38b2283e036496e8dd569fcae33dc5c3.ppt
- Количество слайдов: 22

ANS Embedded Topical meeting June 11, 2008 UNM College of Pharmacy Radiopharmaceutical Sciences Program New Mexico Center for Isotopes in Medicine LANL Isotope Production Program UNM Group Members: Jeff Norenberg, Pharm. D Robert Atcher, Ph. D, MBA - UNM & LANL

Manpower Training Needs/Tiered Training Programs: Based upon NAS and other reports, to assess needs for radiopharmacy, radiochemistry, and biomedical imaging scientists as a variety of training levels. Discuss potential resources to support a Ph. D program in appropriate disciplines

Radiochemistry needs by role Radioisotope Production Reactor based Accelerator based Radiopharmaceutical Production Commercial Setting Academic/Hospital Center Pharma Radiopharmaceutical Research Industry Academic/Government
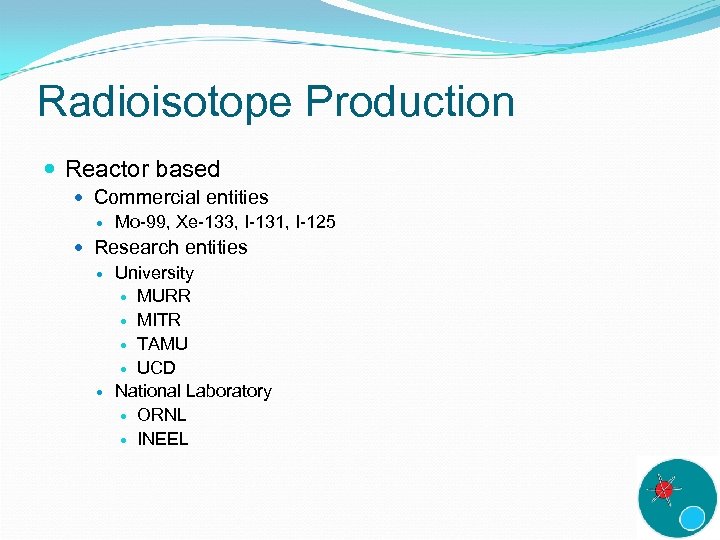
Radioisotope Production Reactor based Commercial entities Mo-99, Xe-133, I-131, I-125 Research entities University MURR MITR TAMU UCD National Laboratory ORNL INEEL
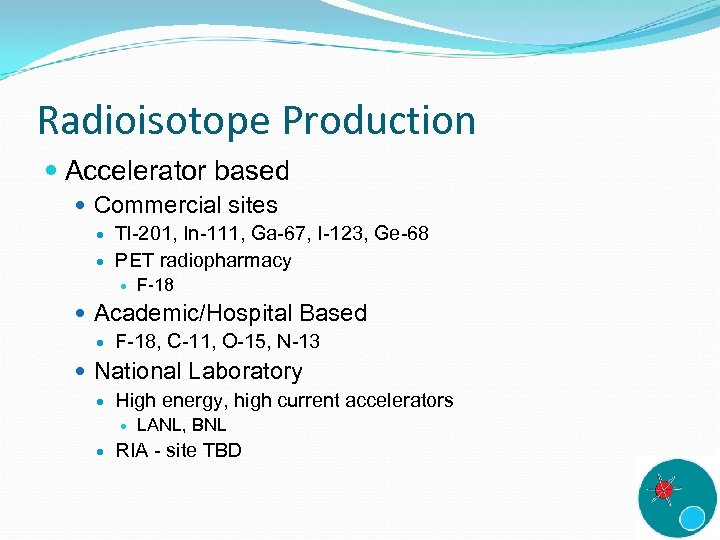
Radioisotope Production Accelerator based Commercial sites Tl-201, In-111, Ga-67, I-123, Ge-68 PET radiopharmacy F-18 Academic/Hospital Based F-18, C-11, O-15, N-13 National Laboratory High energy, high current accelerators LANL, BNL RIA - site TBD

Radiopharmaceutical Production Commercial setting-”Big Radio. Pharma” Perkin Elmer Lantheus GE Siemens Covidien Draximage IBA Bracco Nordion

Radiopharmaceutical Production Commercial - startups MIP Cytogen Trace Sciences AMIC Avid Pharmaceuticals Cyclomedica North American Scientific Nu. View

