123975241ab2857bea2ccf37e3fd3484.ppt
- Количество слайдов: 43
 Anti-TNF-a Strategies in CHF: Data from Randomized, Controlled Clinical Trials Arthritis Advisory Committee March 4, 2003 Ellis F. Unger, M. D. Office of Therapeutics Research and Review (OTRR) Center for Biologics Evaluation and Research (CBER) U. S. Food and Drug Administration (US FDA) 1
Anti-TNF-a Strategies in CHF: Data from Randomized, Controlled Clinical Trials Arthritis Advisory Committee March 4, 2003 Ellis F. Unger, M. D. Office of Therapeutics Research and Review (OTRR) Center for Biologics Evaluation and Research (CBER) U. S. Food and Drug Administration (US FDA) 1
 Why the Interest in Anti-TNF-a Strategies in CHF? • Clinical observations: • elevated TNF-a levels in patients with CHF, especially cardiac cachexia • Preclinical data showing: • TNF-a-induced LV dysfunction • deleterious effects on LV remodeling 2
Why the Interest in Anti-TNF-a Strategies in CHF? • Clinical observations: • elevated TNF-a levels in patients with CHF, especially cardiac cachexia • Preclinical data showing: • TNF-a-induced LV dysfunction • deleterious effects on LV remodeling 2
 Anti-TNF-a Hypotheses in CHF: • TNF-a contributes to the morbidity of CHF • Anti-TNF-a therapies would have salutary effects in patients with CHF 3
Anti-TNF-a Hypotheses in CHF: • TNF-a contributes to the morbidity of CHF • Anti-TNF-a therapies would have salutary effects in patients with CHF 3
 Randomized Controlled Trials of TNF-a Blockers in CHF: • Etanercept 2 Randomized Controlled Studies: • “RENAISSANCE” • “RECOVER” • Infliximab 1 Randomized Controlled Study: • “ATTACH” 4
Randomized Controlled Trials of TNF-a Blockers in CHF: • Etanercept 2 Randomized Controlled Studies: • “RENAISSANCE” • “RECOVER” • Infliximab 1 Randomized Controlled Study: • “ATTACH” 4
 Studies of Etanercept in CHF: “RENAISSANCE” “RECOVER” 5
Studies of Etanercept in CHF: “RENAISSANCE” “RECOVER” 5
 Etanercept in CHF: “RENAISSANCE” - conducted by Immunex in North America; ~900 subjects “RECOVER” - conducted by Wyeth in Europe, Israel, Australia, New Zealand; ~1100 subjects Both: • phase 2/3 • randomized • double-blind • placebo-controlled • multicenter 6
