4f1f7f96d52e6cdcc160d4474161ee23.ppt
- Количество слайдов: 50
 Annual DHAS HIV Coordinator’s Meeting 2010 PRESENTERS: Dr. Evan Cadoff Dr. Eugene Martin Joanne Corbo UMDNJ – Robert Wood Johnson Medical School Somerset, NJ
Annual DHAS HIV Coordinator’s Meeting 2010 PRESENTERS: Dr. Evan Cadoff Dr. Eugene Martin Joanne Corbo UMDNJ – Robert Wood Johnson Medical School Somerset, NJ
 Rapid HIV Testing in NJ Common Issues on Monthly Site Visits Joanne Corbo Program Manager, NJ HIV
Rapid HIV Testing in NJ Common Issues on Monthly Site Visits Joanne Corbo Program Manager, NJ HIV
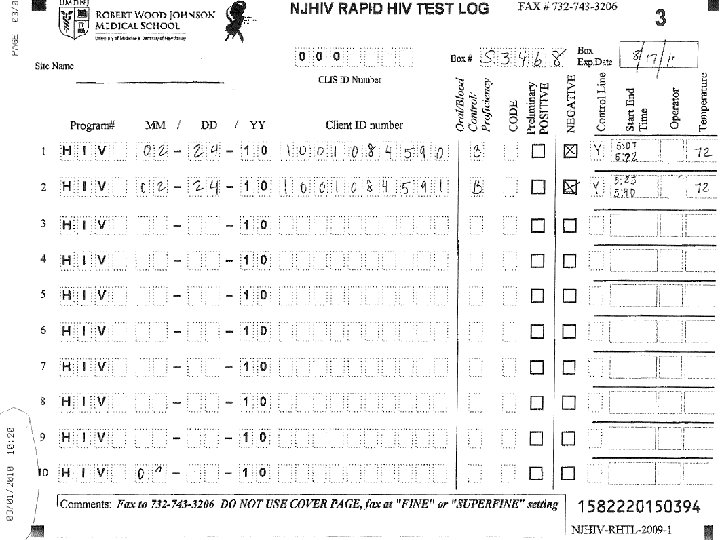
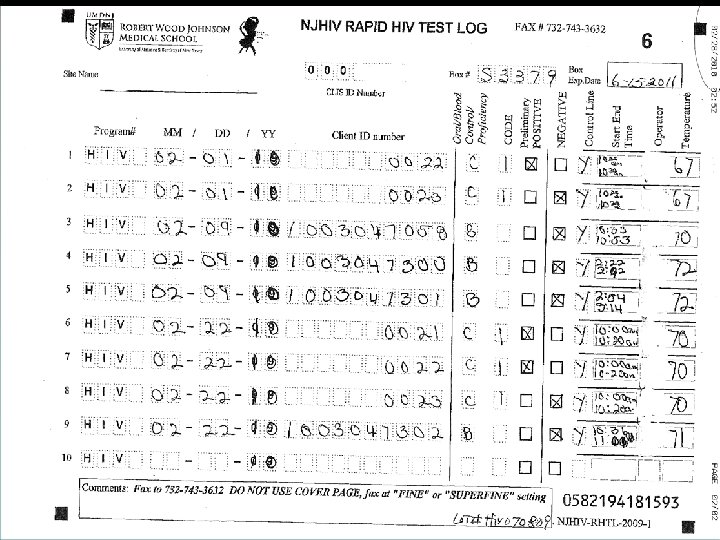
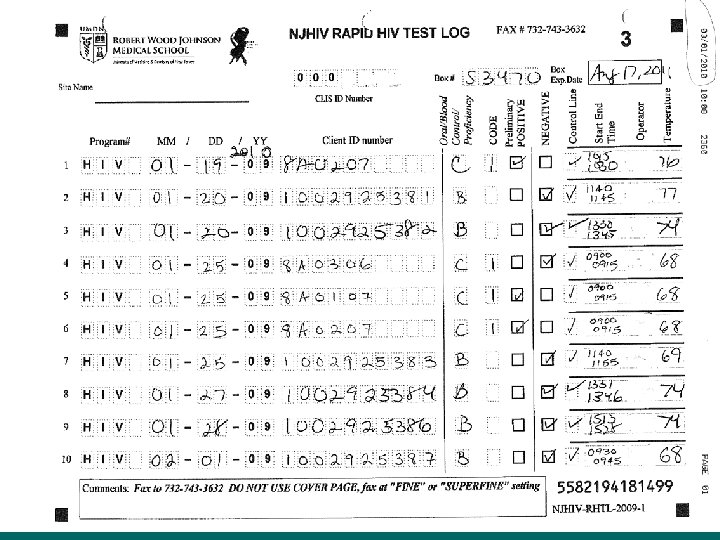
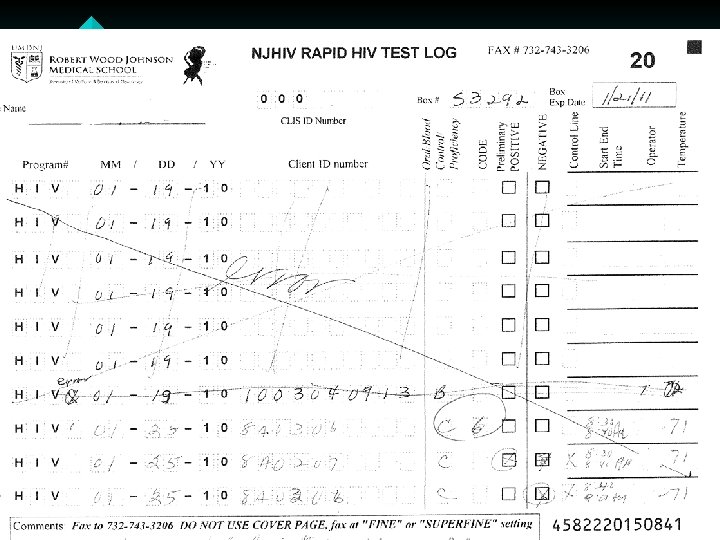
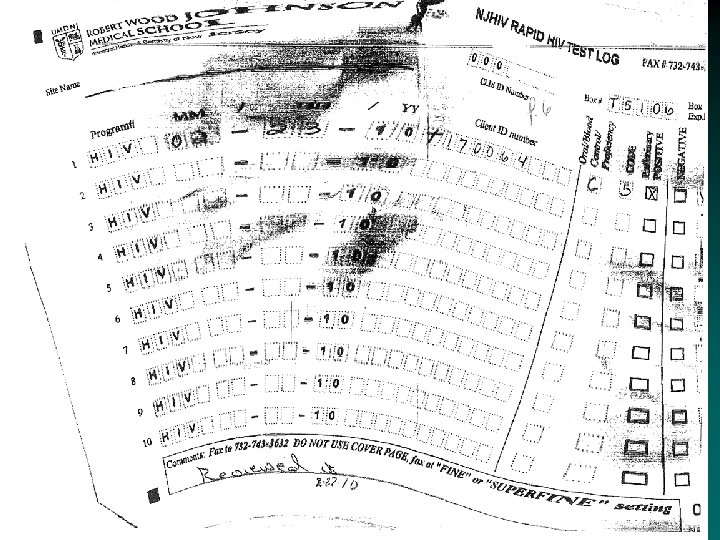
 Common Issues with Record Keeping • Incomplete Cognition log sheets. • Not entering their CLIS ID Number, and/or entering an incorrect CLIS ID Number. • Not writing in the comment box when entering a code – example 3 or 6. If invalid or a manufacturer error please state that and why. • Crossing out the lines on the log sheet and filling in above cross out • Using 09 forms and rewriting over 09 to enter in the year 10 or writing outside the box. • Some sites are not faxing in the log sheets at the end of the month
