314e8f11afdaec960e90d9980b7431fd.ppt
- Количество слайдов: 29

Antibiotic Resistance: Situation Analysis and Needs Assessment in Uganda and Zambia (AR-SANA) Capacity building for laboratory strengthening and detecting antibiotic resistance: findings of a needs assessment in Uganda and Zambia Alliance for the Prudent Use of Antibiotics 1
AMR in Zambia: Key Findings S. pneumoniae resistance rates to penicillin rose from 14. 3% resistance in 1990 s to 53 -67 % in 2007. Infants are most likely to have S. pneumoniae identified from their blood and spinal fluid. Co-trimoxazole resistance of S. pneumoniae is high (80 -100%). Enteric infections that affected Zambian children were due to rotavirus and enteric bacteria (E. coli, V. cholerae, Salmonella spp. , and Shigella spp. ). Available data showed very high resistance among enteric bacteria to gentamicin, cefotaxime, nalidixic acid, ciprofloxacin, co-trimoxazole and cephalexin ranges between 70 -100%. Alliance for the Prudent Use of Antibiotics
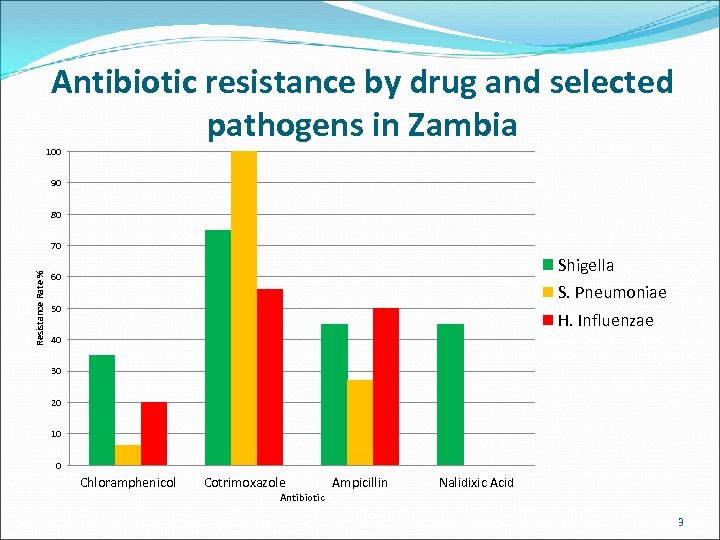
Antibiotic resistance by drug and selected pathogens in Zambia 100 90 80 Resistance Rate % 70 Shigella 60 S. Pneumoniae 50 H. Influenzae 40 30 20 10 0 Chloramphenicol Cotrimoxazole Antibiotic Ampicillin Nalidixic Acid 3
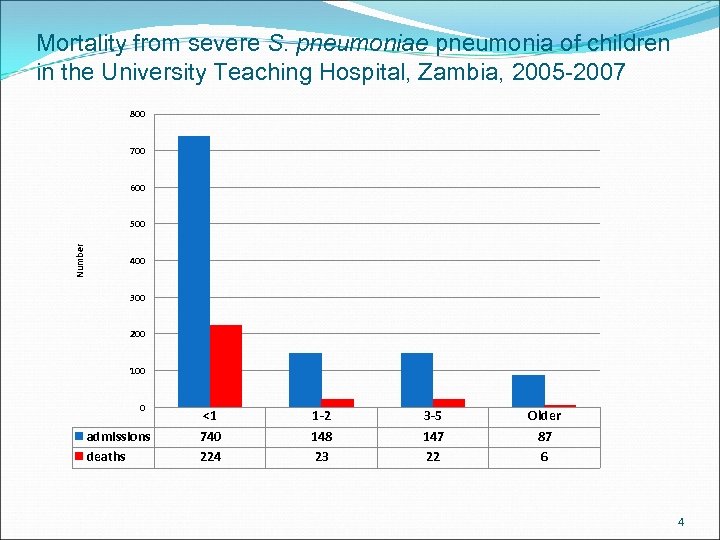
Mortality from severe S. pneumoniae pneumonia of children in the University Teaching Hospital, Zambia, 2005 -2007 800 700 600 Number 500 400 300 200 100 0 admissions deaths <1 740 224 1 -2 148 23 3 -5 147 22 Older 87 6 4
AMR in Uganda: Key Findings Acute respiratory and enteric infections in Uganda are main causes of increased morbidity, mortality and costs. Streptococcus pneumoniae, and Haemophilus influenzae type b (Hib) continue to be the main bacteria responsible for Acute Respiratory Infections (ARI). Viral etiology (mainly Respiratory Syncytial Virus-RSV) in severe pneumonia among infants and children needs to be investigated. § Empirical treatment should be guided by data provided by antibiotic resistance surveillance, particularly in common pathogens. § Available information on Antibiotic Resistance (ABR) is in most cases scattered, incomplete and often unreliable. Alliance for the Prudent Use of Antibiotics
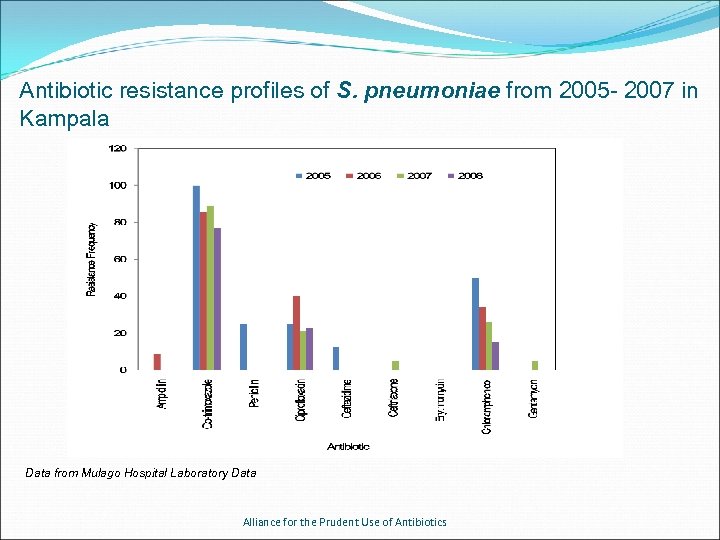
Antibiotic resistance profiles of S. pneumoniae from 2005 - 2007 in Kampala Data from Mulago Hospital Laboratory Data Alliance for the Prudent Use of Antibiotics

