c916dab2faf9db853ce0f8284c314adf.ppt
- Количество слайдов: 66

Antibody structure and function Parham – Chapter 2 H. Hogen. Esch, 2005
Outline • • • H. Hogen. Esch, 2005 Antibody structure Antigens Antigen-antibody interactions Generation of antibody diversity Isotype switching Applications - immunoassays
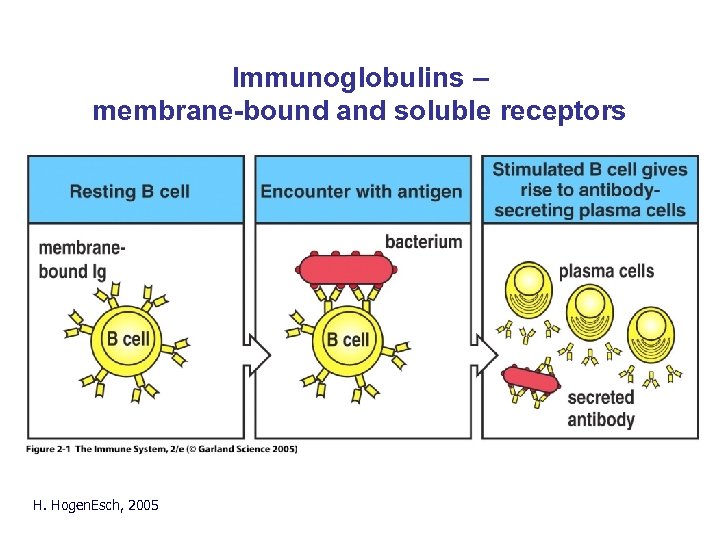
Immunoglobulins – membrane-bound and soluble receptors H. Hogen. Esch, 2005
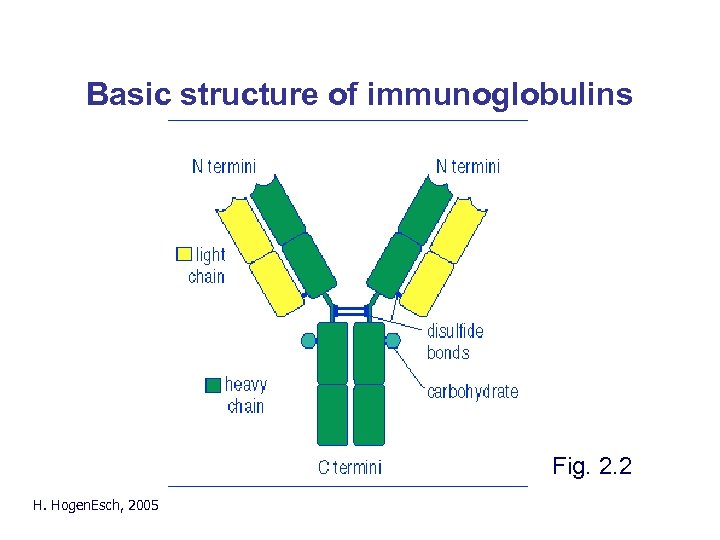
Basic structure of immunoglobulins Fig. 2. 2 H. Hogen. Esch, 2005
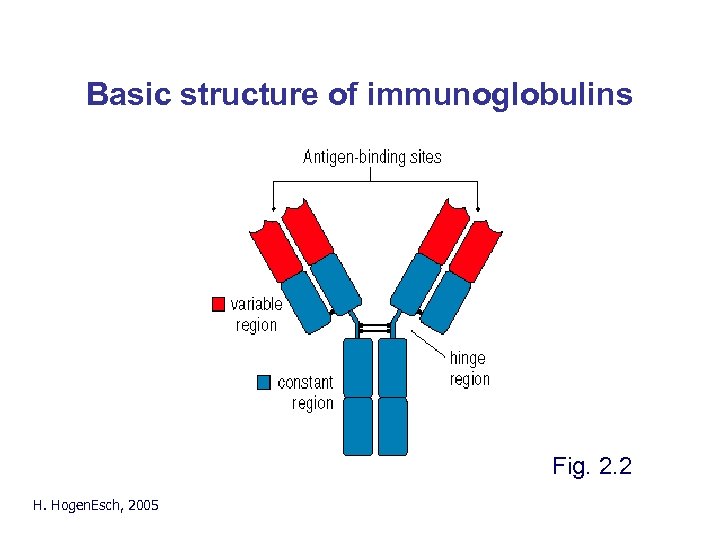
Basic structure of immunoglobulins H. Hogen. Esch, 2005 Fig. 2. 2
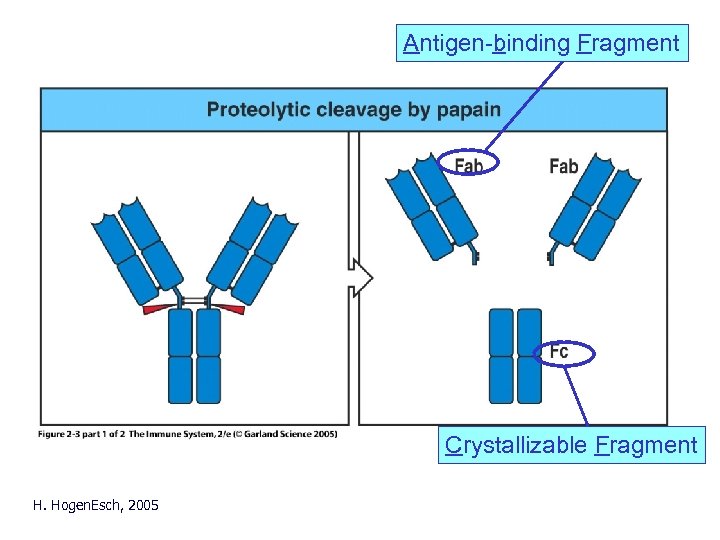
Antigen-binding Fragment Crystallizable Fragment H. Hogen. Esch, 2005
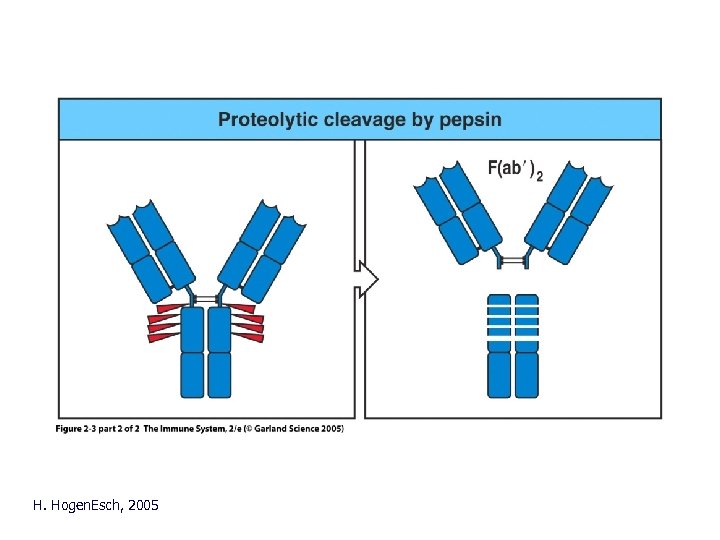
H. Hogen. Esch, 2005
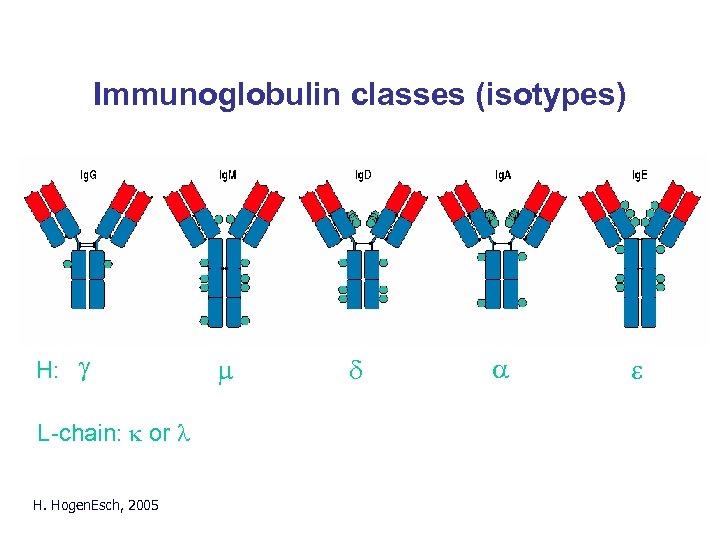
Immunoglobulin classes (isotypes) H: g L-chain: k or l H. Hogen. Esch, 2005 m d a e
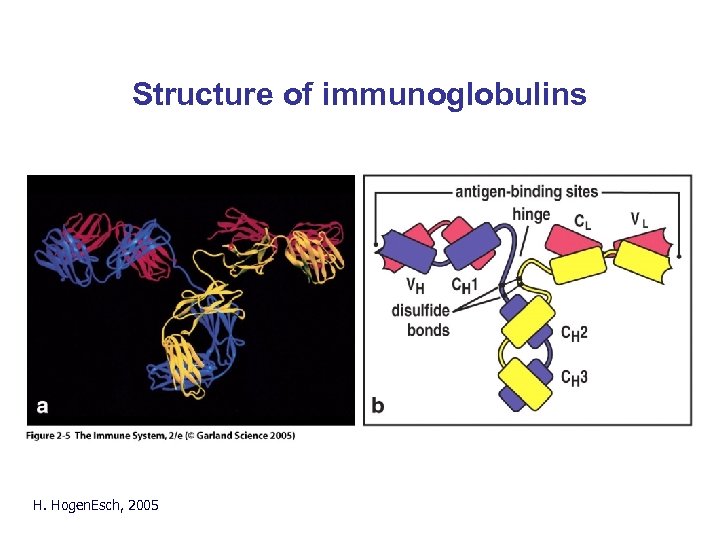
Structure of immunoglobulins H. Hogen. Esch, 2005
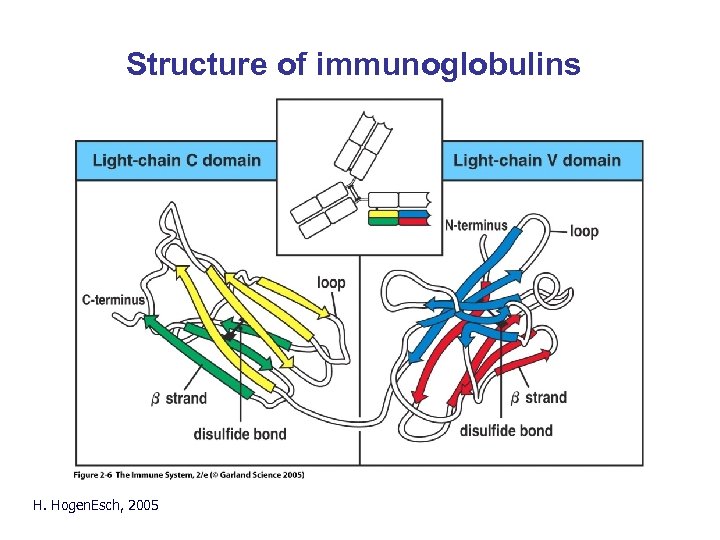
Structure of immunoglobulins H. Hogen. Esch, 2005
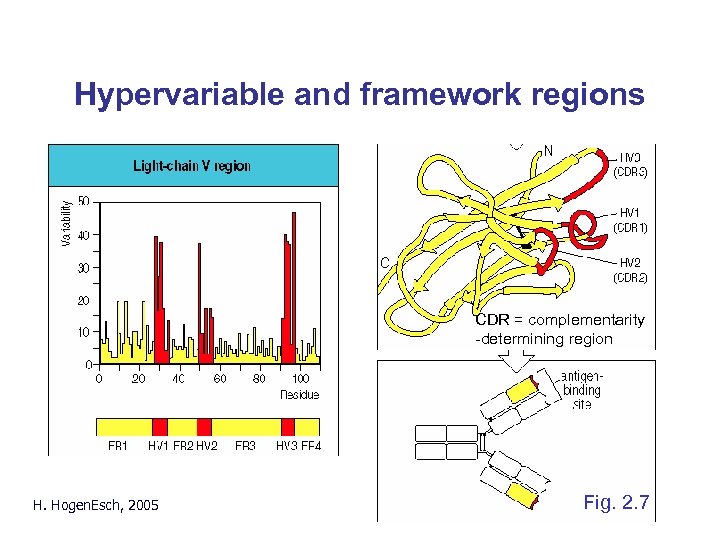
Hypervariable and framework regions CDR = complementarity -determining region H. Hogen. Esch, 2005 Fig. 2. 7