Radiopharmaceutical Production Academic or Hospital setting Routine synthesis Cyclotron based Hot box Custom or Research compounds Cyclotron based Custom configured hot box

Radiopharmaceutical Production Traditional Pharma Utilize clinically proven radiopharmaceuticals FDG, FLT, Custom synthesis of drug candidates PK/PD studies

Radiopharmaceutical Research Industry Market driven Focus on demand availability Waxes and wanes Academic Research driven Utilizes existing or “new” radionuclides Has been steady till recently DOE funding NIH funding

Radiopharmacy Manpower Needs ~1, 000 nuclear pharmacists within the USA 450 nuclear pharmacies within the USA and growing Commercial: CH NPS, Covidien, GE, IBA Molecular, PETNet, Triad Isotopes, Independents (UPPI) Hospital/university-based Nuclear pharmacy was the first specialty practice area recognized by American Pharmacists Association in 1975 First specialty recognized through Board Certification by the Board of Pharmaceutical Specialties 1978 The fastest growing area within nuclear pharmacy practice is in PET Average starting salary for new graduates >$100, 000, parity with hospital and specialty practice settings

Overview of UNM College of Pharmacy Radiopharmaceutical Sciences Programs Education, Research, and Clinical Service First University-based Radiopharmacy Education and Training Program established in 1972 First Commercial Nuclear Pharmacy 1973 -1992 DOE ANMI Nuclear Medicine Education Award for Graduate Radiopharmacy Education $300, 000 total, 2001 -2004 New Mexico Center for Isotopes in Medicine - UNM-Los Alamos National Laboratory established 12/2005
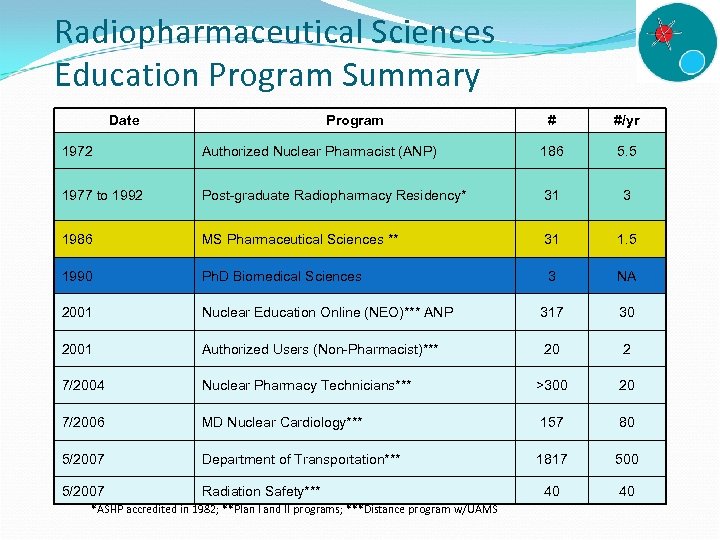
Radiopharmaceutical Sciences Education Program Summary Date Program # #/yr 1972 Authorized Nuclear Pharmacist (ANP) 186 5. 5 1977 to 1992 Post-graduate Radiopharmacy Residency* 31 3 1986 MS Pharmaceutical Sciences ** 31 1. 5 1990 Ph. D Biomedical Sciences 3 NA 2001 Nuclear Education Online (NEO)*** ANP 317 30 2001 Authorized Users (Non-Pharmacist)*** 20 2 7/2004 Nuclear Pharmacy Technicians*** >300 20 7/2006 MD Nuclear Cardiology*** 157 80 5/2007 Department of Transportation*** 1817 500 5/2007 Radiation Safety*** 40 40 *ASHP accredited in 1982; **Plan I and II programs; ***Distance program w/UAMS
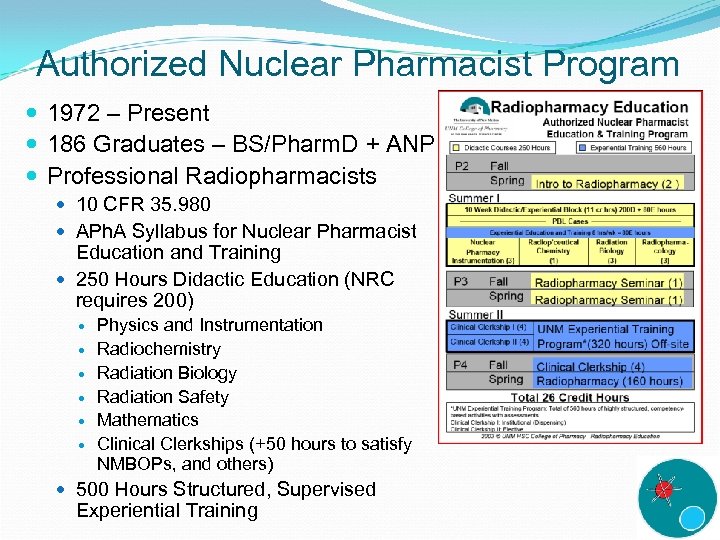
Authorized Nuclear Pharmacist Program 1972 – Present 186 Graduates – BS/Pharm. D + ANP Professional Radiopharmacists 10 CFR 35. 980 APh. A Syllabus for Nuclear Pharmacist Education and Training 250 Hours Didactic Education (NRC requires 200) Physics and Instrumentation Radiochemistry Radiation Biology Radiation Safety Mathematics Clinical Clerkships (+50 hours to satisfy NMBOPs, and others) 500 Hours Structured, Supervised Experiential Training
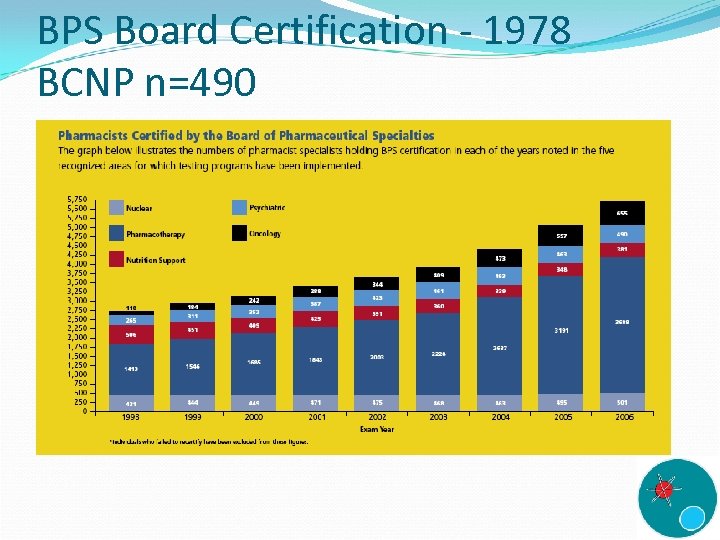
BPS Board Certification - 1978 BCNP n=490