Common Issues with Record Keeping • Incomplete Cognition log sheets. • Not entering their CLIS ID Number, and/or entering an incorrect CLIS ID Number. • Not writing in the comment box when entering a code – example 3 or 6. If invalid or a manufacturer error please state that and why. • Crossing out the lines on the log sheet and filling in above cross out • Using 09 forms and rewriting over 09 to enter in the year 10 or writing outside the box. • Some sites are not faxing in the log sheets at the end of the month
 Common Issues with Record Keeping (Continued) • • Log sheets for the month must be faxed, by the end of the month, even if the sheet is not completed. Do Not copy the log sheets. Do Not send temperature log sheets and cover pages through the Cognition fax number • ONLY the log sheets should come through Cognition (732) 7433206 or (732) 743 -3632. • ALL OTHER forms to (732) 235 -9012 or 866 -238 -1469. • Some Non RWJ sites are not using the log sheets at all. !! Cognition Log sheets need to be used
Common Issues with Record Keeping (Continued) • • Log sheets for the month must be faxed, by the end of the month, even if the sheet is not completed. Do Not copy the log sheets. Do Not send temperature log sheets and cover pages through the Cognition fax number • ONLY the log sheets should come through Cognition (732) 7433206 or (732) 743 -3632. • ALL OTHER forms to (732) 235 -9012 or 866 -238 -1469. • Some Non RWJ sites are not using the log sheets at all. !! Cognition Log sheets need to be used

