d9afa9cc45476d1cf99a2d8043d9ce8d.ppt
- Количество слайдов: 60

Anticoagulant therapies: how do they work? Mary Byrne, St James’s Hospital

Outline of presentation n Anticoagulants n n Warfarin Heparin Dabigatran Laboratory monitoring

Warfarin n Most widely used anticoagulant in world 1% of UK population (8% of >80 yrs) 40, 000 people on Warfarin in Ireland
Clinical indications n n n Treatment of venous thrombosis (VTE), pulmonary embolism (PE) and their extension. Prophylaxis and treatment of thromboembolic complications associated with rheumatic heart disease, atrial fibrillation (AF) and/or prosthetic heart valve replacement. Reduction in the risk of death, recurrent myocardial infarction (MI), and thromboembolic events such as stroke or systemic embolisation after myocardial infarction.

Aim of Warfarin therapy n Maintain a level of anticoagulation n Minimise the risk of thrombosis Minimise the risk of haemorrhagic complication Dependant on the length of time and extent that a persons INR stays outside therapeutic range

History of Warfarin discovery

History of Warfarin discovery n n n 1920 s: prairies of North America and Canada Cattle dying of internal bleeding with no precipitating cause Query dietary problem “Sweet clover disease” Farmers recommended not to feed cattle the mouldy sweet clover hay

History of Warfarin discovery n n Karl Link experimented with “uncoagulated” blood from affected cattle Team isolated compound n n 3, 3’-methylene-bis[4 -hyfroxycoumarin] Oxidised in mouldy hay to form dicoumarol.

History of Warfarin discovery n Research work funded by the: n n Wisconsin Alumni Research Foundation Patented in 1941 Variation of dicoumarol (warfarin) patented as rat poison in 1948 Transition to clinical application (Coumadin)

The need for anticoagulation n Why do thromboses occur? How are they treated? How are they prevented? n Thrombosis and haemostasis n n
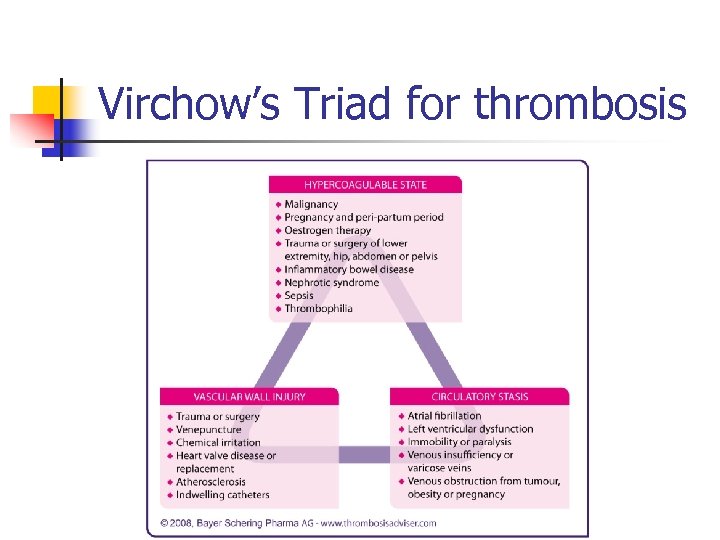
Virchow’s Triad for thrombosis
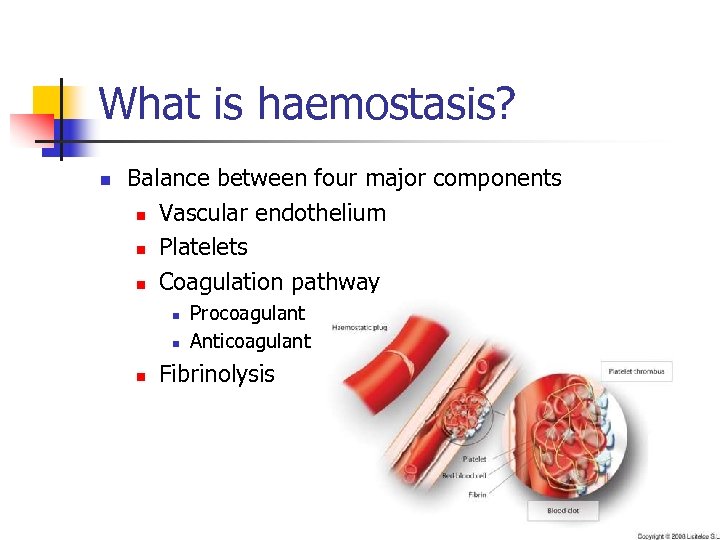
What is haemostasis? n Balance between four major components n Vascular endothelium n Platelets n Coagulation pathway n n n Procoagulant Anticoagulant Fibrinolysis