UNM Radiopharmaceutical Sciences Graduate Program 1986 – Present MS Pharmaceutical Sciences (Radiopharmacy) 31 Graduates Focus on Applied and Translational Research Pharmaceutical Scientists Advanced Clinical Practitioners DOE ANMI NMEA $300, 000 2001 -2004 Linked with COP/BSGP Ph. D programs – 3 graduates
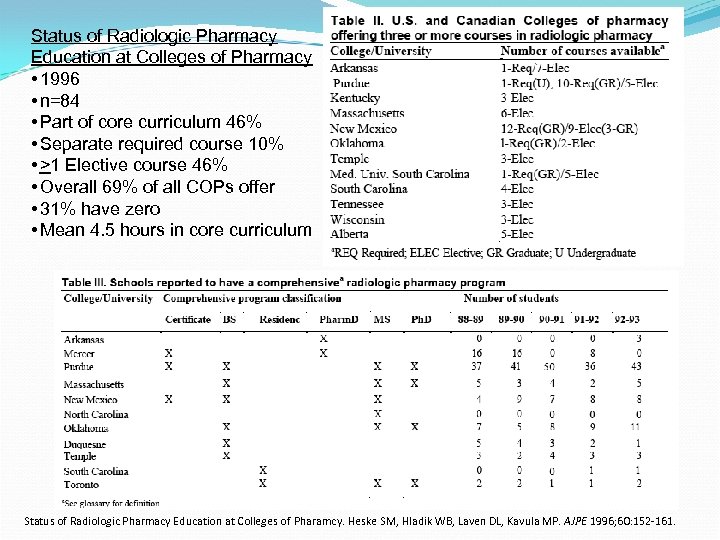
Status of Radiologic Pharmacy Education at Colleges of Pharmacy • 1996 • n=84 • Part of core curriculum 46% • Separate required course 10% • >1 Elective course 46% • Overall 69% of all COPs offer • 31% have zero • Mean 4. 5 hours in core curriculum Status of Radiologic Pharmacy Education at Colleges of Pharamcy. Heske SM, Hladik WB, Laven DL, Kavula MP. AJPE 1996; 60: 152 -161.

