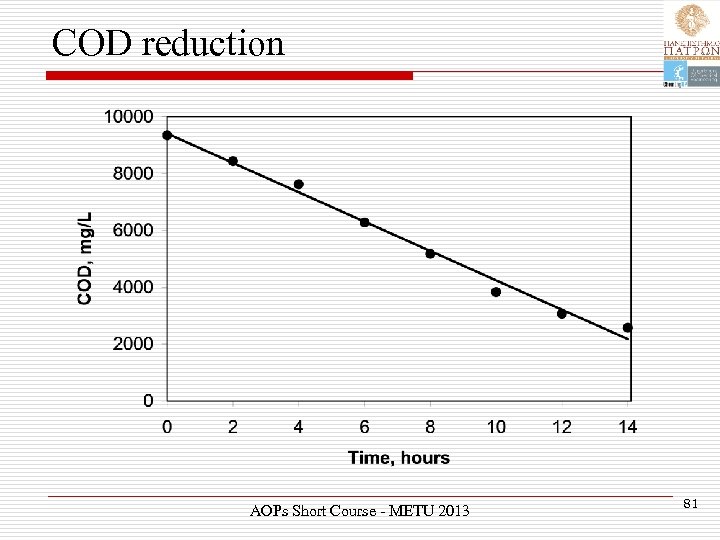 COD reduction AOPs Short Course - METU 2013 81
COD reduction AOPs Short Course - METU 2013 81