Coagulation pathway n n n Procoagulant proteins Anticoagulant proteins Balance between activation and control of coagulation

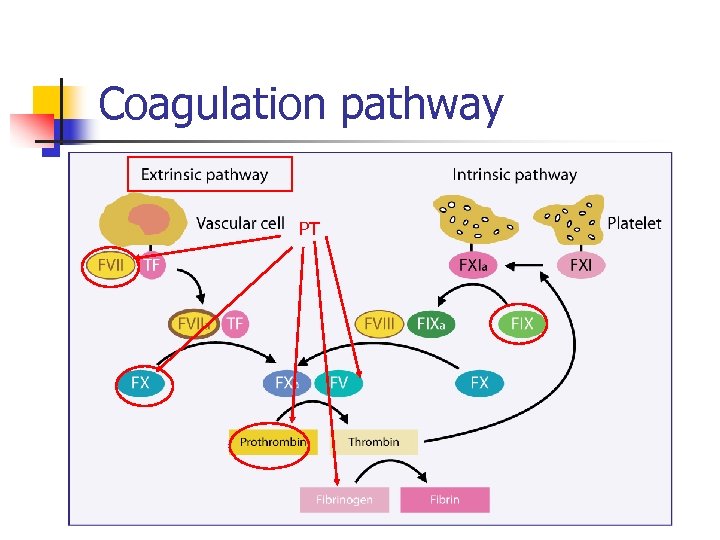
Coagulation pathway
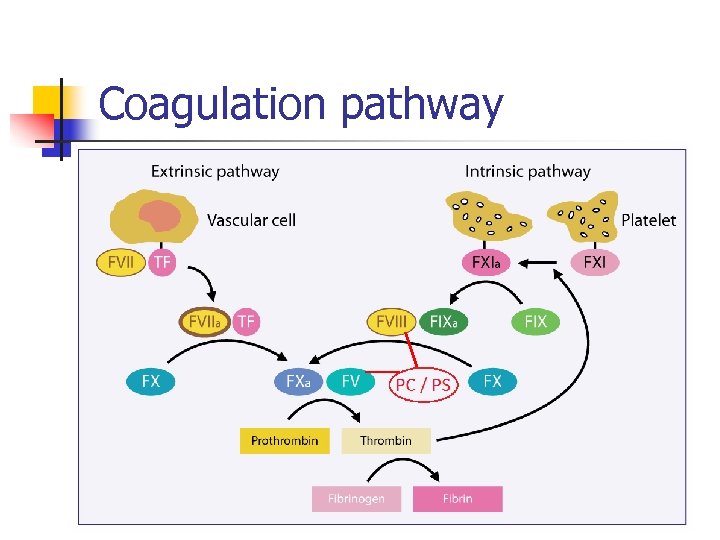
Coagulation pathway

Mechanism of action of Warfarin n n Interferes with the biochemistry of vitamin K dependant coagulation factors in the liver Vitamin K dependant coagulation factors n n Factor II VII IX X Protein C and Protein S Involved in coagulation and anticoagulation pathways in haemostasis

Coagulation pathway Warfarin

Vitamin K cycle and warfarin Active coagulation factors Warfarin

Warfarin therapy n n Inter-individual differences Narrow therapeutic range Bleeding risk Outside anticoagulation range n n n Higher mortality Increased risk of stroke Increased rate of hospitalisation