UNM Radiopharmacy Nuclear Pharmacy Residency 1977 – 1992 31 Graduates ASHP Accredited Advanced Clinical Practice sites UNM Radiopharmacy VAMC Regional Medical Center ASHP-Accredited Programs = 2 SUNY Buffalo School of Pharmacy Edward M. Bednarczyk, Pharm. D. (716) 645 -2828 E-mail: eb@buffalo. edu The National Capital Consortium/National Naval and Walter Reed Army Medical Centers Carol W. Labadie, COL, Pharm. D. (202) 782 -6072 E-mail: Carol. Labadie@amedd. army. mil ACCP-Accredited Programs = 0 Fellowships = 0
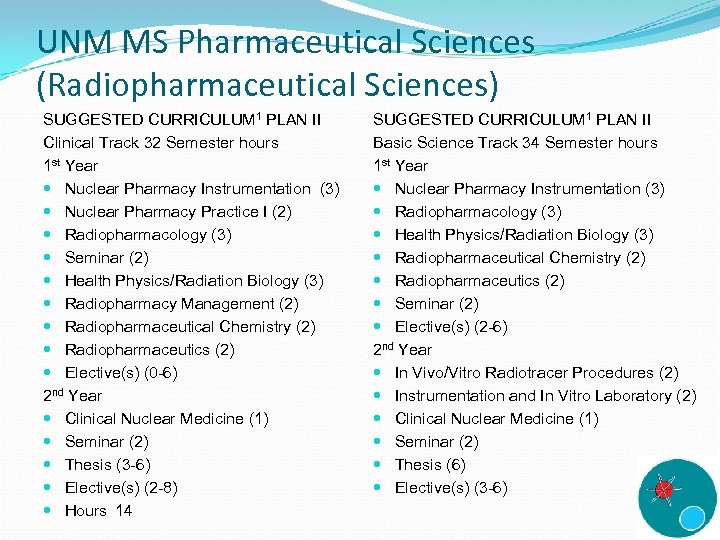
UNM MS Pharmaceutical Sciences (Radiopharmaceutical Sciences) SUGGESTED CURRICULUM 1 PLAN II Clinical Track 32 Semester hours 1 st Year Nuclear Pharmacy Instrumentation (3) Nuclear Pharmacy Practice I (2) Radiopharmacology (3) Seminar (2) Health Physics/Radiation Biology (3) Radiopharmacy Management (2) Radiopharmaceutical Chemistry (2) Radiopharmaceutics (2) Elective(s) (0 -6) 2 nd Year Clinical Nuclear Medicine (1) Seminar (2) Thesis (3 -6) Elective(s) (2 -8) Hours 14 SUGGESTED CURRICULUM 1 PLAN II Basic Science Track 34 Semester hours 1 st Year Nuclear Pharmacy Instrumentation (3) Radiopharmacology (3) Health Physics/Radiation Biology (3) Radiopharmaceutical Chemistry (2) Radiopharmaceutics (2) Seminar (2) Elective(s) (2 -6) 2 nd Year In Vivo/Vitro Radiotracer Procedures (2) Instrumentation and In Vitro Laboratory (2) Clinical Nuclear Medicine (1) Seminar (2) Thesis (6) Elective(s) (3 -6)
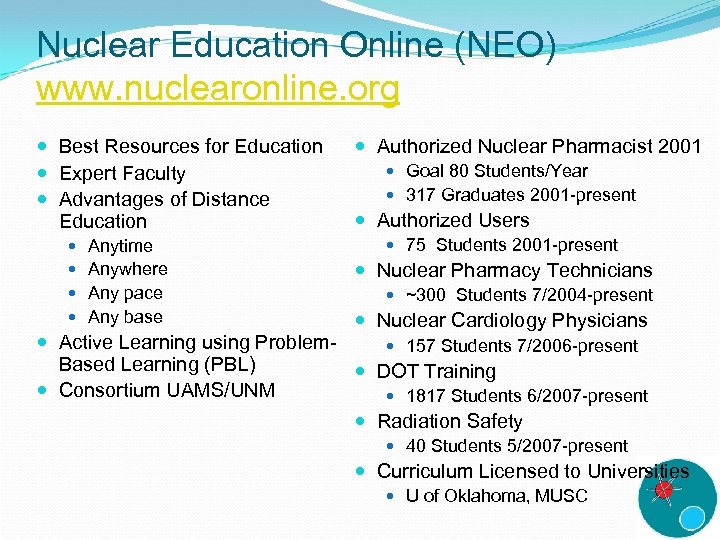
Nuclear Education Online (NEO) www. nuclearonline. org Best Resources for Education Expert Faculty Advantages of Distance Education Anytime Anywhere Any pace Any base Authorized Nuclear Pharmacist 2001 Goal 80 Students/Year 317 Graduates 2001 -present Authorized Users 75 Students 2001 -present Nuclear Pharmacy Technicians ~300 Students 7/2004 -present Nuclear Cardiology Physicians Active Learning using Problem 157 Students 7/2006 -present Based Learning (PBL) DOT Training Consortium UAMS/UNM 1817 Students 6/2007 -present Radiation Safety 40 Students 5/2007 -present Curriculum Licensed to Universities U of Oklahoma, MUSC
UNM Education & Training Capabilities NMCIM = UNM + LANL + NMSU Radiopharmaceuticals • • Discovery, Development, and Translation c. GMP Manufacturing and Formulation Support for Clinical Trials Small-Animal Imaging • • Nano. SPECT/CT and PET Image-based Metrology Los Alamos National Laboratory • • • Isotope Production Facility Chemistry Division Biosciences Division