 Rapid HIV Testing in NJ Discordant Analysis in Rapid HIV Testing Implementation of Rapid-Rapid New Directions Eugene G. Martin, Ph. D. Professor of Pathology and Laboratory Medicine UMDNJ – Robert W. Johnson Medical School
Rapid HIV Testing in NJ Discordant Analysis in Rapid HIV Testing Implementation of Rapid-Rapid New Directions Eugene G. Martin, Ph. D. Professor of Pathology and Laboratory Medicine UMDNJ – Robert W. Johnson Medical School
 Topics • Discordant Analysis 2009 • Rapid-Rapid Initiative 2009 • New Directions in Rapid Testing • • • Trends Rapid Test Product Performance Specificity Oraquick re-formulation of manufacturing process Improved product specificity Narrowing the Detection Window • Acute HIV Initiative • New Products – Determine Combo
Topics • Discordant Analysis 2009 • Rapid-Rapid Initiative 2009 • New Directions in Rapid Testing • • • Trends Rapid Test Product Performance Specificity Oraquick re-formulation of manufacturing process Improved product specificity Narrowing the Detection Window • Acute HIV Initiative • New Products – Determine Combo
 Rapid HIV Testing in NJ 2009 DISCORDANT ANALYSIS
Rapid HIV Testing in NJ 2009 DISCORDANT ANALYSIS
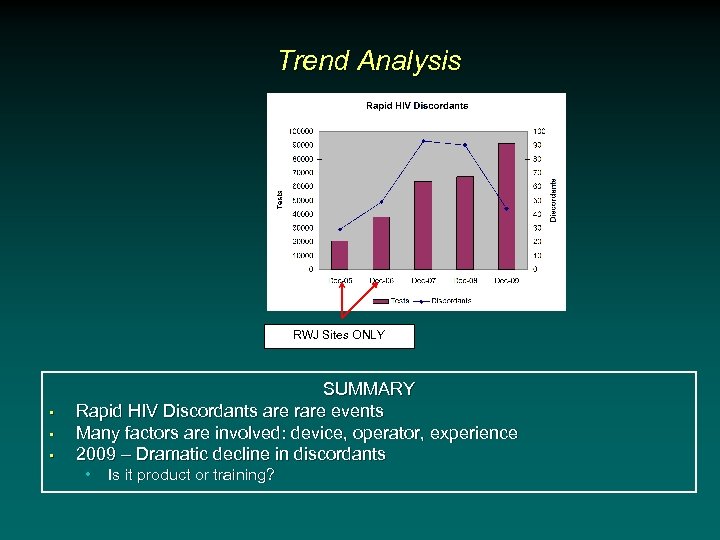 Trend Analysis RWJ Sites ONLY • • • SUMMARY Rapid HIV Discordants are rare events Many factors are involved: device, operator, experience 2009 – Dramatic decline in discordants • Is it product or training?