Purpose of the laboratory survey To examine: I. Laboratory capacity to conduct research on antibiotic resistance. II. Ability of laboratories to deliver accurate results III. Ability of laboratories to detect pathogens and perform antimicrobial sensitivity testing IV. Availability of a system for quality control in the laboratories V. Availability of mechanisms for dissemination of laboratory/ surveillance data VI. Availability of a system for collection, analysis and transmission of the data to be used for antibiotic management decisions VII. Economic situation of the survey laboratories VIII. Availability of the WHONET software for antimicrobial resistance surveillance 7

Method of laboratory survey 17 and 29 laboratories across Zambia and Uganda were surveyed respectively. Structured questionnaires (adapted from the WHO assessment form) were used to conduct the interview. Training of data collectors was carried out The survey was carried out 2009 and 2010. The study obtained ethical approvals from the University of Zambia Ethical Review Board, the Ethical Review Committee of Makerere University College of Health Sciences, Kampala, and Boston Tufts University Institutional Review Board 8
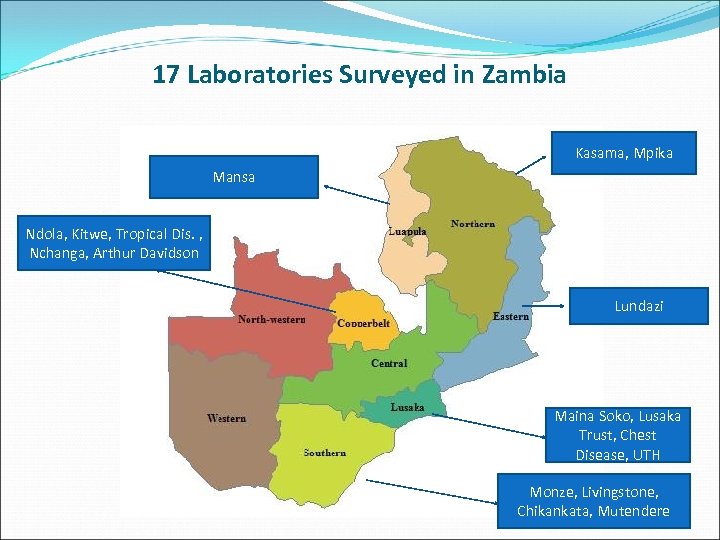
17 Laboratories Surveyed in Zambia Kasama, Mpika Mansa Ndola, Kitwe, Tropical Dis. , Nchanga, Arthur Davidson Lundazi Maina Soko, Lusaka Trust, Chest Disease, UTH Monze, Livingstone, Chikankata, Mutendere 9
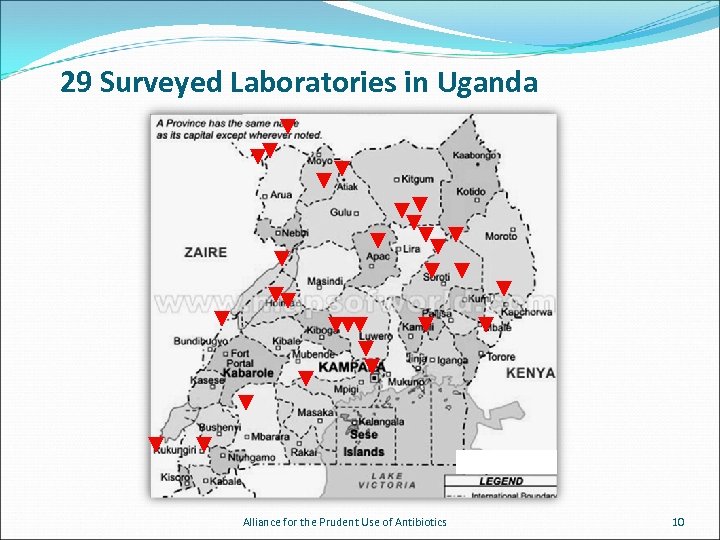
29 Surveyed Laboratories in Uganda Alliance for the Prudent Use of Antibiotics 10