NMCIM Partnerships and Acknowledgements New Mexico Center for Isotopes in Medicine Scott Burchiel, Ph. D UNM Assoc VP Research IAS John Pieper, Pharm. D Dean COP College of Pharmacy, UNM HSC Yubin Miao, Ph. D, Nalini Shenoy, Ph. D (Post-doc) Radiopharmaceutical Sciences Laboratory Tamara Anderson, BS (Associate Scientist) Ben Gershman, MS (Imaging Scientist) Jeremy Howard, BS (Sr. /Lead Biology. Technician) Daniel Irwin, BS (Nuclear Medicine Technologist) Tapan Nayak, BPharm, MS (Ph. D Candidate) Jack Hoppin, Ph. D et al. - Bioscan, Inc. Melanie Bergeron, MS et al. - Gamma. Medica Ideas (AMI) UNM Cancer Research Treatment Center Pathology Richard Larson, MD, Ph. D (LFA-1 Nor. BIRT) David Brown, Ph. D (Survival studies) Gloria Semenuk, Ph. D (Affinity studies) Cell Biology Eric Prossnitz, Ph. D (Receptor biology) C Revankar, Ph. D & Daniel Cimino, Ph. D Center for Molecular High Throughput Screening Larry Sklar, Ph. D (PI Keck Grant) Bruce Edwards, Ph. D & Mark Carter, MS Los Alamos National Laboratory Robert Atcher, Ph. D (Nuclear and Radiochemistry) Jonathon Fitzsimmons, Ph. D (Post-doc) Eugene Peterson, Ph. D (Nuclear Chemistry) NIH/NCI Radiation Oncology Branch Martin Brechbiel, Ph. D (Nuclear and Radiochemistry) Kayhan Garmestani, Ph. D (Radiochemistry) DOE Isotope Production Program Wolfgang Runde, Ph. D Research Support (Norenberg) WM Keck Foundation United States Department of Energy, Advanced Nuclear Medicine Initiative DE-FG 01 -001 NE 23554 University of New Mexico General Clinical Research Centers DHHS/PHS/NIH/NCRR/GCRC, MO 1 RROO 997 UNM-LANL Joint Science and Technology Laboratory Initiatives (JSTL) New Mexico Technology Research Collaborative (TRC) UNM CRTC Translational Science Pilot Award Avid Radiopharmaceuticals, Inc. Bioscan, Inc. Gamma. Medica Ideas (Advanced Molecular Imaging, Inc. ) Molecular Insight Pharmaceuticals, Inc. Tyco/Mallinckrodt Healthcare - Covidien
38b2283e036496e8dd569fcae33dc5c3.ppt