Trend Analysis RWJ Sites ONLY • • • SUMMARY Rapid HIV Discordants are rare events Many factors are involved: device, operator, experience 2009 – Dramatic decline in discordants • Is it product or training?
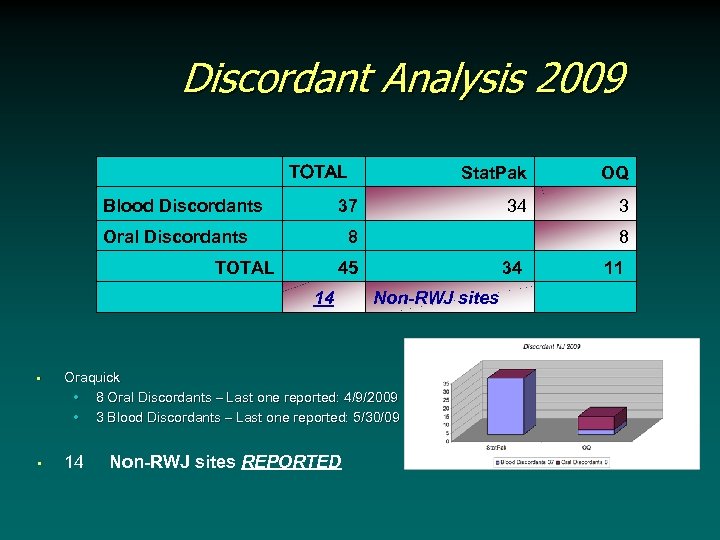 Discordant Analysis 2009 TOTAL Blood Discordants OQ 34 3 37 Oral Discordants 8 TOTAL Stat. Pak 45 14 34 Non-RWJ sites • Oraquick • 8 Oral Discordants – Last one reported: 4/9/2009 • 3 Blood Discordants – Last one reported: 5/30/09 • 14 Non-RWJ sites REPORTED 8 11
Discordant Analysis 2009 TOTAL Blood Discordants OQ 34 3 37 Oral Discordants 8 TOTAL Stat. Pak 45 14 34 Non-RWJ sites • Oraquick • 8 Oral Discordants – Last one reported: 4/9/2009 • 3 Blood Discordants – Last one reported: 5/30/09 • 14 Non-RWJ sites REPORTED 8 11
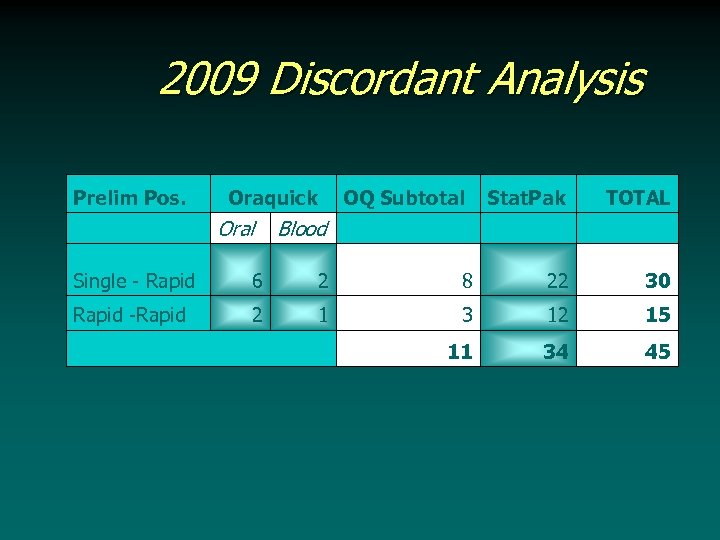 2009 Discordant Analysis Prelim Pos. Oraquick Oral OQ Subtotal Blood Stat. Pak TOTAL Single - Rapid 6 2 8 22 30 Rapid -Rapid 2 1 3 12 15 11 34 45
2009 Discordant Analysis Prelim Pos. Oraquick Oral OQ Subtotal Blood Stat. Pak TOTAL Single - Rapid 6 2 8 22 30 Rapid -Rapid 2 1 3 12 15 11 34 45
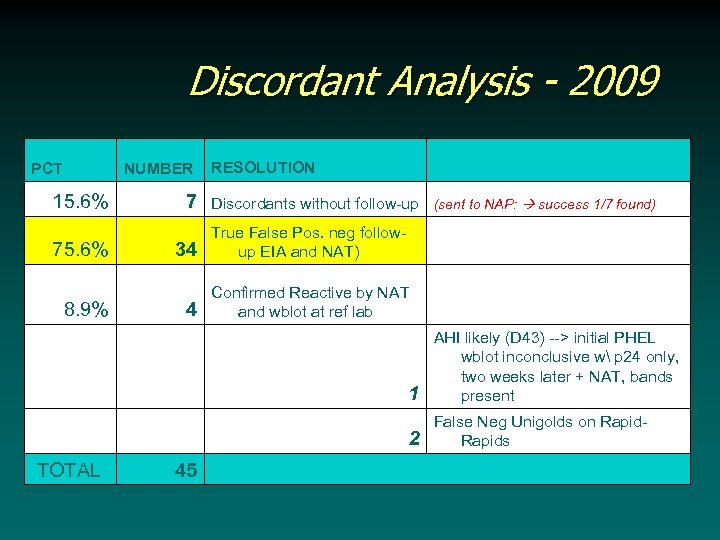 Discordant Analysis - 2009 PCT NUMBER 15. 6% 7 75. 6% 34 8. 9% TOTAL 4 RESOLUTION Discordants without follow-up (sent to NAP: success 1/7 found) True False Pos. neg followup EIA and NAT) Confirmed Reactive by NAT and wblot at ref lab 1 2 45 AHI likely (D 43) --> initial PHEL wblot inconclusive w p 24 only, two weeks later + NAT, bands present False Neg Unigolds on Rapids