Laboratory survey Components I. Laboratory staffing and trainings II. Laboratory equipment III. Laboratory supply logistics IV. Laboratory record keeping for supplies management V. Sources of laboratory reagents VI. Specimen collection, handling and labelling VII. Laboratory specific capacity VIII. Structure of reporting laboratory results IX. Quality control procedures X. Cost of laboratory testing and sources of funding 11

Microbiology Laboratory University Teaching Hospital of Lusaka 12

Microbiology Laboratory University Teaching Hospital of Lusaka
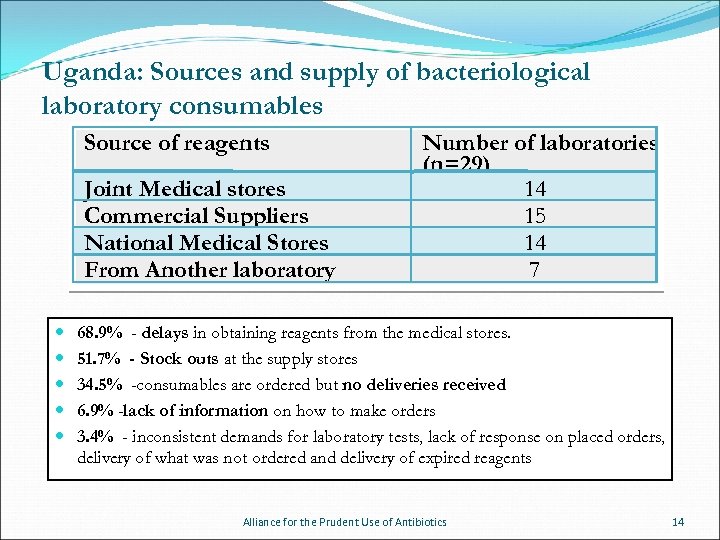
Uganda: Sources and supply of bacteriological laboratory consumables Source of reagents Joint Medical stores Commercial Suppliers National Medical Stores From Another laboratory Number of laboratories (n=29) 14 15 14 7 68. 9% - delays in obtaining reagents from the medical stores. 51. 7% - Stock outs at the supply stores 34. 5% -consumables are ordered but no deliveries received 6. 9% -lack of information on how to make orders 3. 4% - inconsistent demands for laboratory tests, lack of response on placed orders, delivery of what was not ordered and delivery of expired reagents Alliance for the Prudent Use of Antibiotics 14
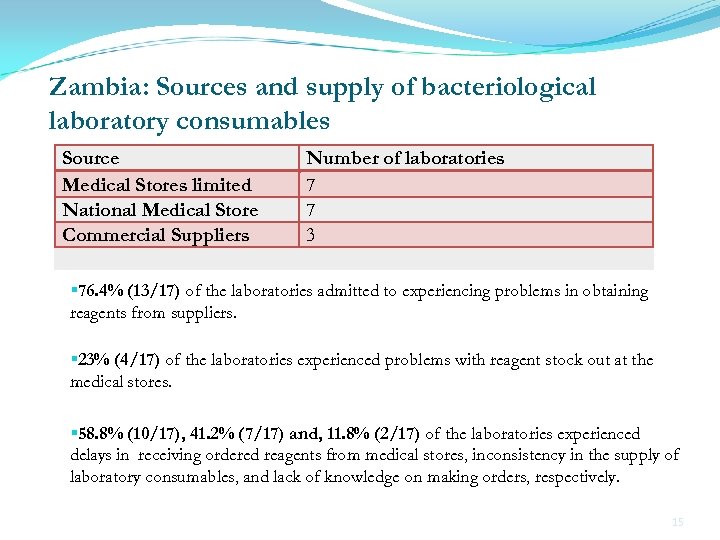
Zambia: Sources and supply of bacteriological laboratory consumables Source Medical Stores limited National Medical Store Commercial Suppliers Number of laboratories 7 7 3 § 76. 4% (13/17) of the laboratories admitted to experiencing problems in obtaining reagents from suppliers. § 23% (4/17) of the laboratories experienced problems with reagent stock out at the medical stores. § 58. 8% (10/17), 41. 2% (7/17) and, 11. 8% (2/17) of the laboratories experienced delays in receiving ordered reagents from medical stores, inconsistency in the supply of laboratory consumables, and lack of knowledge on making orders, respectively. 15