Etanercept in CHF: “RENAISSANCE” - conducted by Immunex in North America; ~900 subjects “RECOVER” - conducted by Wyeth in Europe, Israel, Australia, New Zealand; ~1100 subjects Both: • phase 2/3 • randomized • double-blind • placebo-controlled • multicenter 6
 Inclusion Criteria: • CHF on ischemic or non-ischemic basis • ejection fraction < 30% • symptoms of CHF X 3 months • NYHA Functional Class 2, 3, or 4 • receiving diuretic and ACE inhibitor 7
Inclusion Criteria: • CHF on ischemic or non-ischemic basis • ejection fraction < 30% • symptoms of CHF X 3 months • NYHA Functional Class 2, 3, or 4 • receiving diuretic and ACE inhibitor 7
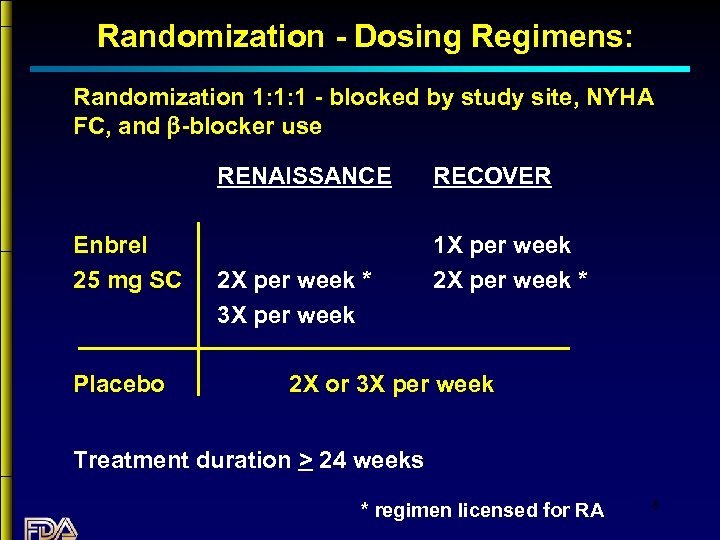 Randomization - Dosing Regimens: Randomization 1: 1: 1 - blocked by study site, NYHA FC, and b-blocker use RENAISSANCE Enbrel 25 mg SC Placebo 2 X per week * 3 X per week RECOVER 1 X per week 2 X per week * 2 X or 3 X per week Treatment duration > 24 weeks * regimen licensed for RA 8
Randomization - Dosing Regimens: Randomization 1: 1: 1 - blocked by study site, NYHA FC, and b-blocker use RENAISSANCE Enbrel 25 mg SC Placebo 2 X per week * 3 X per week RECOVER 1 X per week 2 X per week * 2 X or 3 X per week Treatment duration > 24 weeks * regimen licensed for RA 8
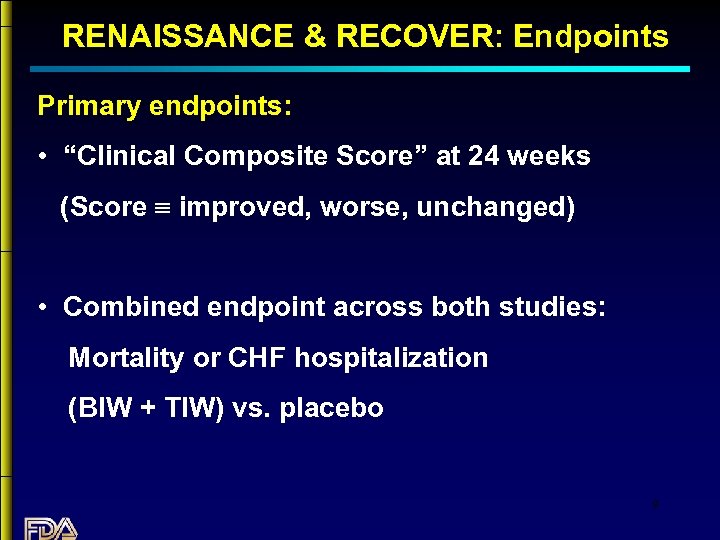 RENAISSANCE & RECOVER: Endpoints Primary endpoints: • “Clinical Composite Score” at 24 weeks (Score º improved, worse, unchanged) • Combined endpoint across both studies: Mortality or CHF hospitalization (BIW + TIW) vs. placebo 9
RENAISSANCE & RECOVER: Endpoints Primary endpoints: • “Clinical Composite Score” at 24 weeks (Score º improved, worse, unchanged) • Combined endpoint across both studies: Mortality or CHF hospitalization (BIW + TIW) vs. placebo 9
 Clinical Composite Score º “Worse” if: · subject died · was hospitalized for CHF · worsened NYHA FC · “Global Assessment” (judged by subject) moderately or markedly worse 10
Clinical Composite Score º “Worse” if: · subject died · was hospitalized for CHF · worsened NYHA FC · “Global Assessment” (judged by subject) moderately or markedly worse 10