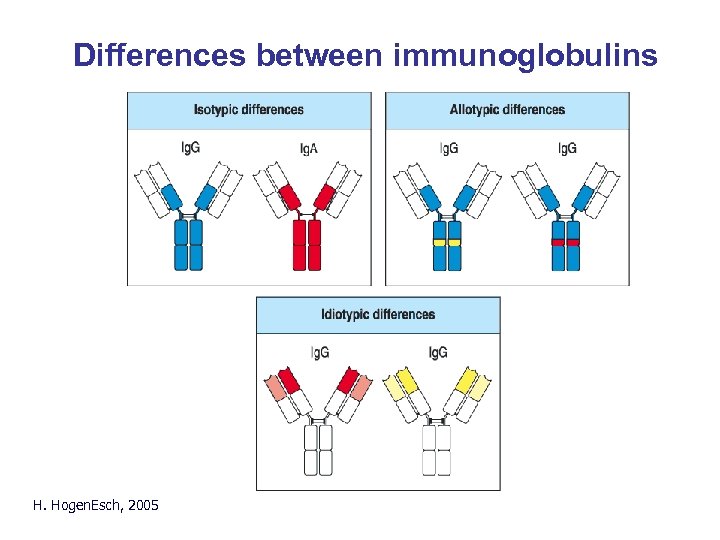
Differences between immunoglobulins H. Hogen. Esch, 2005
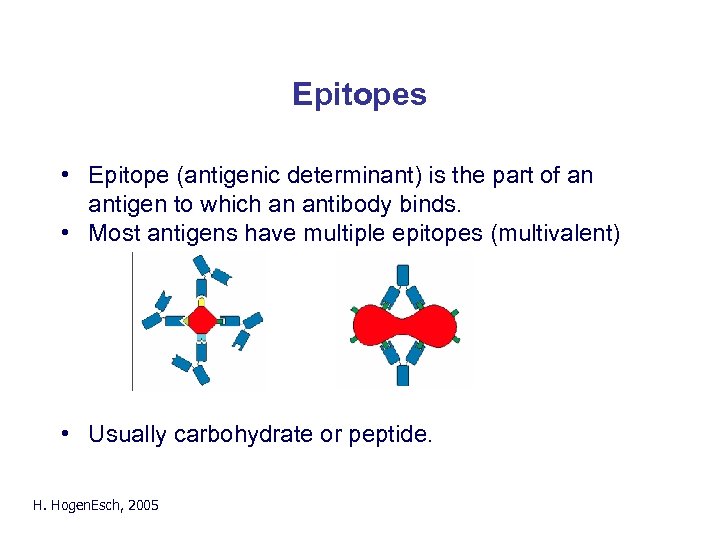
Epitopes • Epitope (antigenic determinant) is the part of an antigen to which an antibody binds. • Most antigens have multiple epitopes (multivalent) Fig. 2. 9 • Usually carbohydrate or peptide. H. Hogen. Esch, 2005
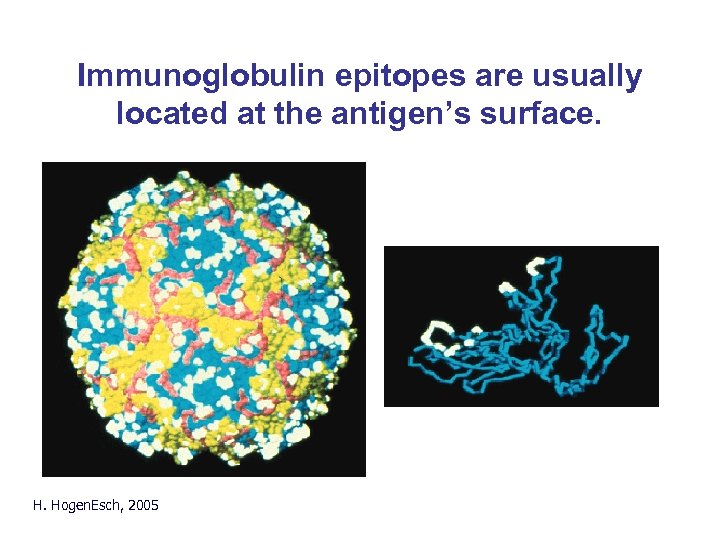
Immunoglobulin epitopes are usually located at the antigen’s surface. Fig. 2. 8 H. Hogen. Esch, 2005
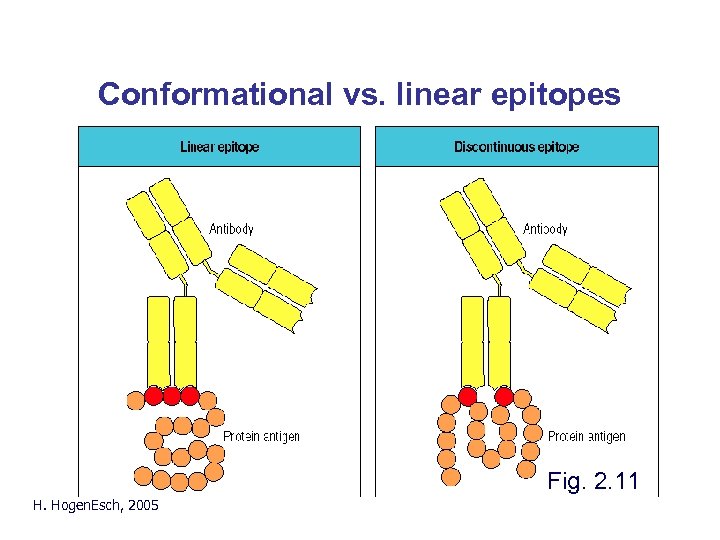
Conformational vs. linear epitopes Fig. 2. 11 H. Hogen. Esch, 2005
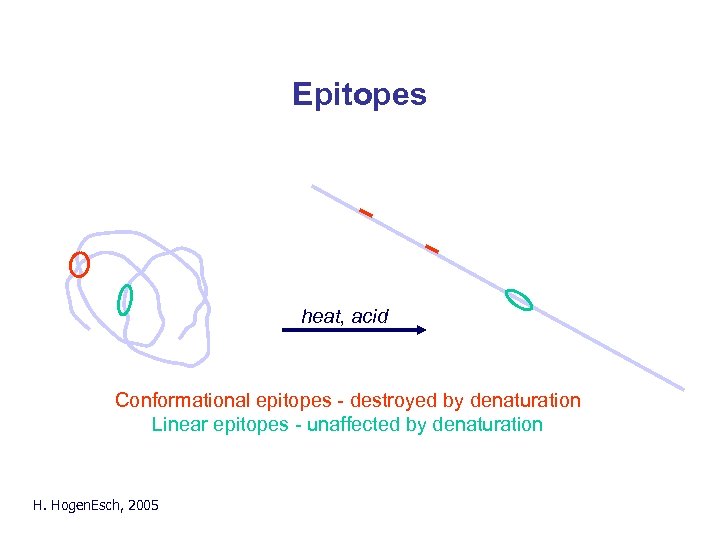
Epitopes heat, acid Conformational epitopes - destroyed by denaturation Linear epitopes - unaffected by denaturation H. Hogen. Esch, 2005
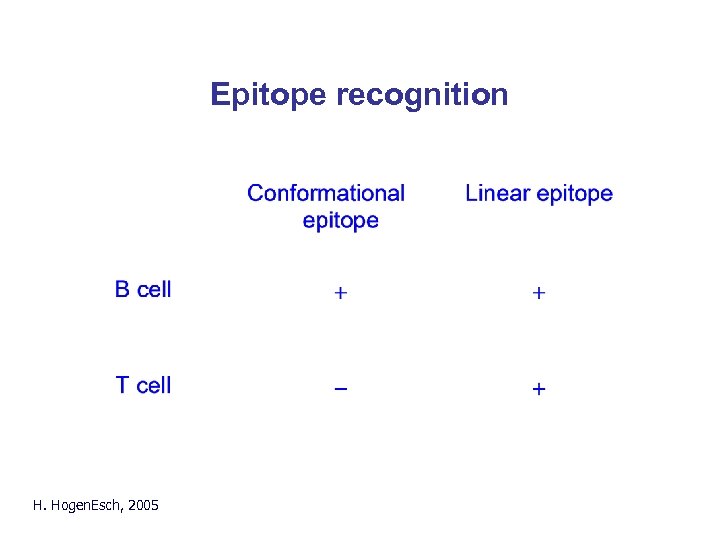
Epitope recognition H. Hogen. Esch, 2005
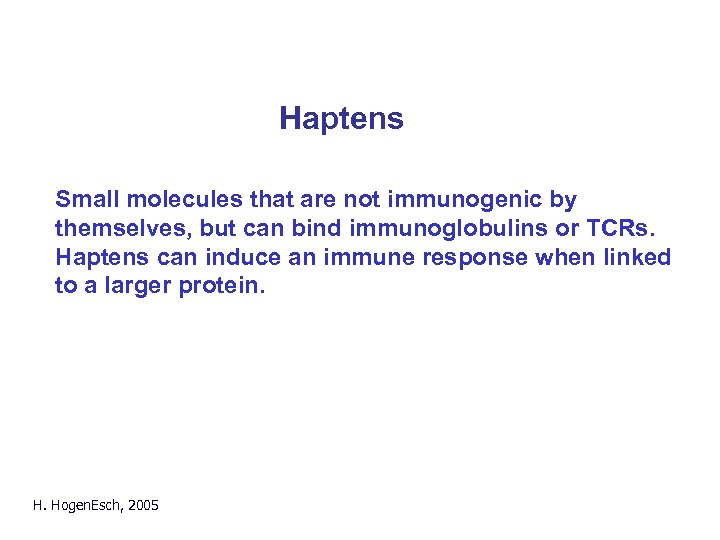
Haptens Small molecules that are not immunogenic by themselves, but can bind immunoglobulins or TCRs. Haptens can induce an immune response when linked to a larger protein. H. Hogen. Esch, 2005
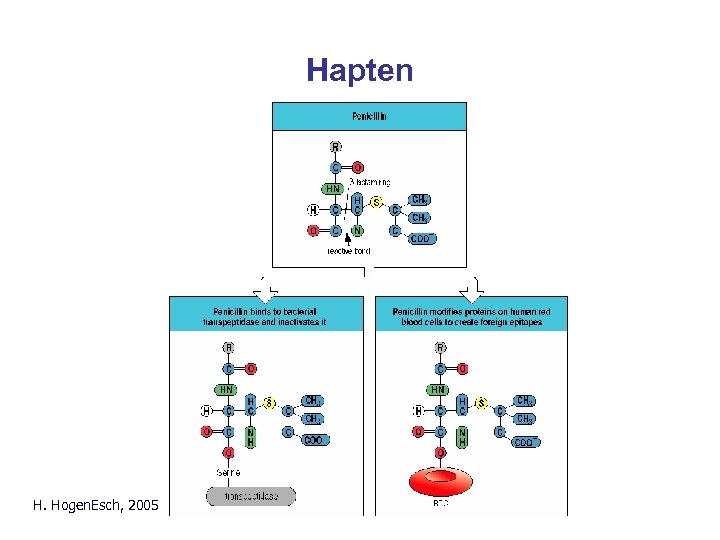
Hapten Parham Fig. 10. 25 H. Hogen. Esch, 2005
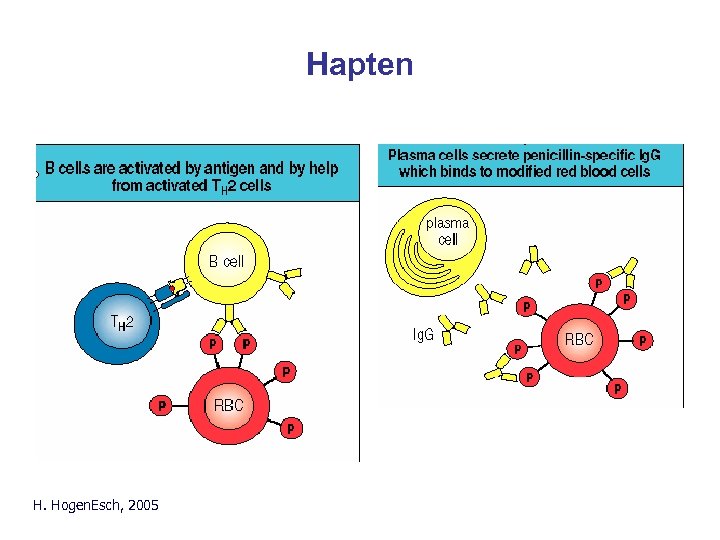
Hapten H. Hogen. Esch, 2005 Parham Fig. 10. 26