Scores of Zambian Laboratories Score Range 0 -49% Lundazi District Hospital Mutendere Mission Hospital Livingstone General Hospital Score Range 50%-74% Maina Soko Military Hospital Mansa General Hospital Chikankata Mission Hospital Mpika General Hospital Kasama General Hospital Kitwe Central Hospital Ndola Central Hospital Monze Mission Hospital Nchanga South Hospital Lusaka Trust Hospital Alliance for the Prudent Use of Antibiotics Score Range >75% University of Zambia Teaching Hospital * Tropical Disease Research Center * (research facility) Arthur Davidson (Pediatric) Hospital Laboratory* Chest Disease Laboratory * (national laboratory) 16
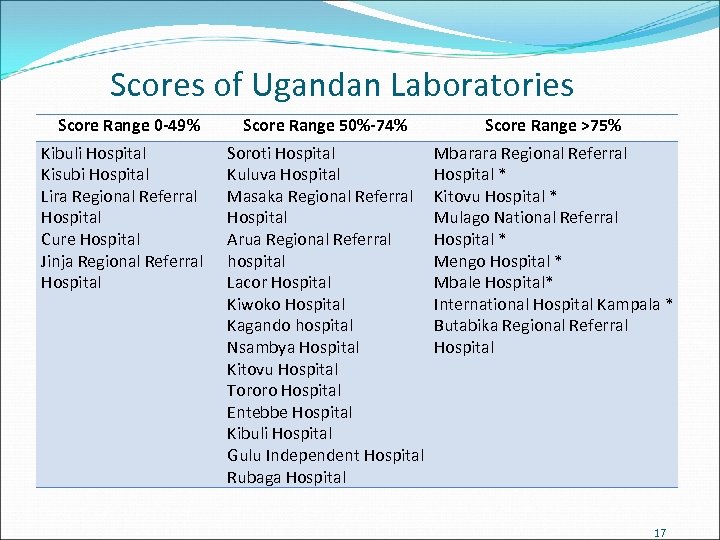
Scores of Ugandan Laboratories Score Range 0 -49% Kibuli Hospital Kisubi Hospital Lira Regional Referral Hospital Cure Hospital Jinja Regional Referral Hospital Score Range 50%-74% Score Range >75% Soroti Hospital Kuluva Hospital Masaka Regional Referral Hospital Arua Regional Referral hospital Lacor Hospital Kiwoko Hospital Kagando hospital Nsambya Hospital Kitovu Hospital Tororo Hospital Entebbe Hospital Kibuli Hospital Gulu Independent Hospital Rubaga Hospital Mbarara Regional Referral Hospital * Kitovu Hospital * Mulago National Referral Hospital * Mengo Hospital * Mbale Hospital* International Hospital Kampala * Butabika Regional Referral Hospital 17
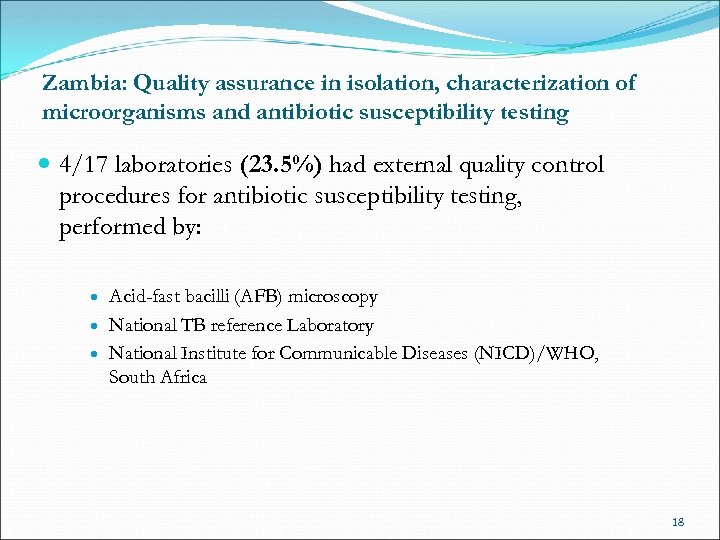
Zambia: Quality assurance in isolation, characterization of microorganisms and antibiotic susceptibility testing 4/17 laboratories (23. 5%) had external quality control procedures for antibiotic susceptibility testing, performed by: Acid-fast bacilli (AFB) microscopy National TB reference Laboratory National Institute for Communicable Diseases (NICD)/WHO, South Africa 18