Warfarin n Environmental factors n n Vitamin K intake Illness Concurrent medication Genetic variation (VKORC 1 and CYP 2 C 9)

Warfarin interactions n Pharmacokinetic interactions n n n Drugs which interfere with clearance Antibiotics which affect intestinal flora Pharmacodynamic interactions n n Drugs which have anti-platelet effect (aspirin and NSAIDS) Drugs associated with falls in the elderly
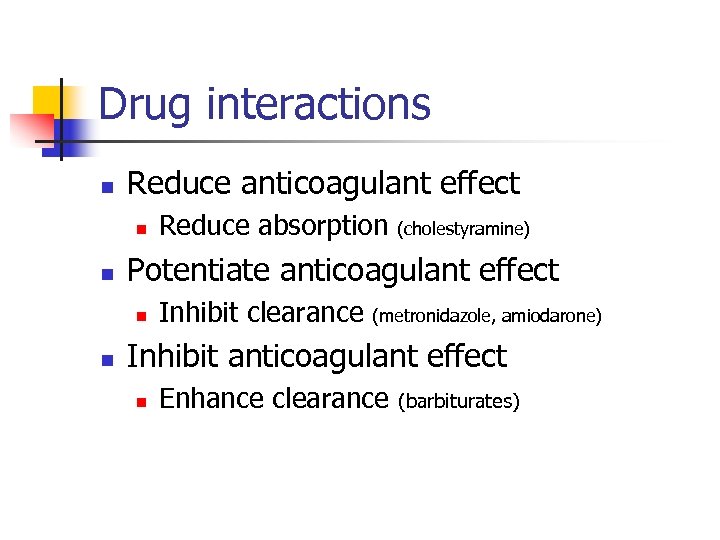
Drug interactions n Reduce anticoagulant effect n n (cholestyramine) Potentiate anticoagulant effect n n Reduce absorption Inhibit clearance (metronidazole, amiodarone) Inhibit anticoagulant effect n Enhance clearance (barbiturates)

Warfarin and bleeding n Major bleeding events 7. 2/100 patient years Fatal bleeding events 1. 3/100 patient years n Bleeding n n n May be lower in specialised anticoagulation clinics More likely within the first 90 days Can occur when INR is raised or within therapeutic range Wadelius M and Pirmohamed M. Pharmacogenetics of warfarin: current status and future challenges. The pharacogenetics Journal (2007) 7, 99 -111

Warfarin and bleeding

Reversal of warfarin n Discontinue warfarin Vitamin K Prothrombin complex concentrates

Monitoring Warfarin therapy n n Laboratory testing Point of care testing Self testing Test = INR (International Normalised Ratio)
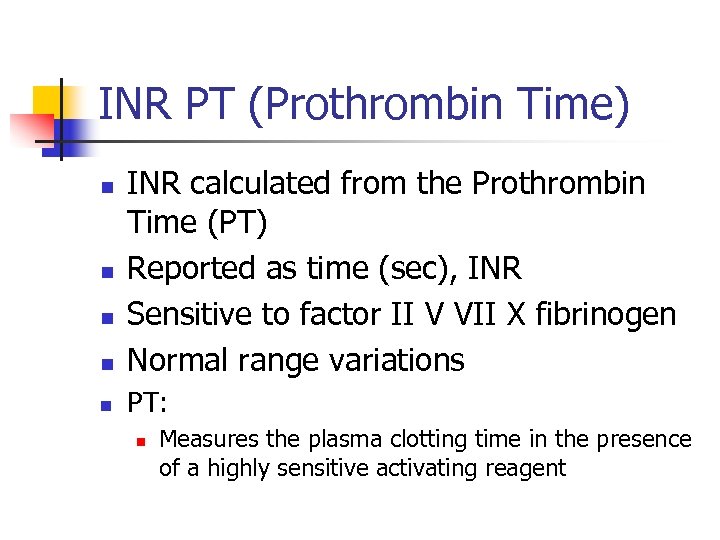
INR PT (Prothrombin Time) n INR calculated from the Prothrombin Time (PT) Reported as time (sec), INR Sensitive to factor II V VII X fibrinogen Normal range variations n PT: n n Measures the plasma clotting time in the presence of a highly sensitive activating reagent