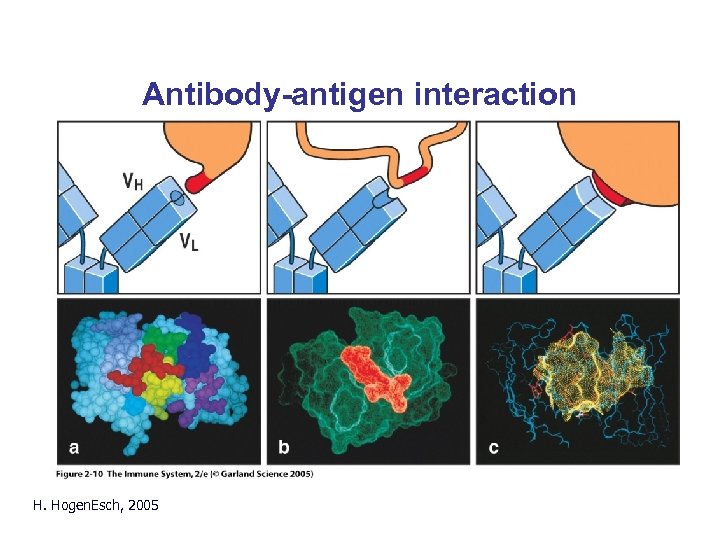
Antibody-antigen interaction H. Hogen. Esch, 2005 Fig. 2. 10
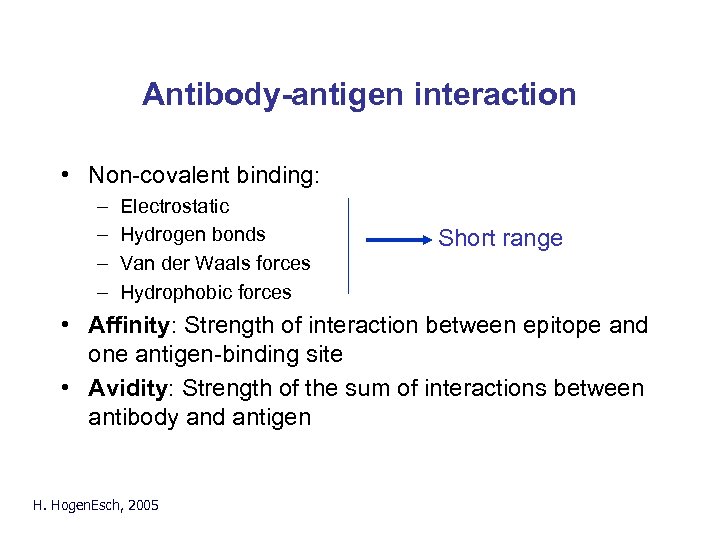
Antibody-antigen interaction • Non-covalent binding: – – Electrostatic Hydrogen bonds Van der Waals forces Hydrophobic forces Short range • Affinity: Strength of interaction between epitope and one antigen-binding site • Avidity: Strength of the sum of interactions between antibody and antigen H. Hogen. Esch, 2005
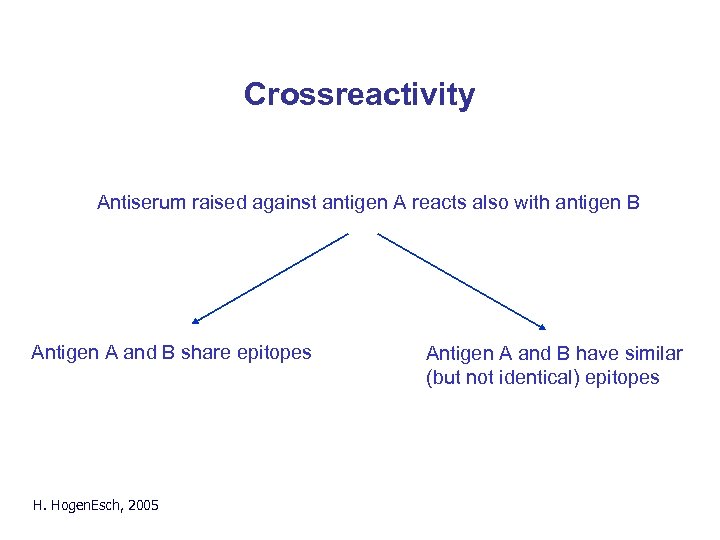
Crossreactivity Antiserum raised against antigen A reacts also with antigen B Antigen A and B share epitopes H. Hogen. Esch, 2005 Antigen A and B have similar (but not identical) epitopes
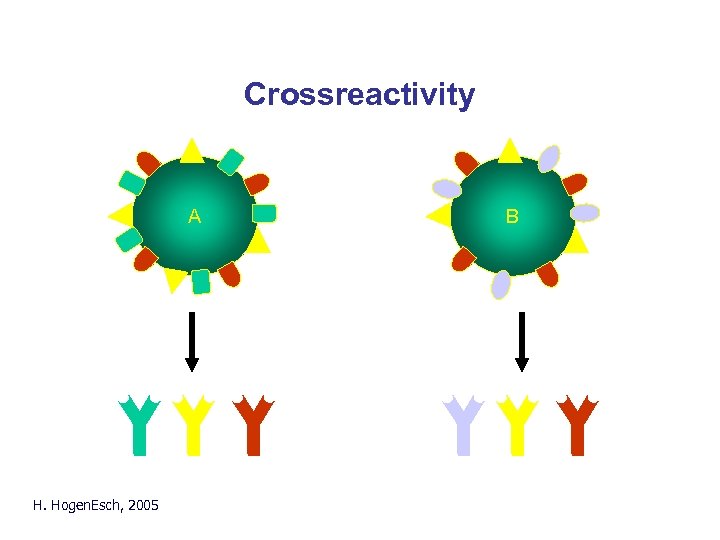
Crossreactivity A H. Hogen. Esch, 2005 B
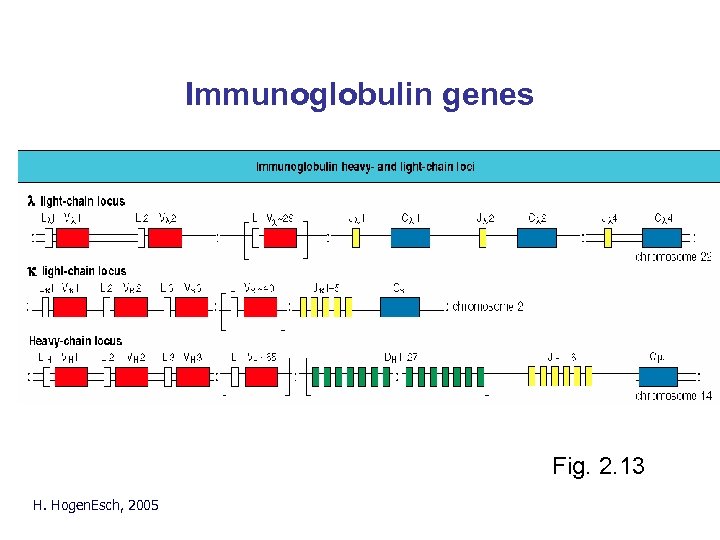
Immunoglobulin genes Fig. 2. 13 H. Hogen. Esch, 2005
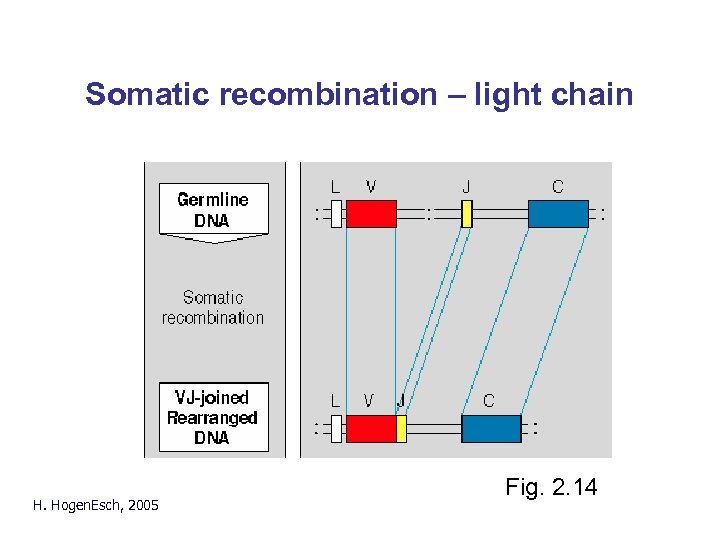
Somatic recombination – light chain H. Hogen. Esch, 2005 Fig. 2. 14
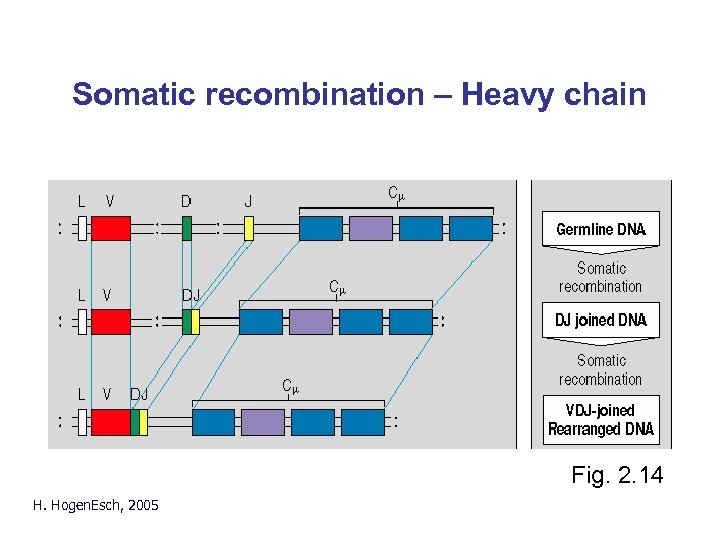
Somatic recombination – Heavy chain Fig. 2. 14 H. Hogen. Esch, 2005
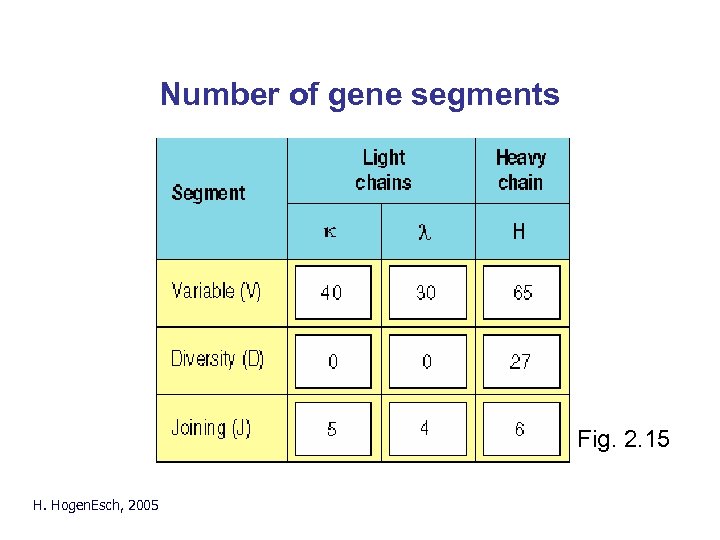
Number of gene segments Fig. 2. 15 H. Hogen. Esch, 2005
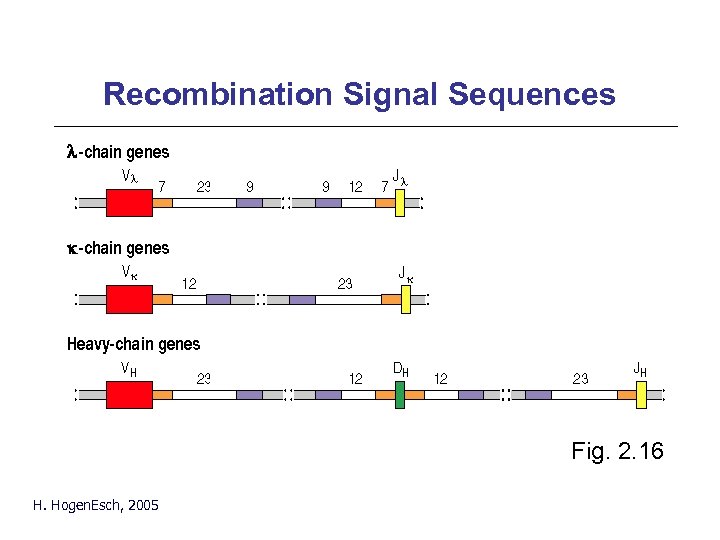
Recombination Signal Sequences Fig. 2. 16 H. Hogen. Esch, 2005