 Clinical Composite Score º “Improved” if: · the Clinical Composite Score was not worse AND · NYHA FC is improved OR · “Global Assessment” moderately or markedly improved 11
Clinical Composite Score º “Improved” if: · the Clinical Composite Score was not worse AND · NYHA FC is improved OR · “Global Assessment” moderately or markedly improved 11
 Clinical Composite Score º “Unchanged” if: Clinical Composite Score neither better nor worse. 12
Clinical Composite Score º “Unchanged” if: Clinical Composite Score neither better nor worse. 12
 Results 13
Results 13
 March 2001: Studies Stopped for Futility At a planned interim review, the DSMB recommended that both RENAISSANCE and RECOVER be halted because the pre-specified results indicating futility had been observed. Median follow-up: RENAISSANCE - 12. 7 months RECOVER - 5. 7 months 14
March 2001: Studies Stopped for Futility At a planned interim review, the DSMB recommended that both RENAISSANCE and RECOVER be halted because the pre-specified results indicating futility had been observed. Median follow-up: RENAISSANCE - 12. 7 months RECOVER - 5. 7 months 14
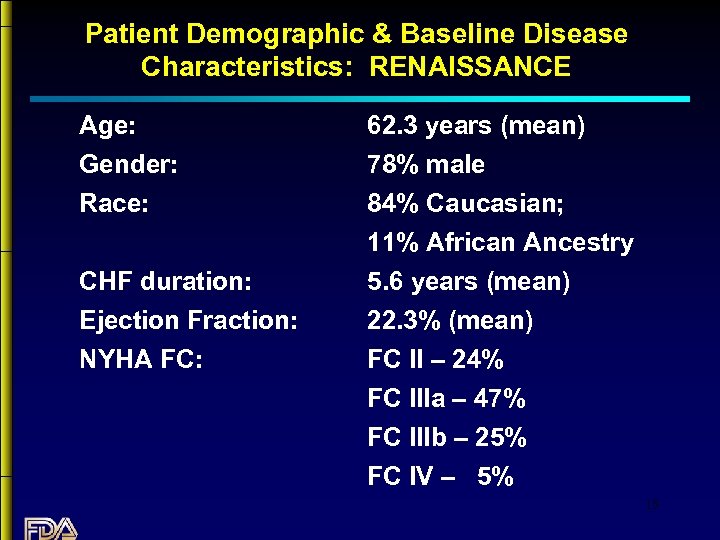 Patient Demographic & Baseline Disease Characteristics: RENAISSANCE Age: Gender: Race: CHF duration: Ejection Fraction: NYHA FC: 62. 3 years (mean) 78% male 84% Caucasian; 11% African Ancestry 5. 6 years (mean) 22. 3% (mean) FC II – 24% FC IIIa – 47% FC IIIb – 25% FC IV – 5% 15
Patient Demographic & Baseline Disease Characteristics: RENAISSANCE Age: Gender: Race: CHF duration: Ejection Fraction: NYHA FC: 62. 3 years (mean) 78% male 84% Caucasian; 11% African Ancestry 5. 6 years (mean) 22. 3% (mean) FC II – 24% FC IIIa – 47% FC IIIb – 25% FC IV – 5% 15
 Imbalances in Patient Demographics & Disease Characteristics: RENAISSANCE Treatment groups well balanced with respect to demographic and baseline characteristics 4 notable exceptions. For the placebo group, on average, baseline: • BP was higher • 6 -minute walk was longer • antiarrhythmic use was less frequent • atrial fib/flutter was less frequent These imbalances were small, but all would be associated with a more favorable prognosis in the placebo group. 16