Discordant Analysis - 2009 PCT NUMBER 15. 6% 7 75. 6% 34 8. 9% TOTAL 4 RESOLUTION Discordants without follow-up (sent to NAP: success 1/7 found) True False Pos. neg followup EIA and NAT) Confirmed Reactive by NAT and wblot at ref lab 1 2 45 AHI likely (D 43) --> initial PHEL wblot inconclusive w p 24 only, two weeks later + NAT, bands present False Neg Unigolds on Rapids
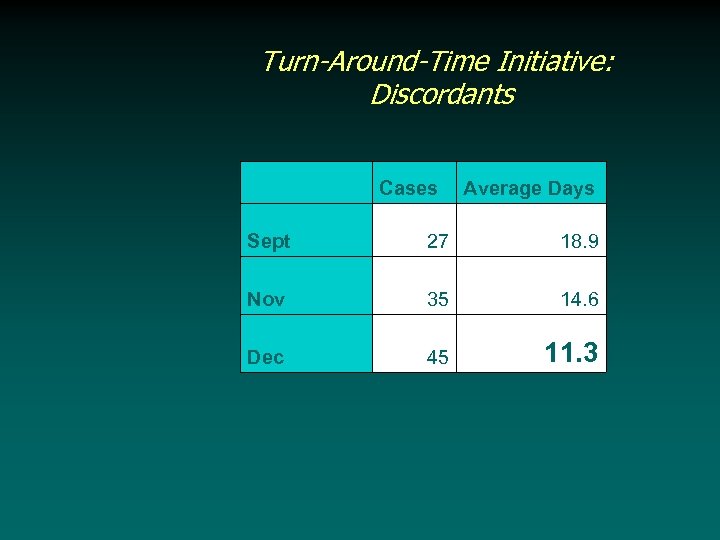 Turn-Around-Time Initiative: Discordants Cases Average Days Sept 27 18. 9 Nov 35 14. 6 Dec 45 11. 3
Turn-Around-Time Initiative: Discordants Cases Average Days Sept 27 18. 9 Nov 35 14. 6 Dec 45 11. 3
 Rapid HIV Testing in NJ STATUS OF RAPID-RAPID IMPLEMENTATION
Rapid HIV Testing in NJ STATUS OF RAPID-RAPID IMPLEMENTATION
 Status of the Rapid-Rapid Initiative • What is ‘Rapid-Rapid’ • What is the NJ implementation process • Volume/performance figures 2009 • The CDC Surveillance Taskforce data - two rapids verify a positive HIV test 99. 2% of the time • AHEAD: Efforts to recruit higher prevalence, non-RWJ sites to participate in the next phase of roll-out
Status of the Rapid-Rapid Initiative • What is ‘Rapid-Rapid’ • What is the NJ implementation process • Volume/performance figures 2009 • The CDC Surveillance Taskforce data - two rapids verify a positive HIV test 99. 2% of the time • AHEAD: Efforts to recruit higher prevalence, non-RWJ sites to participate in the next phase of roll-out
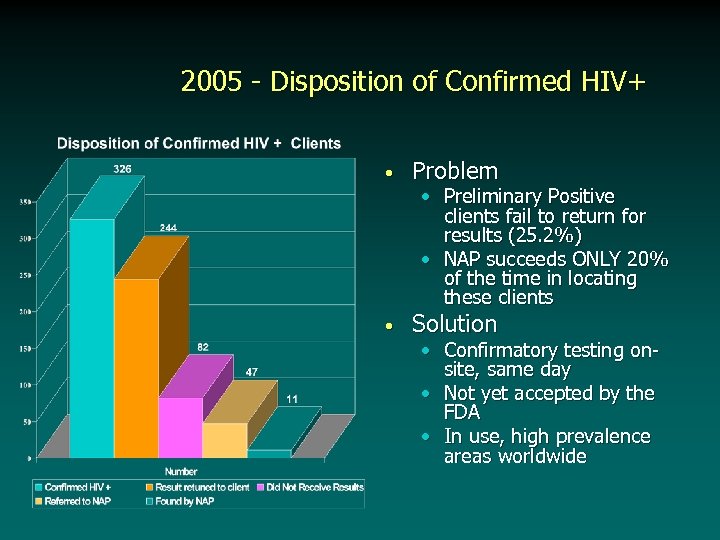 2005 - Disposition of Confirmed HIV+ • Problem • Solution • Preliminary Positive clients fail to return for results (25. 2%) • NAP succeeds ONLY 20% of the time in locating these clients • Confirmatory testing onsite, same day • Not yet accepted by the FDA • In use, high prevalence areas worldwide