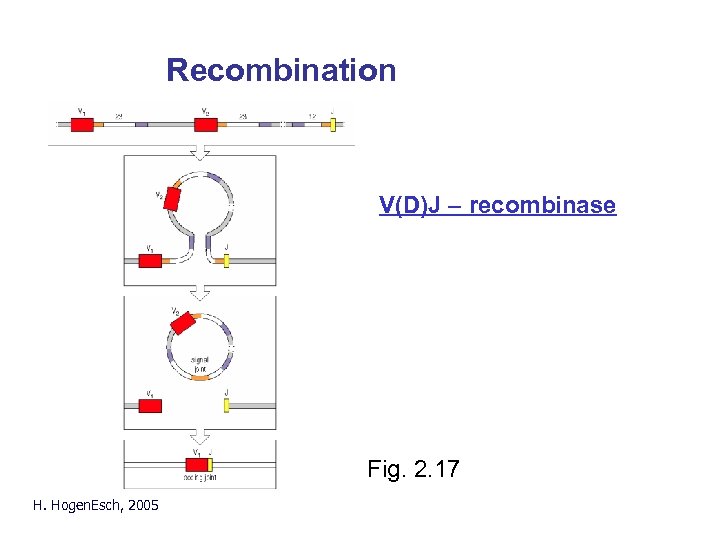
Recombination V(D)J – recombinase Fig. 2. 17 H. Hogen. Esch, 2005
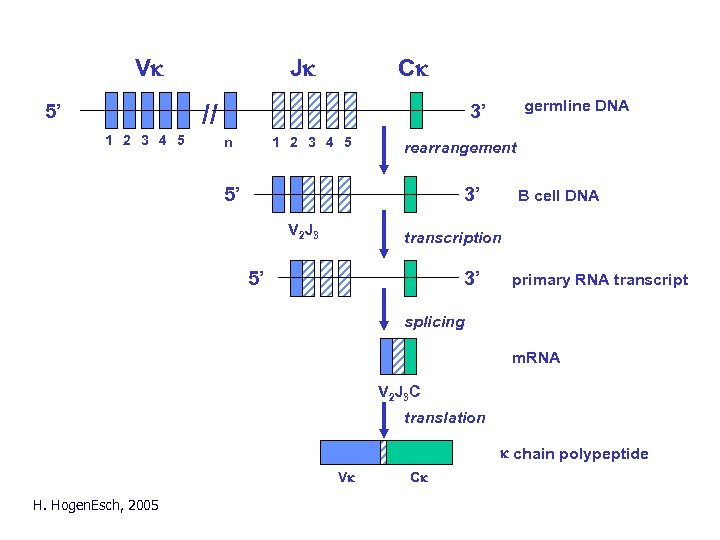
Vk Jk Ck // 5’ 1 2 3 4 5 germline DNA 3’ n 1 2 3 4 5 rearrangement 5’ 3’ V 2 J 3 B cell DNA transcription 5’ 3’ primary RNA transcript splicing m. RNA V 2 J 3 C translation k chain polypeptide Vk H. Hogen. Esch, 2005 Ck
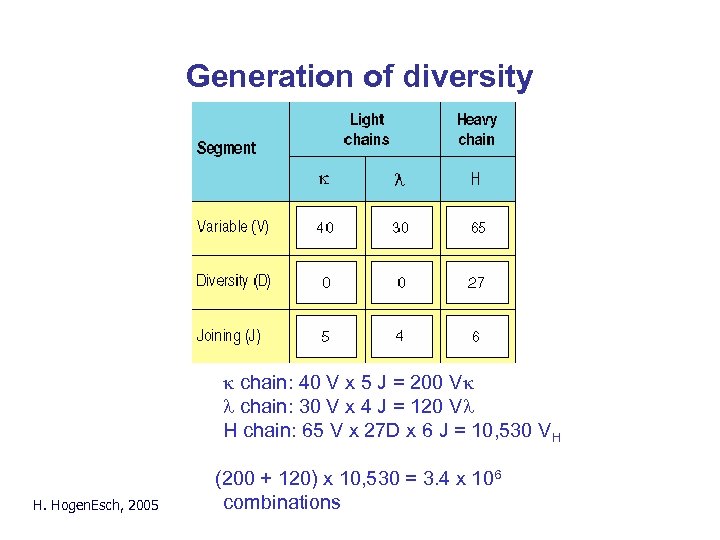
Generation of diversity k chain: 40 V x 5 J = 200 Vk l chain: 30 V x 4 J = 120 Vl H chain: 65 V x 27 D x 6 J = 10, 530 VH H. Hogen. Esch, 2005 (200 + 120) x 10, 530 = 3. 4 x 106 combinations
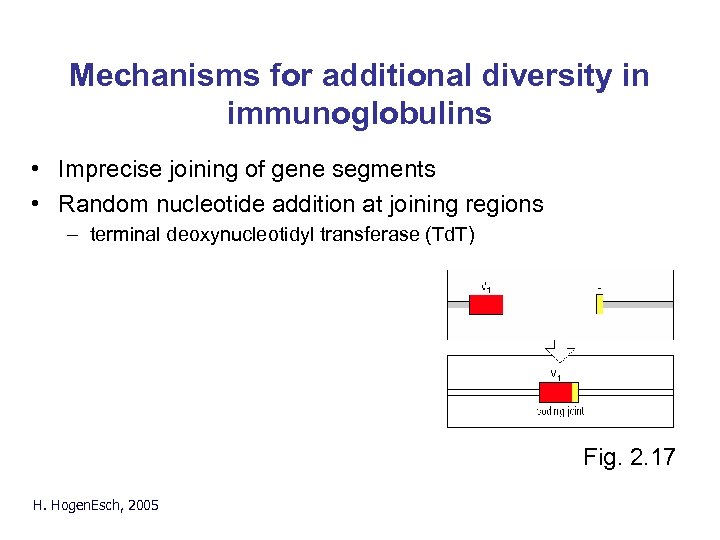
Mechanisms for additional diversity in immunoglobulins • Imprecise joining of gene segments • Random nucleotide addition at joining regions – terminal deoxynucleotidyl transferase (Td. T) Fig. 2. 17 H. Hogen. Esch, 2005
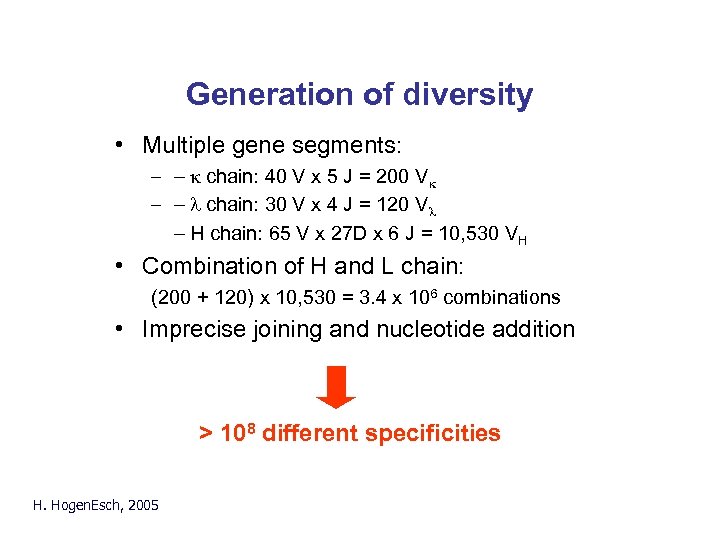
Generation of diversity • Multiple gene segments: – - k chain: 40 V x 5 J = 200 Vk – - l chain: 30 V x 4 J = 120 Vl - H chain: 65 V x 27 D x 6 J = 10, 530 VH • Combination of H and L chain: (200 + 120) x 10, 530 = 3. 4 x 106 combinations • Imprecise joining and nucleotide addition > 108 different specificities H. Hogen. Esch, 2005