Imbalances in Patient Demographics & Disease Characteristics: RENAISSANCE Treatment groups well balanced with respect to demographic and baseline characteristics 4 notable exceptions. For the placebo group, on average, baseline: • BP was higher • 6 -minute walk was longer • antiarrhythmic use was less frequent • atrial fib/flutter was less frequent These imbalances were small, but all would be associated with a more favorable prognosis in the placebo group. 16
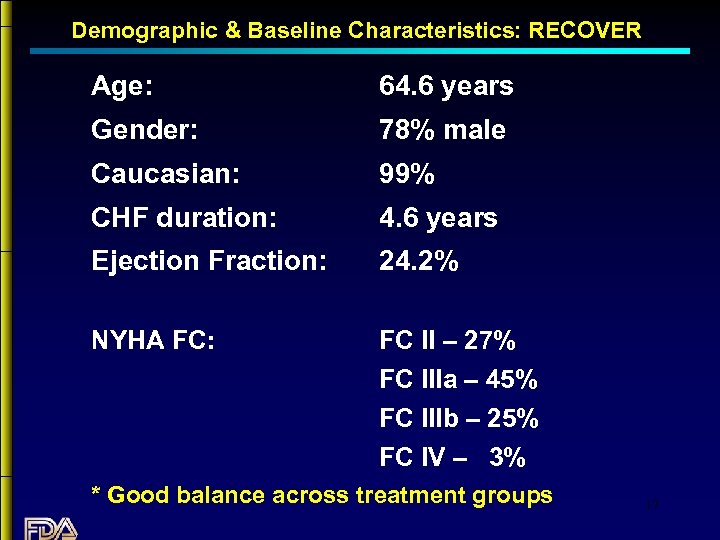 Demographic & Baseline Characteristics: RECOVER Age: 64. 6 years Gender: 78% male Caucasian: 99% CHF duration: 4. 6 years Ejection Fraction: 24. 2% NYHA FC: FC II – 27% FC IIIa – 45% FC IIIb – 25% FC IV – 3% * Good balance across treatment groups 17
Demographic & Baseline Characteristics: RECOVER Age: 64. 6 years Gender: 78% male Caucasian: 99% CHF duration: 4. 6 years Ejection Fraction: 24. 2% NYHA FC: FC II – 27% FC IIIa – 45% FC IIIb – 25% FC IV – 3% * Good balance across treatment groups 17
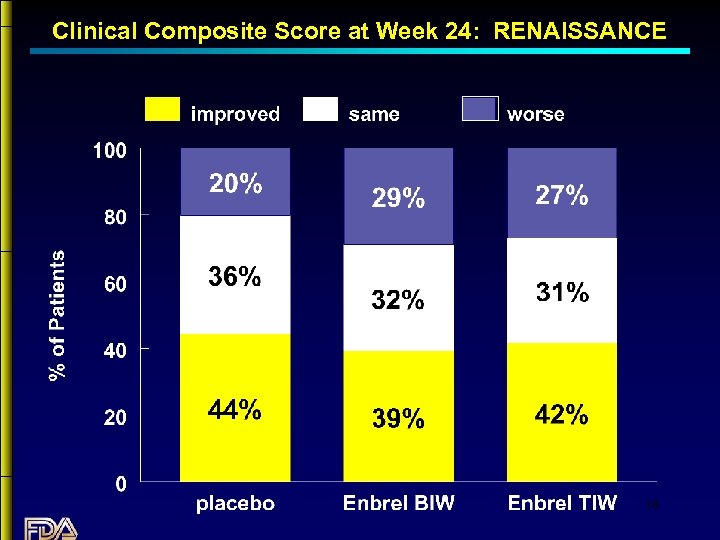 Clinical Composite Score at Week 24: RENAISSANCE 18
Clinical Composite Score at Week 24: RENAISSANCE 18
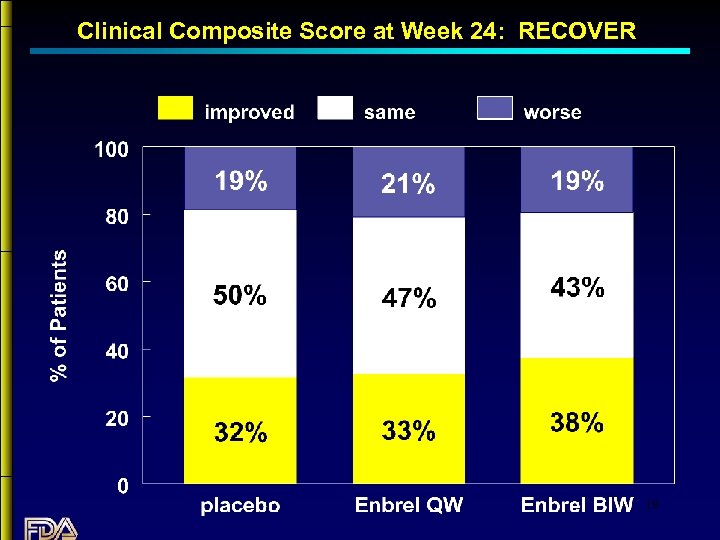 Clinical Composite Score at Week 24: RECOVER 19
Clinical Composite Score at Week 24: RECOVER 19
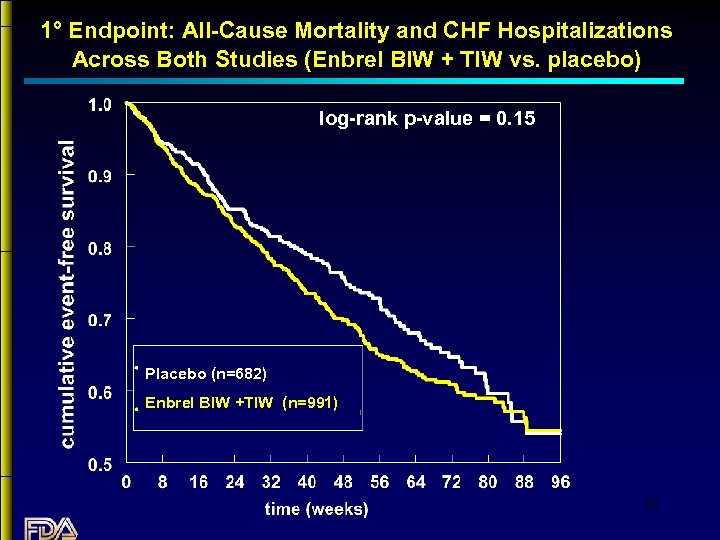 1° Endpoint: All-Cause Mortality and CHF Hospitalizations Across Both Studies (Enbrel BIW + TIW vs. placebo) log-rank p-value = 0. 15 Placebo (n=682) Enbrel BIW +TIW (n=991) 20
1° Endpoint: All-Cause Mortality and CHF Hospitalizations Across Both Studies (Enbrel BIW + TIW vs. placebo) log-rank p-value = 0. 15 Placebo (n=682) Enbrel BIW +TIW (n=991) 20
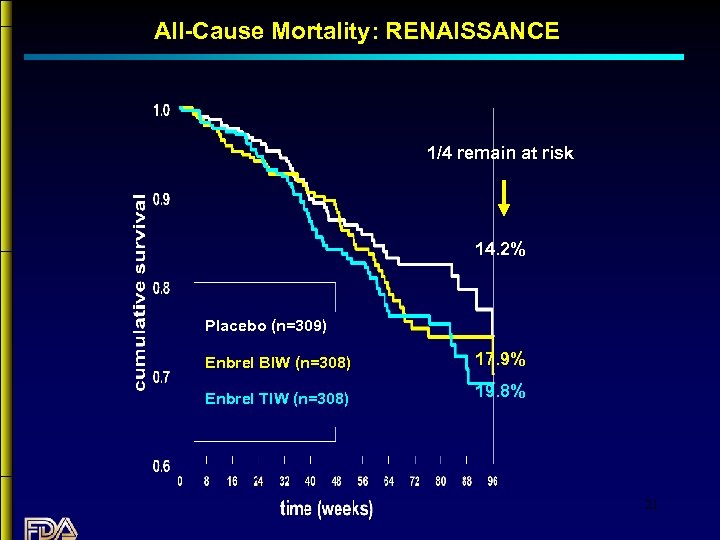 All-Cause Mortality: RENAISSANCE 1/4 remain at risk 14. 2% Placebo (n=309) Enbrel BIW (n=308) 17. 9% Enbrel TIW (n=308) 19. 8% 21
All-Cause Mortality: RENAISSANCE 1/4 remain at risk 14. 2% Placebo (n=309) Enbrel BIW (n=308) 17. 9% Enbrel TIW (n=308) 19. 8% 21