INR n n Surrogate measure of the effectiveness of Warfarin therapy Different reagent and analytical systems are widely used
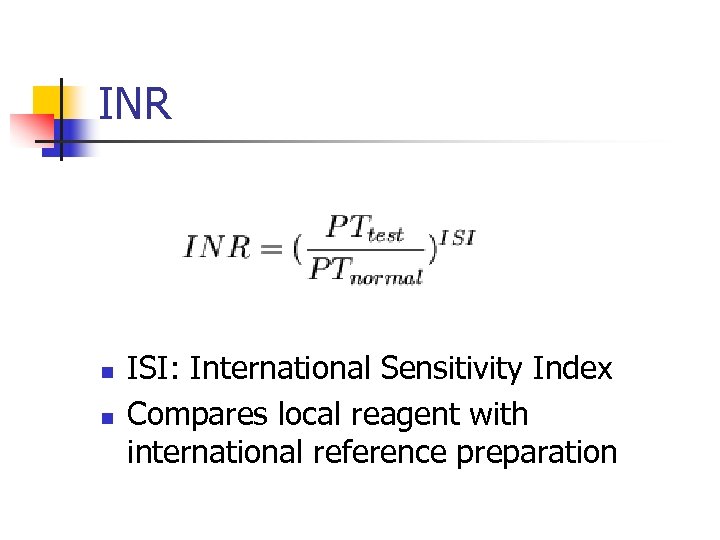
INR n n ISI: International Sensitivity Index Compares local reagent with international reference preparation
Coagulation pathway PT

Delivery of Warfarin monitoring n n In-patient Out-patient n n Warfarin clinic (SJH: 1500 active patients) Primary Care Team n Warfarin clinic

Delivery of Warfarin monitoring n n Point of care / self testing Coagucheck XS Plus Hemosense INRatio Protime 3 (ITC)

n n n Published 2009 162 patients recruited Crossover study Self selected group On long tem anticoagulant


Other anticoagulants n n Indirect Xa inhibitors (Heparin) Direct thrombin inhibitors (Dabigitran, Argatroban) Direct Xa inhibitors Different modes of action on coagulation cascade

Other anticoagulants n n n Indirect Xa inhibitors (Heparin) Direct thrombin inhibitors (Dabigatran, Argatroban) Different modes of action on coagulation cascade

Indirect Xa inhibitors n Enhance action of antithrombin n Heparin n n unfractionated UFH low molecular weight LMWH

Heparin n n n Discovered in 1916 Utilised when there is need for rapid anticoagulant effect Prevention of VTE and treatment of DVT and PE Early treatment of unstable angina and MI Cardiac surgery, bypass, vascular surgery, and coronary angioplasty Selected patients with disseminated intravascular coagulation Heparin and Low-Molecular-Weight Heparin, Mechanisms of Action, Pharmacokinetics, Dosing, Monitoring, Efficacy, and Safety Hirsh J et al CHEST 2001; 119: 64 S– 94 S
Low Molecular Weight Heparin n n Prevention of VTE and treatment of DVT and PE Early treatment of unstable angina and MI