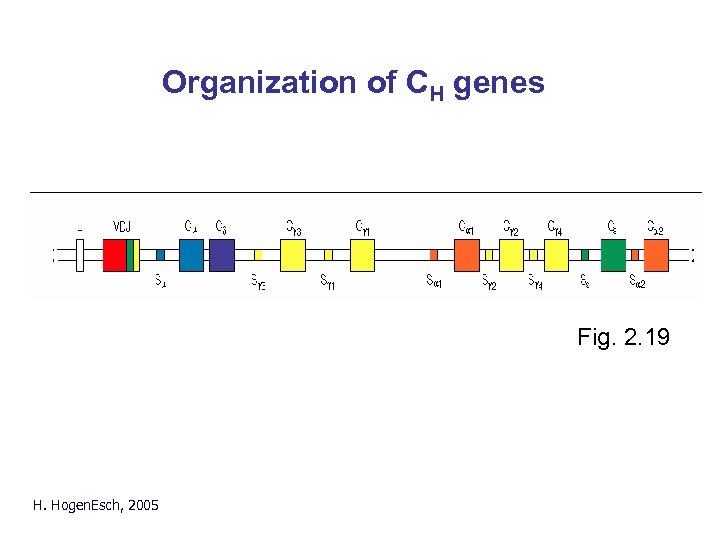
Organization of CH genes Fig. 2. 19 H. Hogen. Esch, 2005
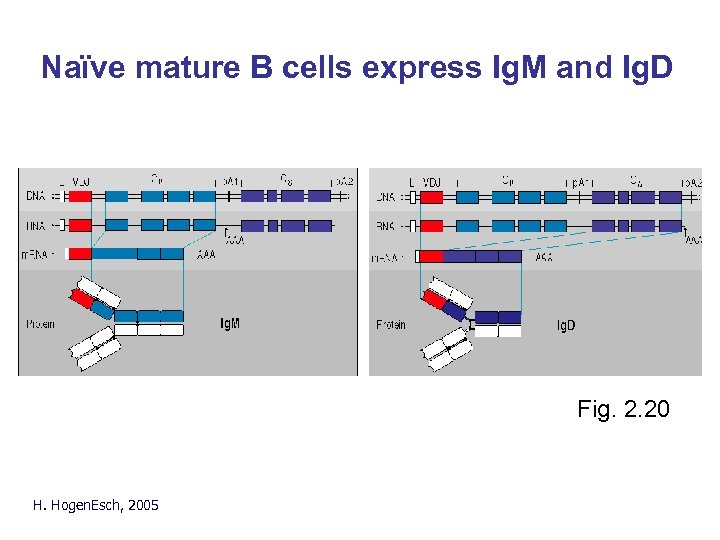
Naïve mature B cells express Ig. M and Ig. D Fig. 2. 20 H. Hogen. Esch, 2005
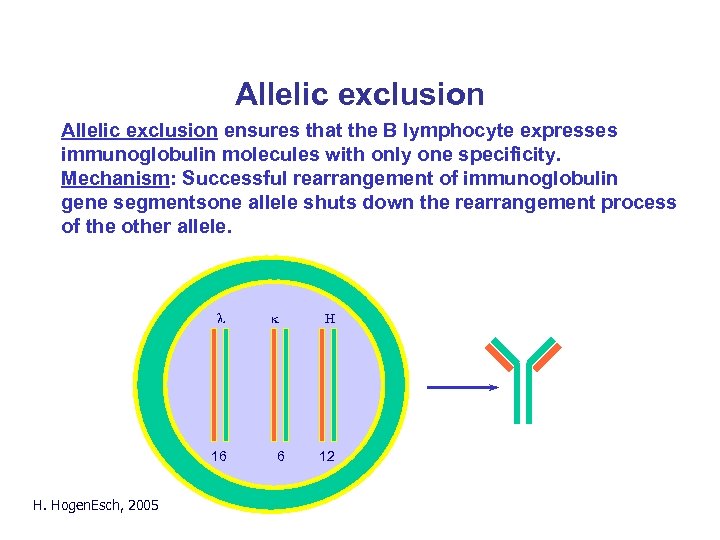
Allelic exclusion ensures that the B lymphocyte expresses immunoglobulin molecules with only one specificity. Mechanism: Successful rearrangement of immunoglobulin gene segmentsone allele shuts down the rearrangement process of the other allele. l 16 H. Hogen. Esch, 2005 k 6 H 12
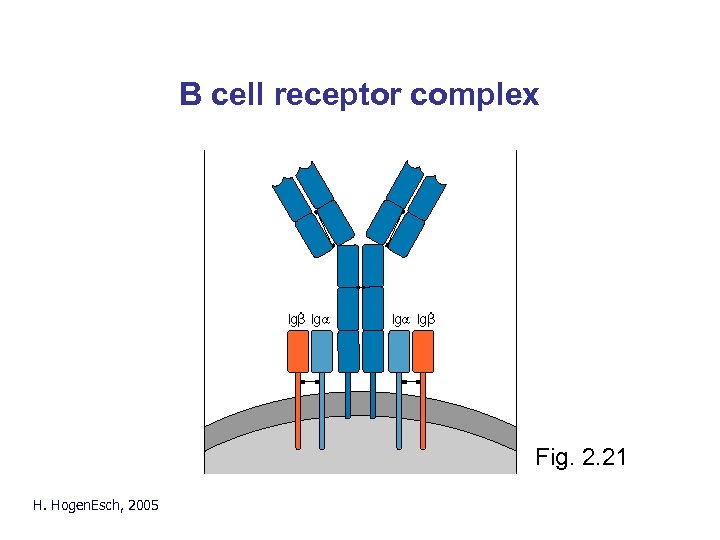
B cell receptor complex Fig. 2. 21 H. Hogen. Esch, 2005
Changes in B cells after activation by antigen • Somatic mutation – additional diversity • Isotype switching H. Hogen. Esch, 2005
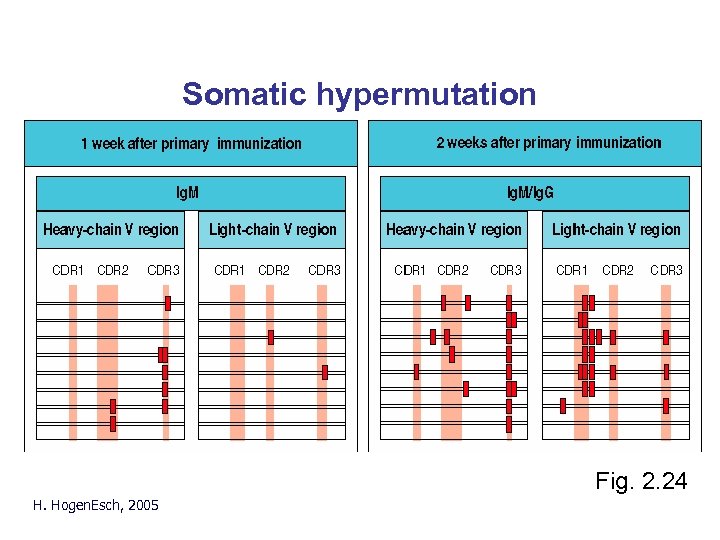
Somatic hypermutation Fig. 2. 24 H. Hogen. Esch, 2005
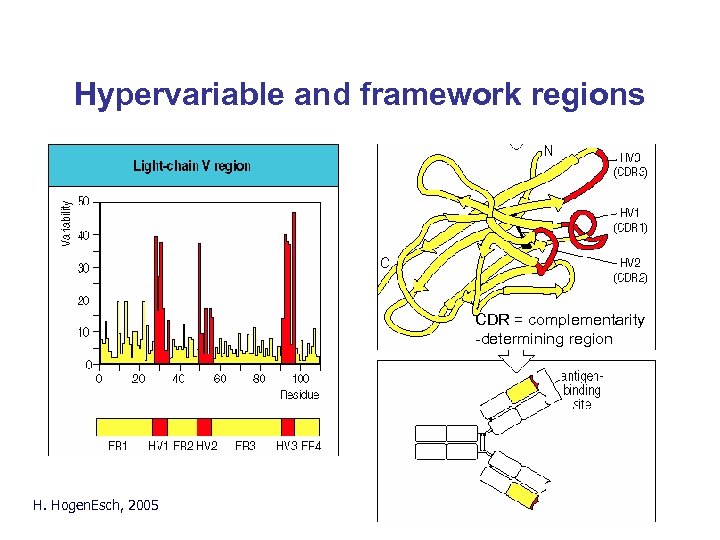
Hypervariable and framework regions CDR = complementarity -determining region H. Hogen. Esch, 2005 Fig. 2. 7
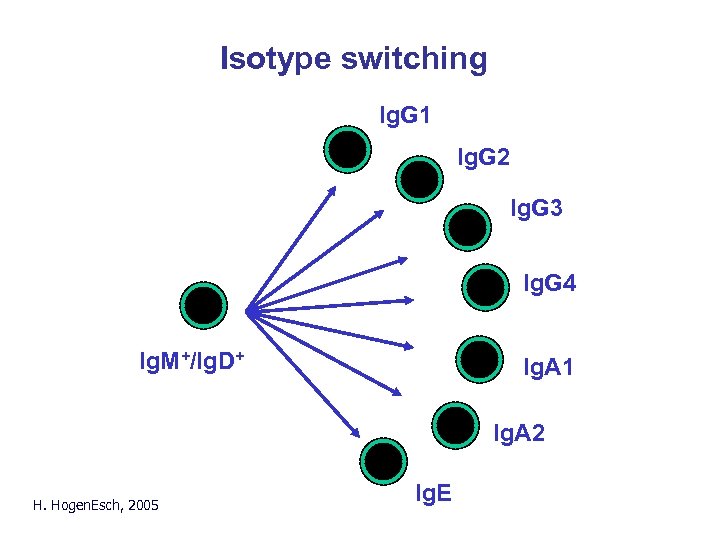
Isotype switching Ig. G 1 Ig. G 2 Ig. G 3 Ig. G 4 Ig. M+/Ig. D+ Ig. A 1 Ig. A 2 H. Hogen. Esch, 2005 Ig. E
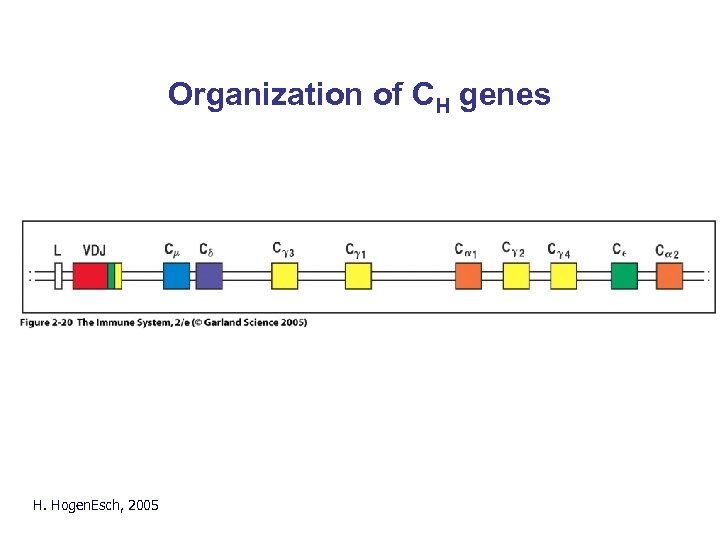
Organization of CH genes Fig. 2. 19 H. Hogen. Esch, 2005
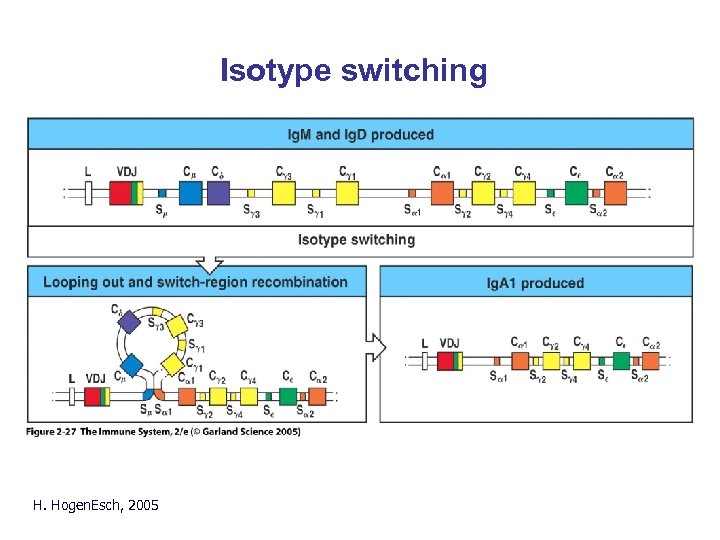