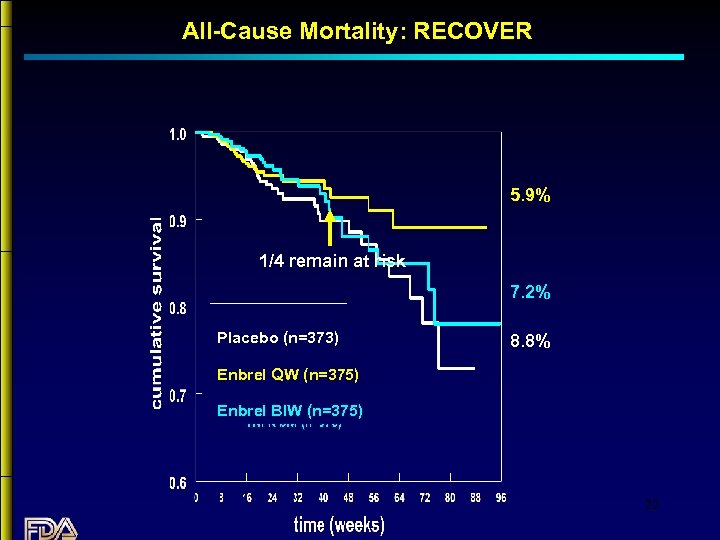 All-Cause Mortality: RECOVER 5. 9% 1/4 remain at risk 7. 2% Placebo (n=373) 8. 8% Enbrel QW (n=375) Enbrel BIW (n=375) 22
All-Cause Mortality: RECOVER 5. 9% 1/4 remain at risk 7. 2% Placebo (n=373) 8. 8% Enbrel QW (n=375) Enbrel BIW (n=375) 22
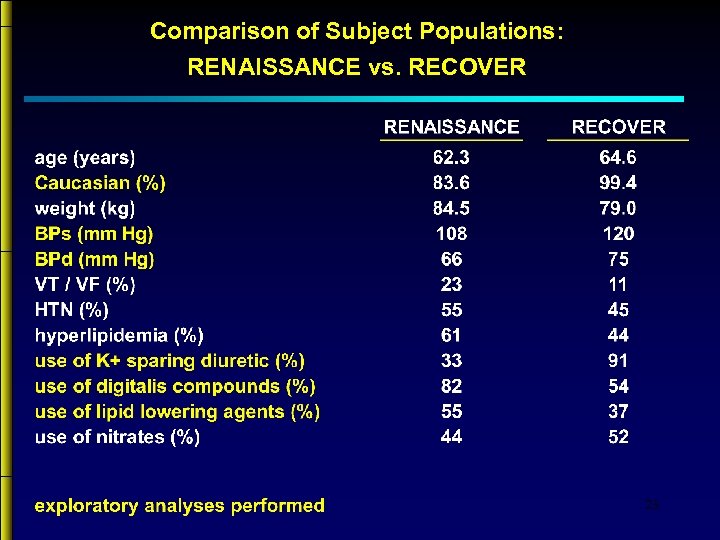 Comparison of Subject Populations: RENAISSANCE vs. RECOVER 23
Comparison of Subject Populations: RENAISSANCE vs. RECOVER 23
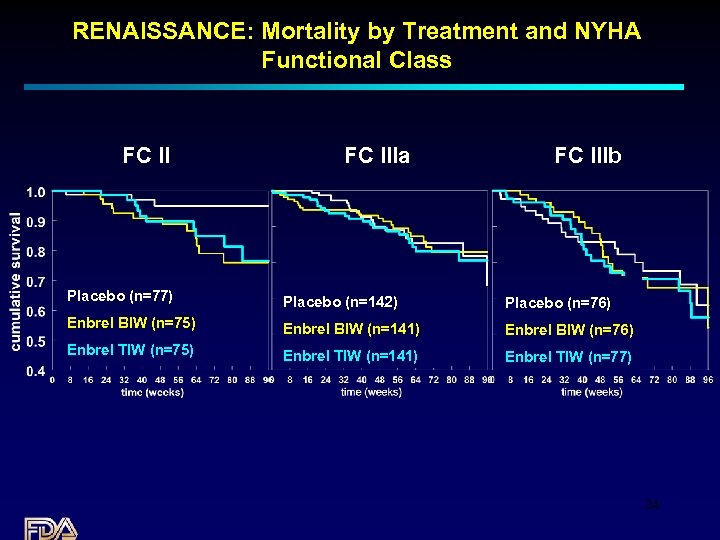 RENAISSANCE: Mortality by Treatment and NYHA Functional Class FC IIIa FC IIIb Placebo (n=77) Placebo (n=142) Placebo (n=76) Enbrel BIW (n=75) Enbrel BIW (n=141) Enbrel BIW (n=76) Enbrel TIW (n=75) Enbrel TIW (n=141) Enbrel TIW (n=77) 24
RENAISSANCE: Mortality by Treatment and NYHA Functional Class FC IIIa FC IIIb Placebo (n=77) Placebo (n=142) Placebo (n=76) Enbrel BIW (n=75) Enbrel BIW (n=141) Enbrel BIW (n=76) Enbrel TIW (n=75) Enbrel TIW (n=141) Enbrel TIW (n=77) 24
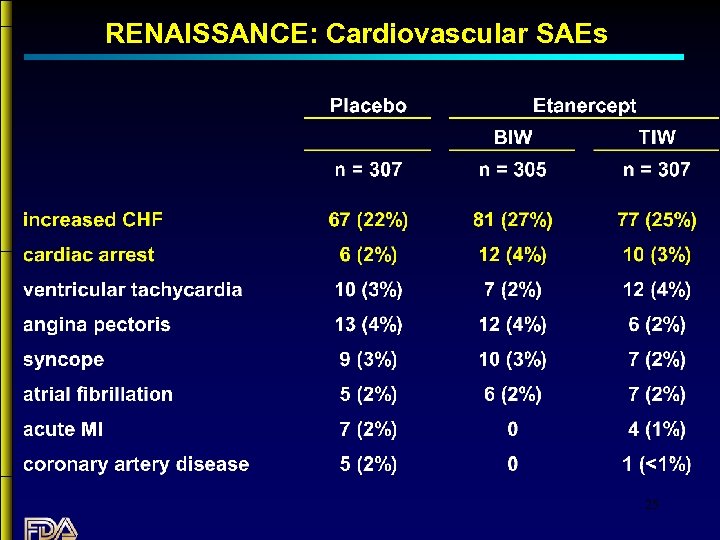 RENAISSANCE: Cardiovascular SAEs 25
RENAISSANCE: Cardiovascular SAEs 25
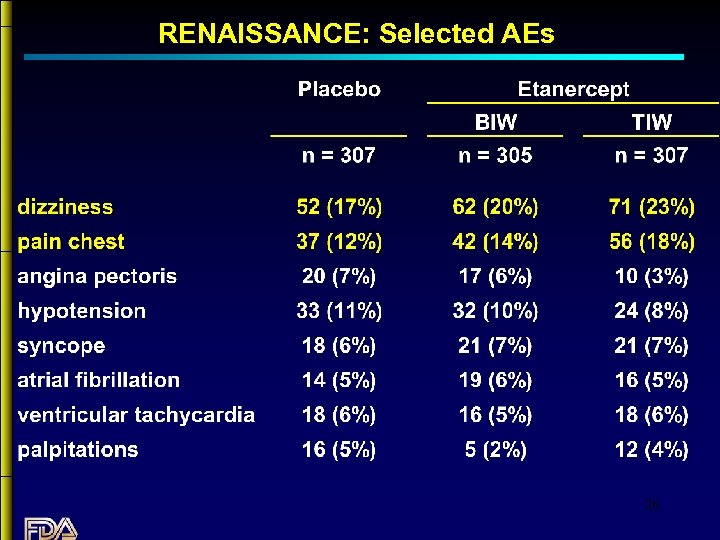 RENAISSANCE: Selected AEs 26
RENAISSANCE: Selected AEs 26
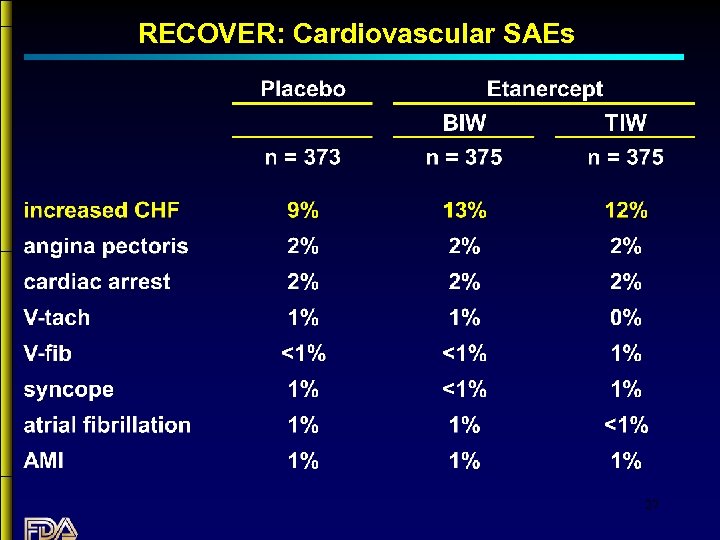 RECOVER: Cardiovascular SAEs 27
RECOVER: Cardiovascular SAEs 27
 Summary: Etanercept in CHF (1) • No evidence that Etanercept is beneficial in CHF • The data suggest harm, though the results are not conclusive. • The key finding of concern was a trend towards higher mortality in Etanercept-treated subjects in RENAISSANCE; this was heightened by the apparent dose-response relation. • The results of RECOVER do not substantiate the findings of Renaissance with respect to Etanerceptinduced mortality in CHF. • The greatest concern is for an Enbrel dose higher than that currently licensed for RA in the US. 28
Summary: Etanercept in CHF (1) • No evidence that Etanercept is beneficial in CHF • The data suggest harm, though the results are not conclusive. • The key finding of concern was a trend towards higher mortality in Etanercept-treated subjects in RENAISSANCE; this was heightened by the apparent dose-response relation. • The results of RECOVER do not substantiate the findings of Renaissance with respect to Etanerceptinduced mortality in CHF. • The greatest concern is for an Enbrel dose higher than that currently licensed for RA in the US. 28