Zambia: Availability and use of the WHONET software Only the University Teaching Hospital, Lusaka laboratory is currently using WHONET software (version 5. 1 installed in May 2009) There is no national policy on antibiotic resistance surveillance 19
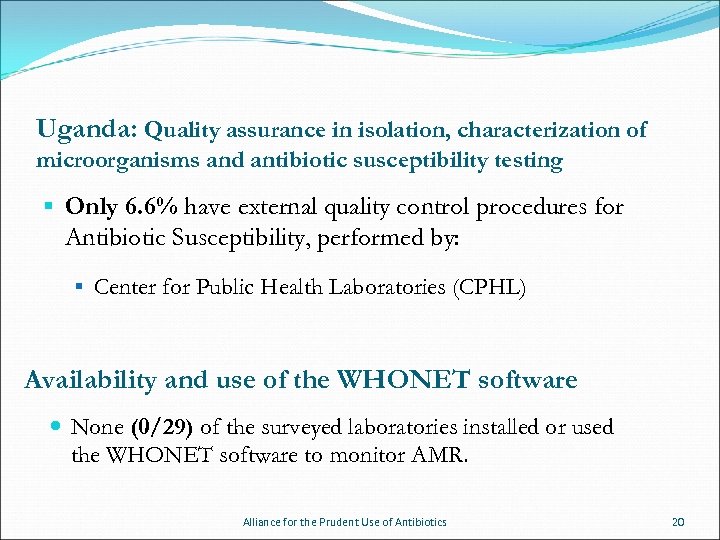
Uganda: Quality assurance in isolation, characterization of microorganisms and antibiotic susceptibility testing § Only 6. 6% have external quality control procedures for Antibiotic Susceptibility, performed by: § Center for Public Health Laboratories (CPHL) Availability and use of the WHONET software None (0/29) of the surveyed laboratories installed or used the WHONET software to monitor AMR. Alliance for the Prudent Use of Antibiotics 20

Availability of Laboratory Equipment Bactec at the Lusaka University Teaching Hospital Microbiology Laboratory, 2009 The survey of laboratory equipment examined the following: I. Availability of the essential equipment required to provide routine clinical diagnostics II. Functioning of equipment III. Equipment operation and maintenance standards IV. Equipment storage conditions, and the records of equipment calibration 21

Availability of Laboratory Equipment §Most of the surveyed laboratories had the essential equipment needed to perform clinical diagnostics §Some of this equipment was not in working condition. §Most of the laboratory equipment was not regularly calibrated and maintained. 22