Isotype switching H. Hogen. Esch, 2005
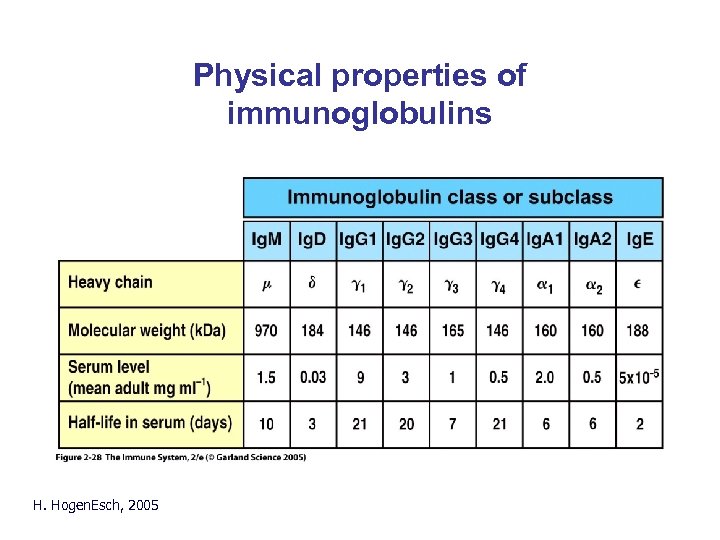
Physical properties of immunoglobulins H. Hogen. Esch, 2005
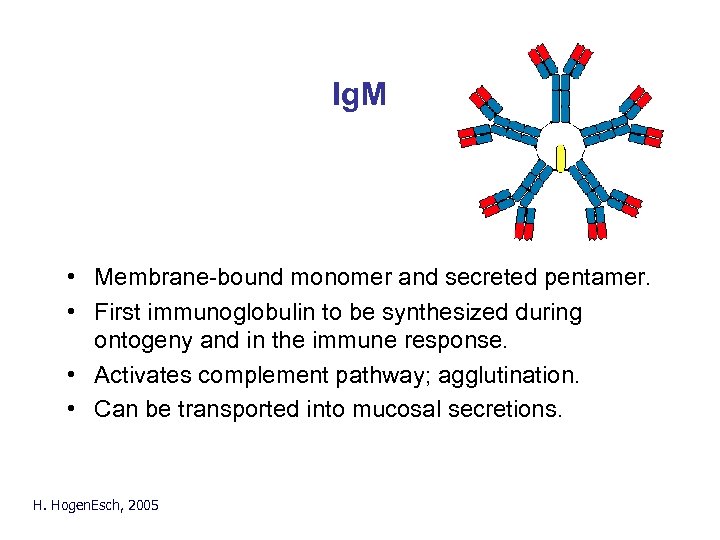
Ig. M • Membrane-bound monomer and secreted pentamer. • First immunoglobulin to be synthesized during ontogeny and in the immune response. • Activates complement pathway; agglutination. • Can be transported into mucosal secretions. H. Hogen. Esch, 2005
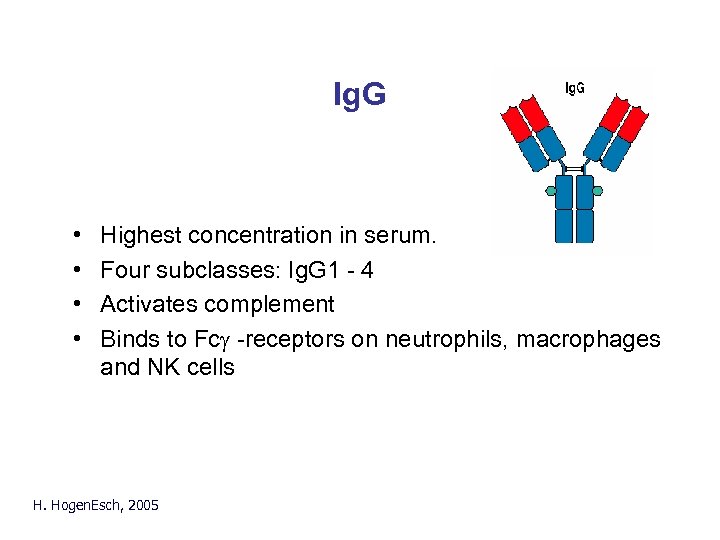
Ig. G • • Highest concentration in serum. Four subclasses: Ig. G 1 - 4 Activates complement Binds to Fcg -receptors on neutrophils, macrophages and NK cells H. Hogen. Esch, 2005
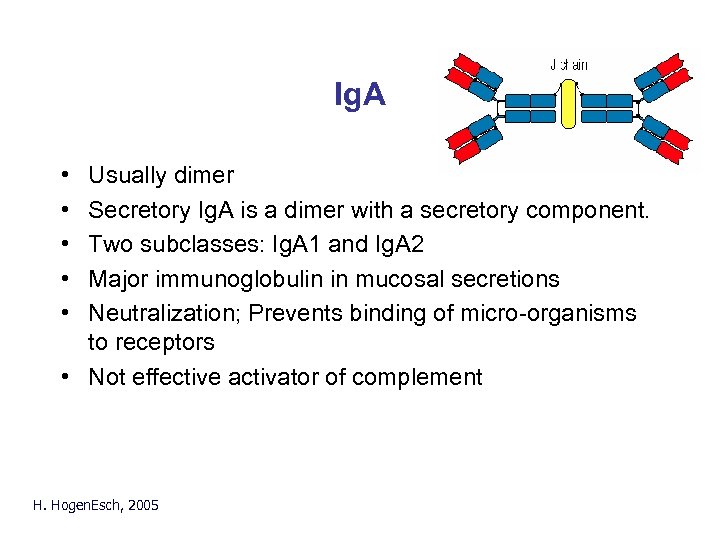
Ig. A • • • Usually dimer Secretory Ig. A is a dimer with a secretory component. Two subclasses: Ig. A 1 and Ig. A 2 Major immunoglobulin in mucosal secretions Neutralization; Prevents binding of micro-organisms to receptors • Not effective activator of complement H. Hogen. Esch, 2005
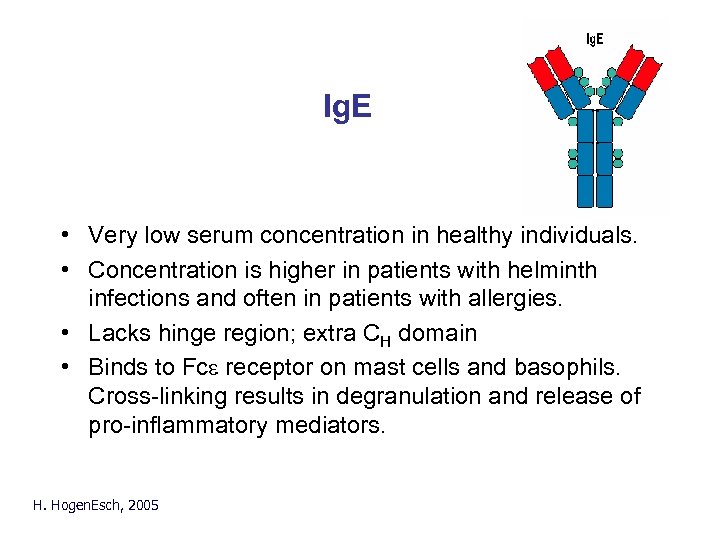
Ig. E • Very low serum concentration in healthy individuals. • Concentration is higher in patients with helminth infections and often in patients with allergies. • Lacks hinge region; extra CH domain • Binds to Fce receptor on mast cells and basophils. Cross-linking results in degranulation and release of pro-inflammatory mediators. H. Hogen. Esch, 2005