 Summary: Etanercept in CHF (2) • The data do not suggest a specific mechanism of action leading to Etanercept-related adverse outcomes in the CHF patient population. • Exploratory analyses failed to identify specific factors associated with increased risk of adverse events. In particular, patients in Renaissance with milder CHF (NYHA FC II) did not appear to be at a lower risk of adverse outcomes. In labeling, there is no basis to provide: • a measure of reassurance for patients with mild forms of CHF; • a listing of factors that appear to predispose to worsening CHF 29
Summary: Etanercept in CHF (2) • The data do not suggest a specific mechanism of action leading to Etanercept-related adverse outcomes in the CHF patient population. • Exploratory analyses failed to identify specific factors associated with increased risk of adverse events. In particular, patients in Renaissance with milder CHF (NYHA FC II) did not appear to be at a lower risk of adverse outcomes. In labeling, there is no basis to provide: • a measure of reassurance for patients with mild forms of CHF; • a listing of factors that appear to predispose to worsening CHF 29
 Study of Infliximab in CHF: “ATTACH” 30
Study of Infliximab in CHF: “ATTACH” 30
 ATTACH: • phase 2 pilot trial • randomized • double-blind • placebo-controlled • multicenter (32 centers in USA) 31
ATTACH: • phase 2 pilot trial • randomized • double-blind • placebo-controlled • multicenter (32 centers in USA) 31
 Randomization - Dosing Regimens: 150 subjects randomized 1: 1: 1 to: · infliximab 5 mg/kg at 0, 2 and 6 weeks · infliximab 10 mg/kg at 0, 2 and 6 weeks · placebo at 0, 2 and 6 weeks 32
Randomization - Dosing Regimens: 150 subjects randomized 1: 1: 1 to: · infliximab 5 mg/kg at 0, 2 and 6 weeks · infliximab 10 mg/kg at 0, 2 and 6 weeks · placebo at 0, 2 and 6 weeks 32