2005 - Disposition of Confirmed HIV+ • Problem • Solution • Preliminary Positive clients fail to return for results (25. 2%) • NAP succeeds ONLY 20% of the time in locating these clients • Confirmatory testing onsite, same day • Not yet accepted by the FDA • In use, high prevalence areas worldwide
 Evolving Issues in RAPID TESTING • Sensitivity Issues: • How Sensitive are rapid HIV tests? • Rapid HIV Tests Measures Antibodies to HIV • They DO NOT Measure HIV RNA or DNA • At least as sensitive as more complex EIA technology used in hospitals and laboratories • In some cases more sensitive than the Western blot, the so-called ‘Gold Standard’ for validation. … this creates problems
Evolving Issues in RAPID TESTING • Sensitivity Issues: • How Sensitive are rapid HIV tests? • Rapid HIV Tests Measures Antibodies to HIV • They DO NOT Measure HIV RNA or DNA • At least as sensitive as more complex EIA technology used in hospitals and laboratories • In some cases more sensitive than the Western blot, the so-called ‘Gold Standard’ for validation. … this creates problems
 Why run a second test? • Specificity of a testing algorithm • Builds upon the specificity of a test • ALL laboratory tests have a • • • A sensitivity – i. e. the ability to call a true positive, positive A specificity – i. e. the ability to call a true negative, negative Traditionally the Western blot, improves the overall specificity of the testing algorithm.