Heparin: mechanism of action n n Mechanism is mediated through antithrombin in the coagulation cascade Inhibits platelet function
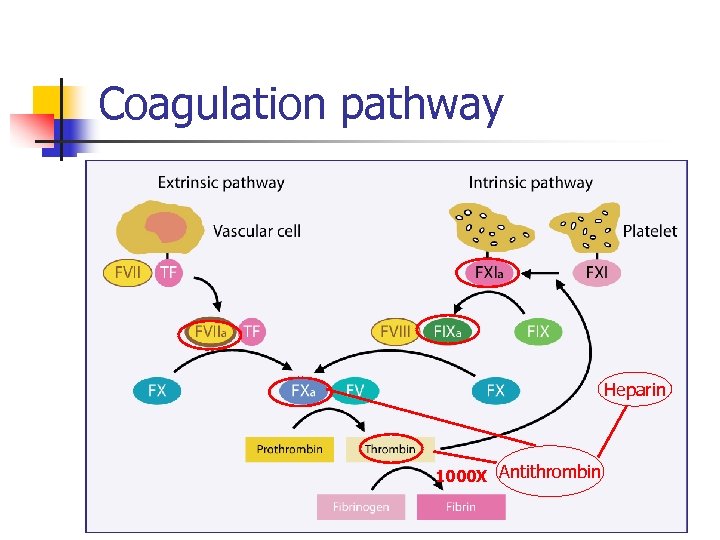
Coagulation pathway Heparin 1000 X Antithrombin
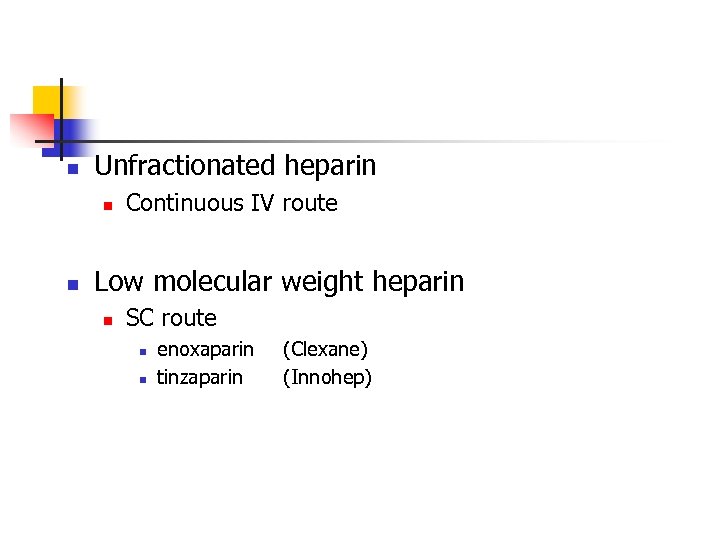
n Unfractionated heparin n n Continuous IV route Low molecular weight heparin n SC route n n enoxaparin tinzaparin (Clexane) (Innohep)
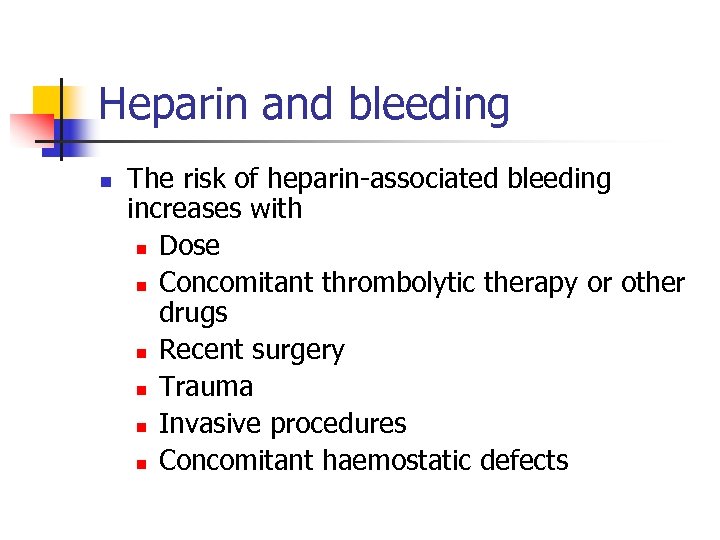
Heparin and bleeding n The risk of heparin-associated bleeding increases with n Dose n Concomitant thrombolytic therapy or other drugs n Recent surgery n Trauma n Invasive procedures n Concomitant haemostatic defects

Heparin and bleeding n Reversal of heparin n Stop treatment (Half life = 90 minutes) n Protamine Sulphate
Limitations of heparin n n Osteopoenia Heparin Induced Thrombocytopoenia

Monitoring heparin therapy n n Relationship between heparin dose, efficacy and safety Need for laboratory monitoring n n APTT (Unfractionated heparin) Anti Xa assay (Low molecular weight heparin)

APTT n Activated Partial Thromboplastin Time APTT ratio calculated from the APTT Sensitive to factor VIII IX XI XII Normal range variations n APTT: n n Measures the clotting time of plasma after the activation of the coagulation cascade with Silica.