 ATTACH: Inclusion Criteria • symptoms of CHF X 3 months • NYHA functional class 3, or 4 • LV ejection fraction £ 35% • receiving diuretic and ACE inhibitor 33
ATTACH: Inclusion Criteria • symptoms of CHF X 3 months • NYHA functional class 3, or 4 • LV ejection fraction £ 35% • receiving diuretic and ACE inhibitor 33
 ATTACH: Primary Endpoint “Clinical Status” at 14 weeks: improved, worse, or unchanged 34
ATTACH: Primary Endpoint “Clinical Status” at 14 weeks: improved, worse, or unchanged 34
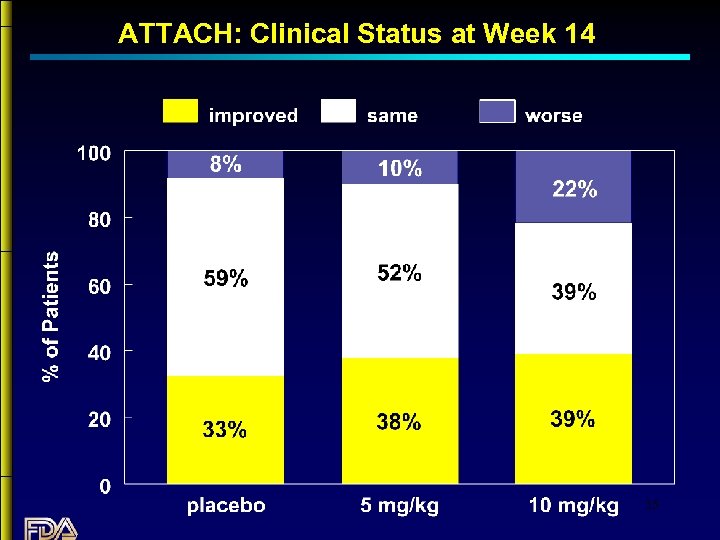 ATTACH: Clinical Status at Week 14 35
ATTACH: Clinical Status at Week 14 35
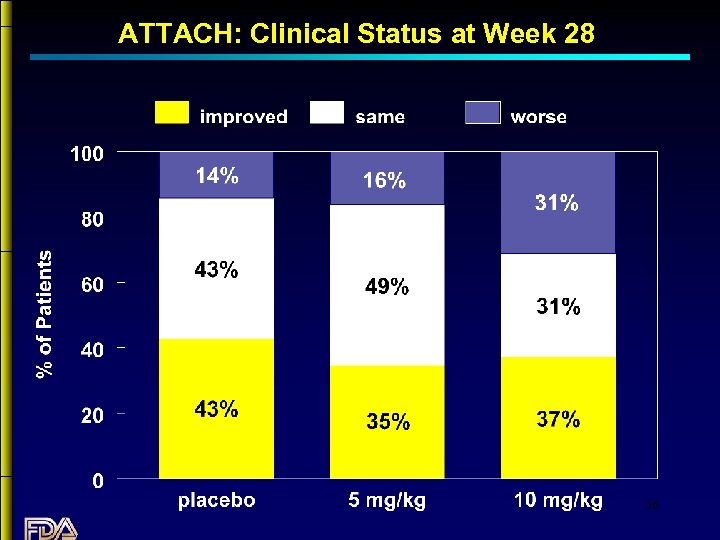 ATTACH: Clinical Status at Week 28 36
ATTACH: Clinical Status at Week 28 36
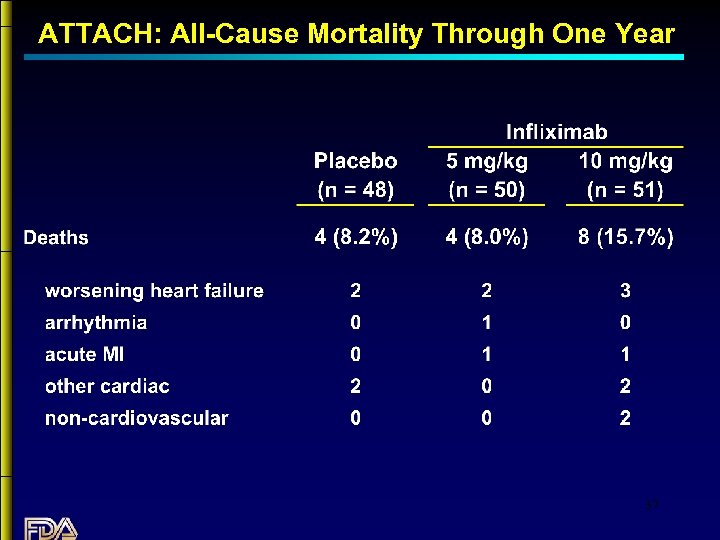 ATTACH: All-Cause Mortality Through One Year 37
ATTACH: All-Cause Mortality Through One Year 37
 Infliximab: Dear Healthcare Professional letter issued October 18, 2001 38
Infliximab: Dear Healthcare Professional letter issued October 18, 2001 38
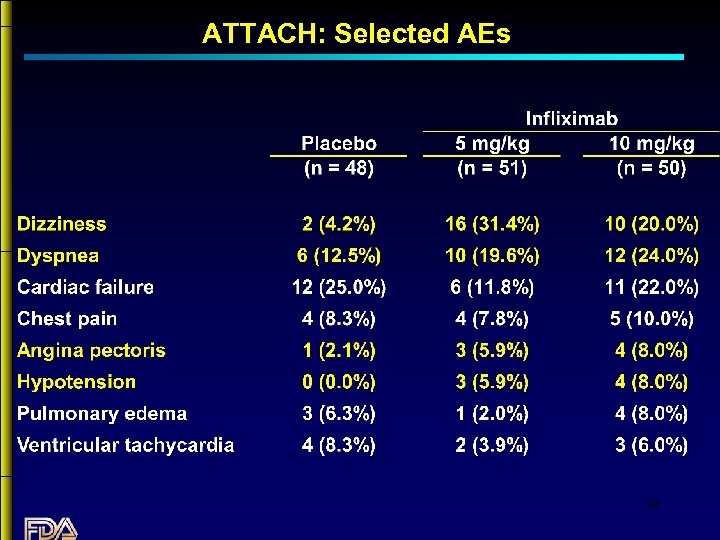 ATTACH: Selected AEs 39
ATTACH: Selected AEs 39
 Summary: Infliximab in CHF • No evidence that Infliximab is beneficial in patients with CHF. • Although the numbers of subjects treated are small, there is a strong trend suggesting increased mortality in CHF patients treated with Infliximab. • The data do not show an increase in mortality with the 5 mg/kg dose; however, adverse event data suggest that the 5 mg/kg dose is deleterious. • The mechanism underlying this apparent effect is unclear. 40
Summary: Infliximab in CHF • No evidence that Infliximab is beneficial in patients with CHF. • Although the numbers of subjects treated are small, there is a strong trend suggesting increased mortality in CHF patients treated with Infliximab. • The data do not show an increase in mortality with the 5 mg/kg dose; however, adverse event data suggest that the 5 mg/kg dose is deleterious. • The mechanism underlying this apparent effect is unclear. 40