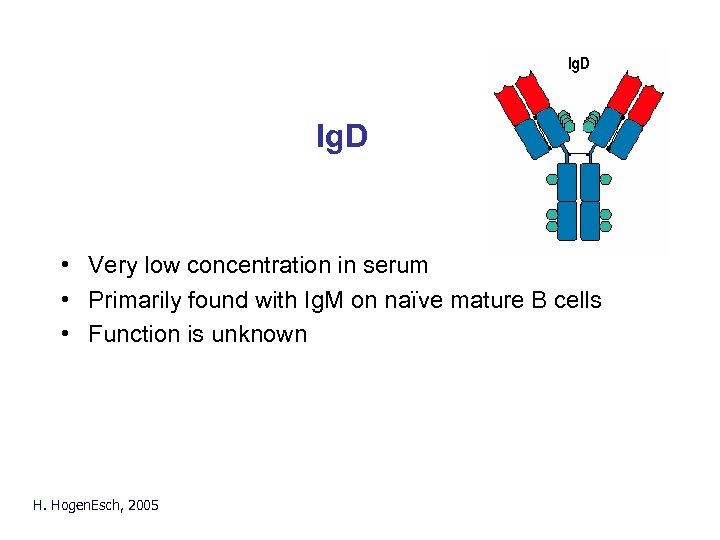
Ig. D • Very low concentration in serum • Primarily found with Ig. M on naïve mature B cells • Function is unknown H. Hogen. Esch, 2005
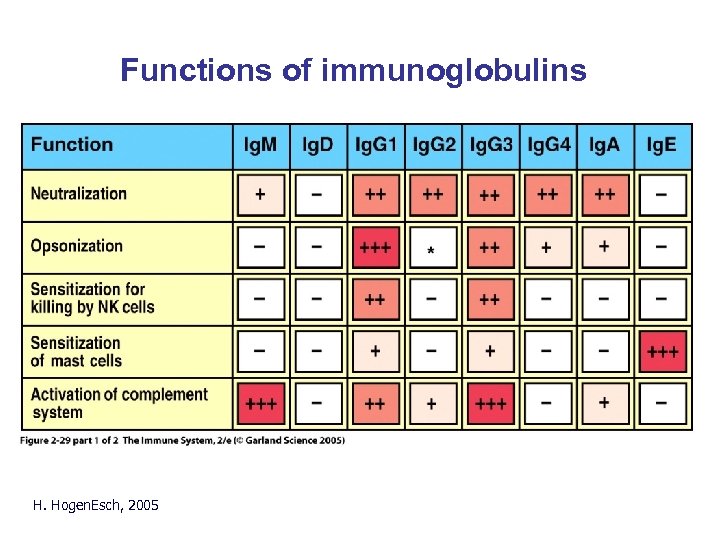
Functions of immunoglobulins H. Hogen. Esch, 2005
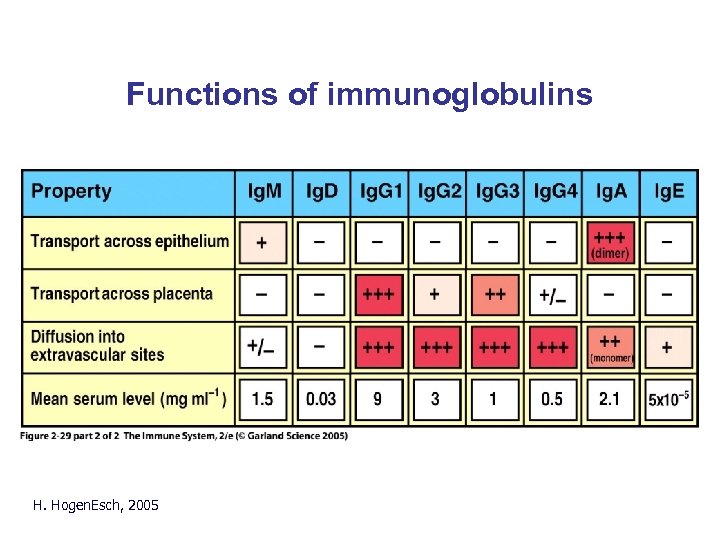
Functions of immunoglobulins H. Hogen. Esch, 2005
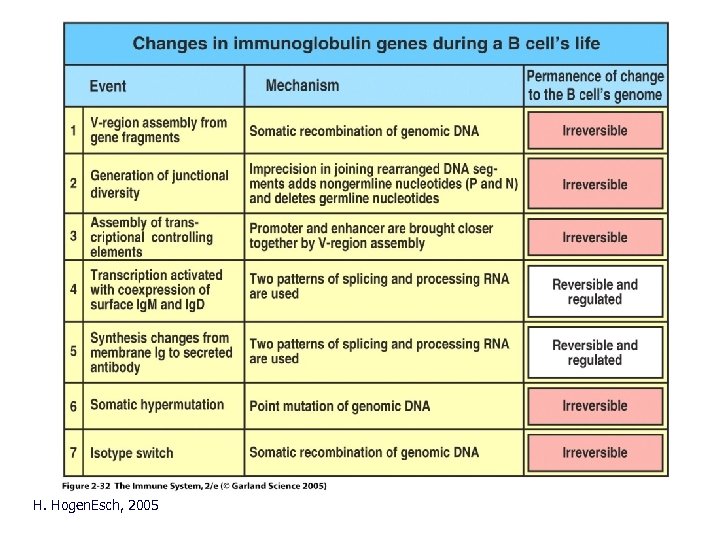
H. Hogen. Esch, 2005
Polyclonal vs. monoclonal antibodies • Polyclonal antibodies – purified from serum of immunized animals, often goats or rabbits. – Multiple specificities and affinities – Variation from batch to batch • Monoclonal antibodies – Produced by immortalized plasma cells, usually mouse origin. – Single specificity and affinity – Unlimited supply of identical antibody molecules H. Hogen. Esch, 2005
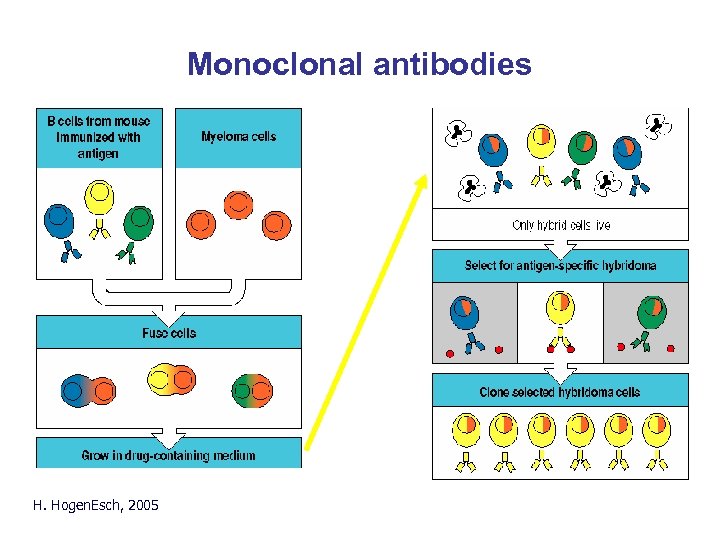
Monoclonal antibodies H. Hogen. Esch, 2005
Immunoassays • • H. Hogen. Esch, 2005 Precipitation assay Agglutination assay Enzyme-linked immunosorbent assay (ELISA) Radioimmunoassay (RIA) Western blotting Immunofluorescence Flow cytometry
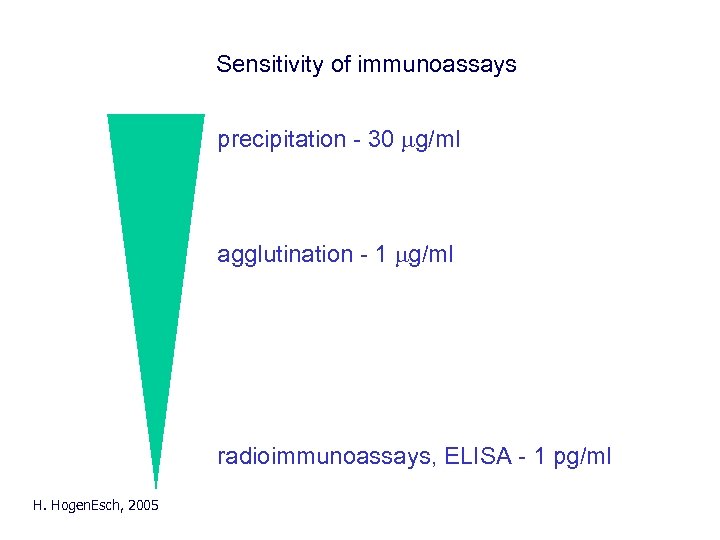