Coagulation pathway APTT Heparin 1000 X Antithrombin

Direct Thrombin Inhibitors n n (DTI) Dabigatran Inhibits thrombin directly, no cofactor required as in heparin Predictable anticoagulant response Dabigatran etexilate, oral prodrug that is converted to dabigatran

Dabigatran n n n Rapid onset of action Lack of interaction with food and drugs No need for routine monitoring Broad therapeutic window Fixed dose administration Renal excretion
Dabigatran licence (EU) n 2008 n n Prevention of VTE after elective TKR or THR 2011 n Stroke prevention in and systemic embolism in adult patients with nonvalvular atrial fibrillation (AF)
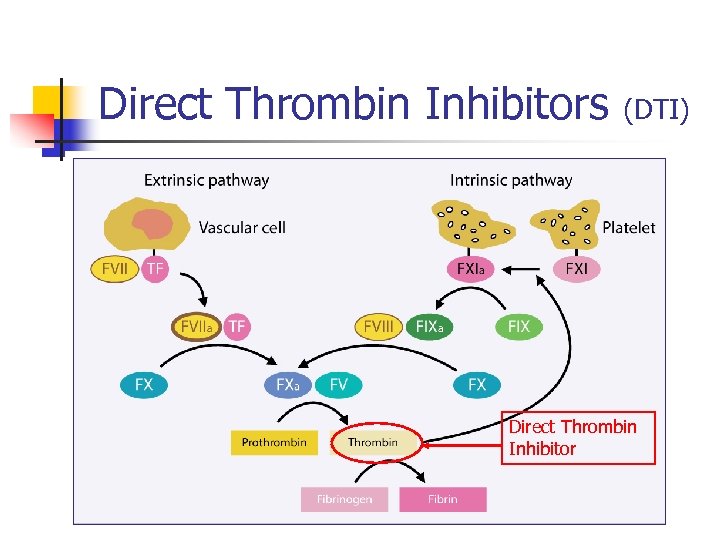
Direct Thrombin Inhibitors (DTI) Direct Thrombin Inhibitor

Dabigatran trials n n n REDEEM (post MI) RE-LY (AF) RE-NOVATE (DVT prophylaxis) RE-MODEL RE-MOBILISE

n n n Non-inferiority trial, 18113 patients recruited AF and risk of stroke Rates of stroke and systemic embolism n n n Dose of 110 mg: same as warfarin Dose of 150 mg: lower than warfarin Rates of major haemorrhage n n Dose of 110 mg: lower than warfarin Dose of 150 mg: same rate as warfarin

Considerations with dabigatran n n Non compliance No reversible agent Safety vs efficacy at extremes of body weight Renal impairment Cost

Dabigatran and bleeding n n No reversal agent or antidote currently Supportive care and control of bleeding Eliminate by natural excretion through kidney unless renal impairment Plasma half life: 12 – 17 hrs

Laboratory monitoring n n Not necessary generally Rarely needed n Suspected overdose n Bleeding n Need for emergency surgery n Impaired renal function n Pregnancy n Obesity n Children

Laboratory monitoring n n n APTT of limited use Specific test using a snake venom called Ecarin Not widely available

Dabigatran headlines Bleeding Risk with Dabigatran in the Frail Elderly N ENGL J MED 2012; 366: 864 -866 March 1, 2012 Pradaxa (dabigatran etexilate mesylate): Drug Safety Communication - Safety Review of Post-Market Reports of Serious Bleeding Events Posted 12/07/2011 Journal of Neurosurgery, published online March 6, 2012; Irreversible catastrophic brain haemorrhage after minor injury in a patient on dabigatran

Thank you for your attention!
d9afa9cc45476d1cf99a2d8043d9ce8d.ppt