Mulago National Referral Hospital & Makerere School of Medicine, Kampala Alliance for the Prudent Use of Antibiotics 23
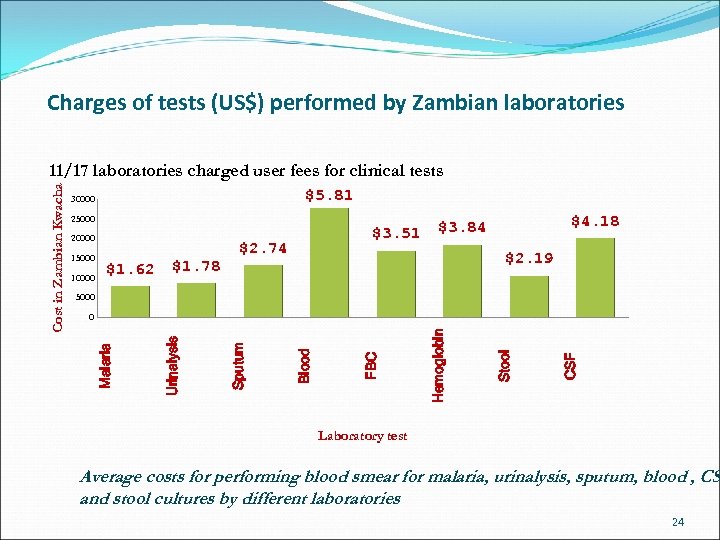
Charges of tests (US$) performed by Zambian laboratories $5. 81 30000 25000 20000 15000 10000 $1. 62 $1. 78 $3. 51 $2. 74 $4. 18 $3. 84 $2. 19 5000 CSF Stool Hemoglobin FBC Blood Sputum Urinalysis 0 Malaria Cost in Zambian Kwacha 11/17 laboratories charged user fees for clinical tests Laboratory test Average costs for performing blood smear for malaria, urinalysis, sputum, blood , CS and stool cultures by different laboratories 24
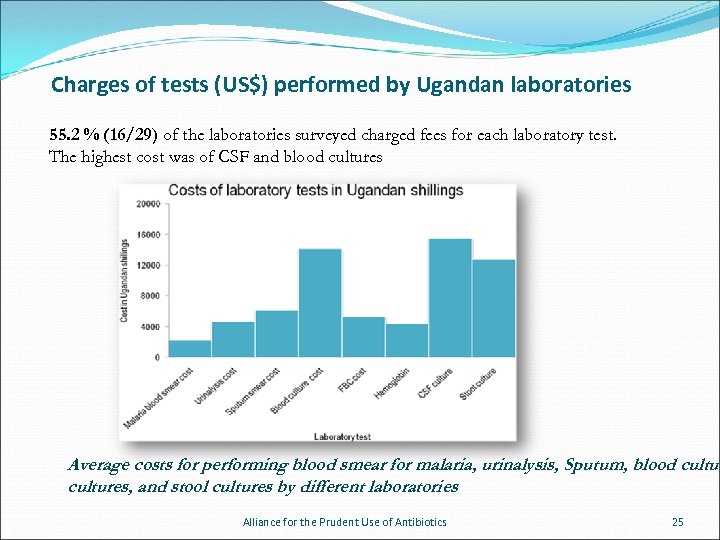
Charges of tests (US$) performed by Ugandan laboratories 55. 2 % (16/29) of the laboratories surveyed charged fees for each laboratory test. The highest cost was of CSF and blood cultures Average costs for performing blood smear for malaria, urinalysis, Sputum, blood cultures, and stool cultures by different laboratories Alliance for the Prudent Use of Antibiotics 25

Specimen handling Some laboratories discarded specimens a few days after testing. Most of the laboratories had no criteria for sample disposal. Alliance for the Prudent Use of Antibiotics 26

Conclusions and Major Constraints 1. Limited antibiotics susceptibility testing capabilities. 2. Essential equipment is available in most laboratories, but often, the equipment is not maintained, calibrated, or in working condition 3. No standard specimen handling procedures 4. No sample disposal procedures 5. No antibiotic resistance surveillance systems in place in most hospitals 27

Conclusion and major constrains (continued) 6. Lack of adequate funding for laboratory equipment, reagents, staff, stationery, and consumables 7. No standard procedures on antibiotic susceptibility testing 8. Problems with reagent stock-outs from suppliers and medical stores 9. Delays in receiving laboratory supplies 10. Inconsistent reporting of notable diseases to national and district health authorities 28

Funded by the Bill & Melinda Gates Foundation for a two year period, from November 2008 -January 2011. 29
314e8f11afdaec960e90d9980b7431fd.ppt