Sensitivity of immunoassays precipitation - 30 mg/ml agglutination - 1 mg/ml radioimmunoassays, ELISA - 1 pg/ml H. Hogen. Esch, 2005
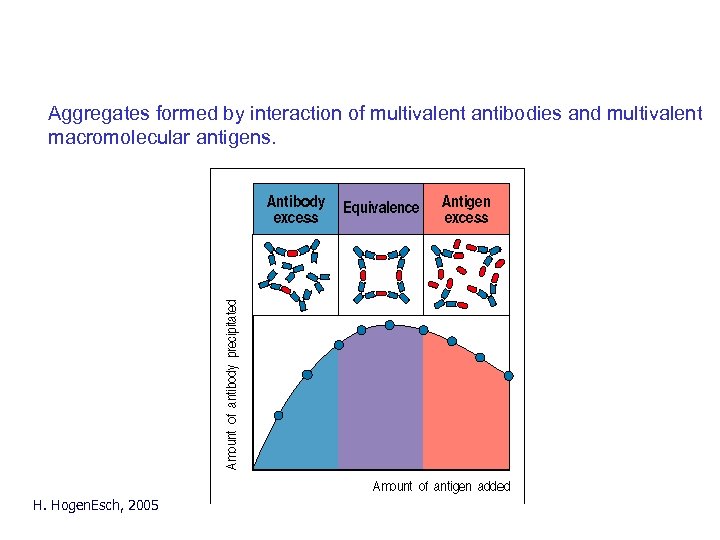
Precipitation reaction Aggregates formed by interaction of multivalent antibodies and multivalent macromolecular antigens. H. Hogen. Esch, 2005
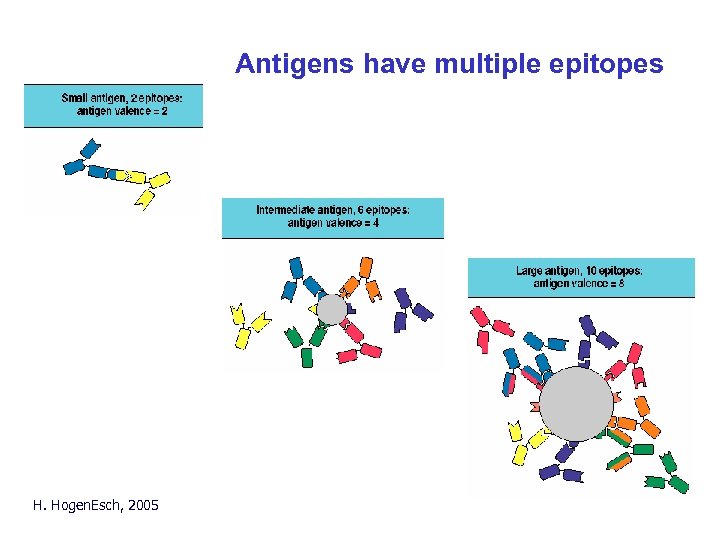
Antigens have multiple epitopes H. Hogen. Esch, 2005
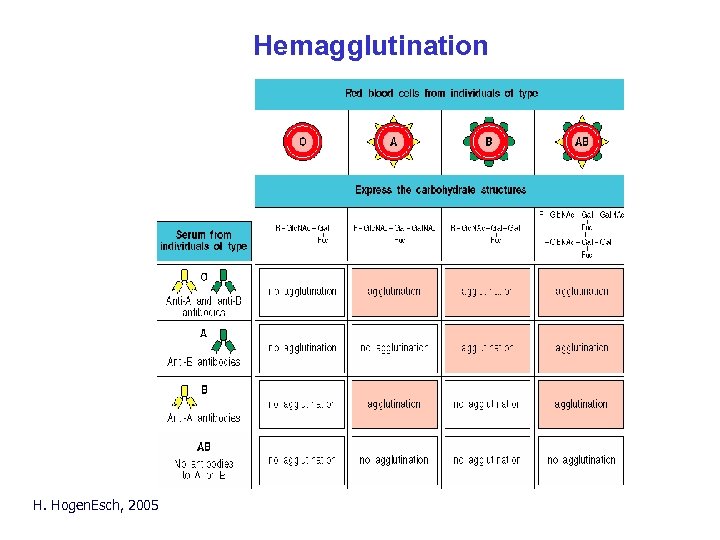
Hemagglutination H. Hogen. Esch, 2005
Coombs test • Direct: Add anti-human immunoglobulin antibodies (Coombs’ reagent) to red blood cells. Agglutination occurs if the red blood cells are coated with antibodies. • Indirect: Incubate test serum with red blood cells. Wash red blood cells. Add anti-human immunoglobulin antibodies. H. Hogen. Esch, 2005
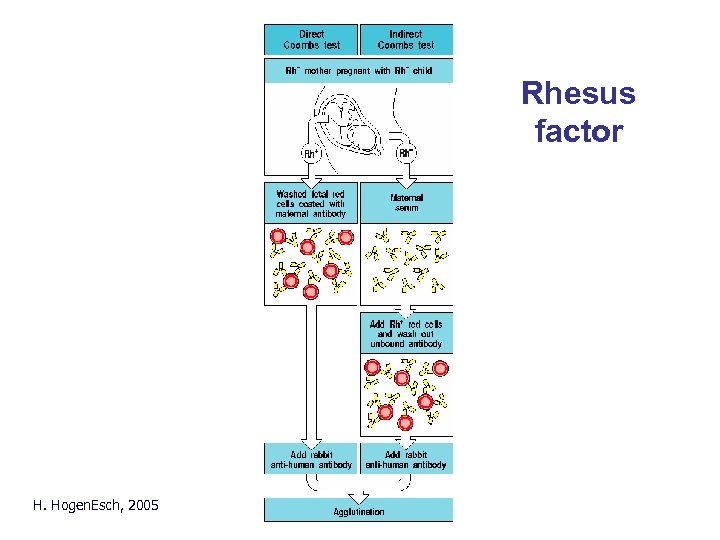
Rhesus factor H. Hogen. Esch, 2005
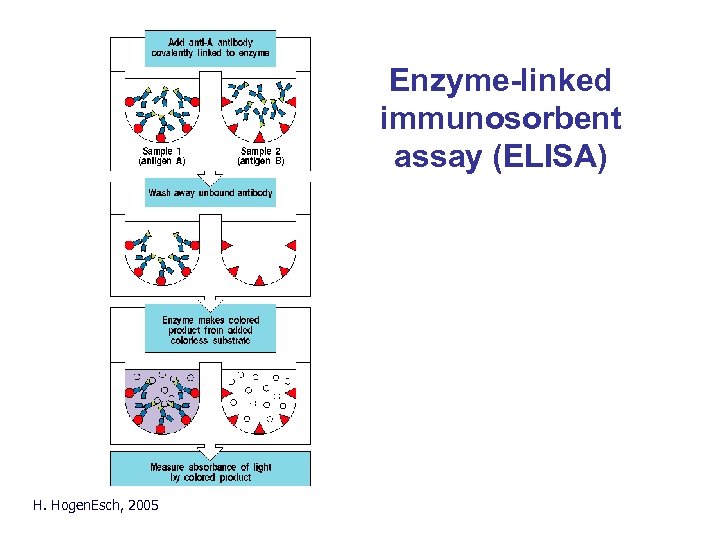
Enzyme-linked immunosorbent assay (ELISA) Principle of ELISA/RIA H. Hogen. Esch, 2005
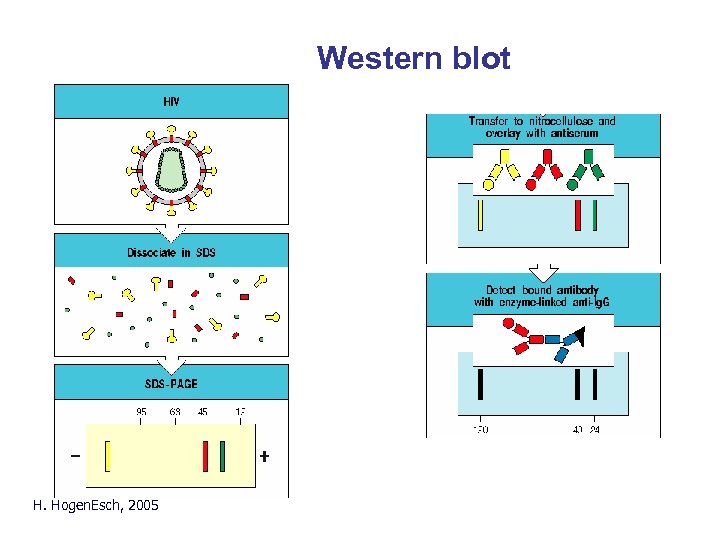
Western blotting Western blot H. Hogen. Esch, 2005
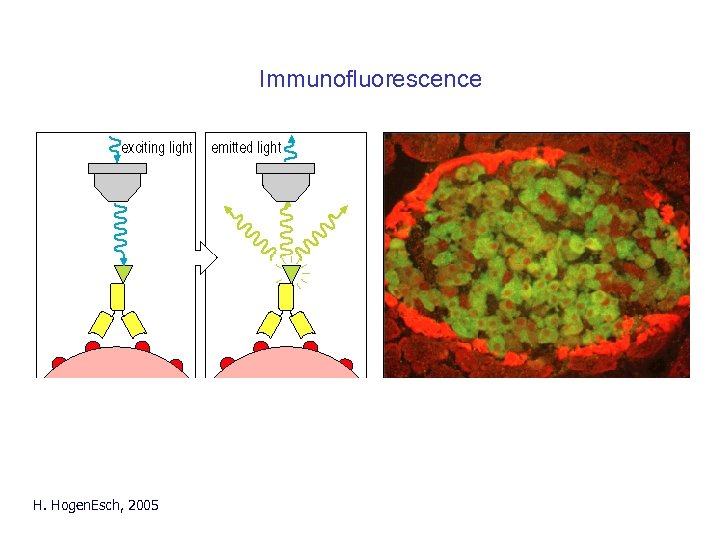
Immunofluorescence H. Hogen. Esch, 2005
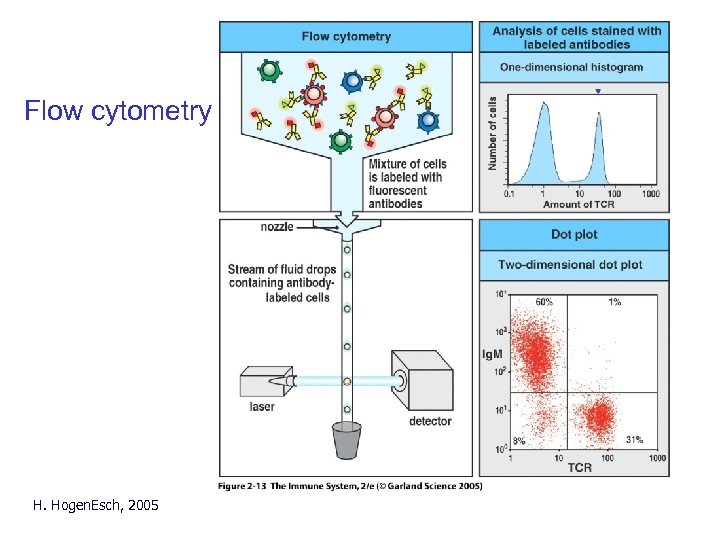
Flow cytometry H. Hogen. Esch, 2005
c916dab2faf9db853ce0f8284c314adf.ppt