 Post-Marketing Reports of CHF: 51 cases reported as of February, 2002: 30 received Etanercept; 21 received Infliximab • 42 reports of new onset CHF - - half with no identifiable risk factors • 9 reports of CHF exacerbation Median age = 64 yrs (range 19 to 87 years) Median time to onset 3. 5 months (range: 24 hours to 24 months) 20% were < 50 years old 41
Post-Marketing Reports of CHF: 51 cases reported as of February, 2002: 30 received Etanercept; 21 received Infliximab • 42 reports of new onset CHF - - half with no identifiable risk factors • 9 reports of CHF exacerbation Median age = 64 yrs (range 19 to 87 years) Median time to onset 3. 5 months (range: 24 hours to 24 months) 20% were < 50 years old 41
 CHF Cases in Patients Under 50 Years Old • 10 cases: – 6 received infliximab & 4 received etanercept – Median ejection fraction = 20% (range 10 to 45%, n=9) – 3 with underlying risk factors for CHF – After discontinuation of TNF antagonists and HF treatment: • 3 reported complete resolution of CHF • 6 reported improvement • 1 reported death 42
CHF Cases in Patients Under 50 Years Old • 10 cases: – 6 received infliximab & 4 received etanercept – Median ejection fraction = 20% (range 10 to 45%, n=9) – 3 with underlying risk factors for CHF – After discontinuation of TNF antagonists and HF treatment: • 3 reported complete resolution of CHF • 6 reported improvement • 1 reported death 42
 Summary: TNF Blockers and CHF: • Significant overlap between CHF and RA in the general population, to a lesser extent CHF & Crohn’s Disease • Data from RCT’s in the CHF population raise concerns about the safety of Infliximab and Etanercept. • Post-marketing data raise concern regarding new-onset CHF. • CBER plans comprehensive analyses of the RCT databases of all 3 TNF-blockers. • Specific language for labeling is under discussion. 43
Summary: TNF Blockers and CHF: • Significant overlap between CHF and RA in the general population, to a lesser extent CHF & Crohn’s Disease • Data from RCT’s in the CHF population raise concerns about the safety of Infliximab and Etanercept. • Post-marketing data raise concern regarding new-onset CHF. • CBER plans comprehensive analyses of the RCT databases of all 3 TNF-blockers. • Specific language for labeling is under discussion. 43