Why run a second test? • Specificity of a testing algorithm • Builds upon the specificity of a test • ALL laboratory tests have a • • • A sensitivity – i. e. the ability to call a true positive, positive A specificity – i. e. the ability to call a true negative, negative Traditionally the Western blot, improves the overall specificity of the testing algorithm.
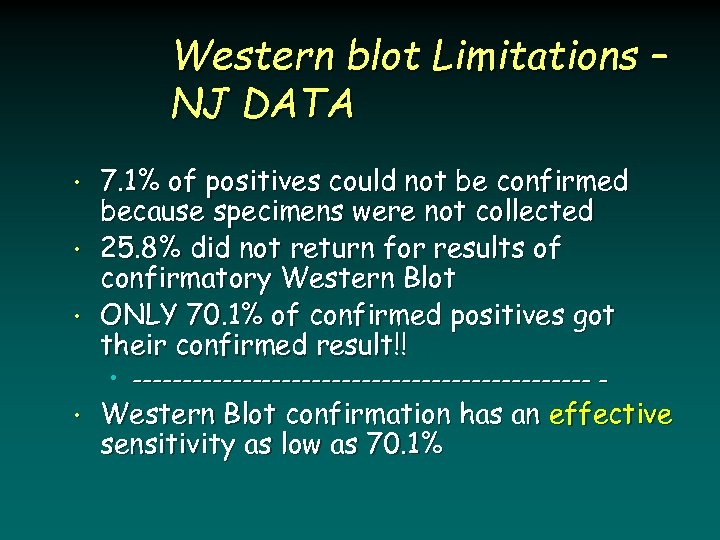 Western blot Limitations – NJ DATA • • • 7. 1% of positives could not be confirmed because specimens were not collected 25. 8% did not return for results of confirmatory Western Blot ONLY 70. 1% of confirmed positives got their confirmed result!! • ----------------------- - • Western Blot confirmation has an effective sensitivity as low as 70. 1%
Western blot Limitations – NJ DATA • • • 7. 1% of positives could not be confirmed because specimens were not collected 25. 8% did not return for results of confirmatory Western Blot ONLY 70. 1% of confirmed positives got their confirmed result!! • ----------------------- - • Western Blot confirmation has an effective sensitivity as low as 70. 1%
 Rapid Testing Algorithms “Rapid-Rapid” • Principle: • Two different immunoassays that employ different HIV antigens to search for HIV antibodies will verify the HIV result >99% of the time • Outcome • Could we potentially eliminate the western blot as a confirmatory assay and substitute a second rapid HIV test? ? ?
Rapid Testing Algorithms “Rapid-Rapid” • Principle: • Two different immunoassays that employ different HIV antigens to search for HIV antibodies will verify the HIV result >99% of the time • Outcome • Could we potentially eliminate the western blot as a confirmatory assay and substitute a second rapid HIV test? ? ?
 Rolling out ‘Rapid-Rapid’ • • • VALIDATION OF THE CONCEPT SHARING THE EFFORT PRESENTATION at numerous national conferences: APHA, HIV Prevention, IDSA, etc. IMPLEMENTATION DECISION: Rapid-Rapid testing at NJ HIV sites directed by RWJMS – 2009 HIV Coordinators Conference IMPLEMENTATION PROCESS: Funding from supplemental funding from CDC, we began the roll-out in December, 2009.
Rolling out ‘Rapid-Rapid’ • • • VALIDATION OF THE CONCEPT SHARING THE EFFORT PRESENTATION at numerous national conferences: APHA, HIV Prevention, IDSA, etc. IMPLEMENTATION DECISION: Rapid-Rapid testing at NJ HIV sites directed by RWJMS – 2009 HIV Coordinators Conference IMPLEMENTATION PROCESS: Funding from supplemental funding from CDC, we began the roll-out in December, 2009.
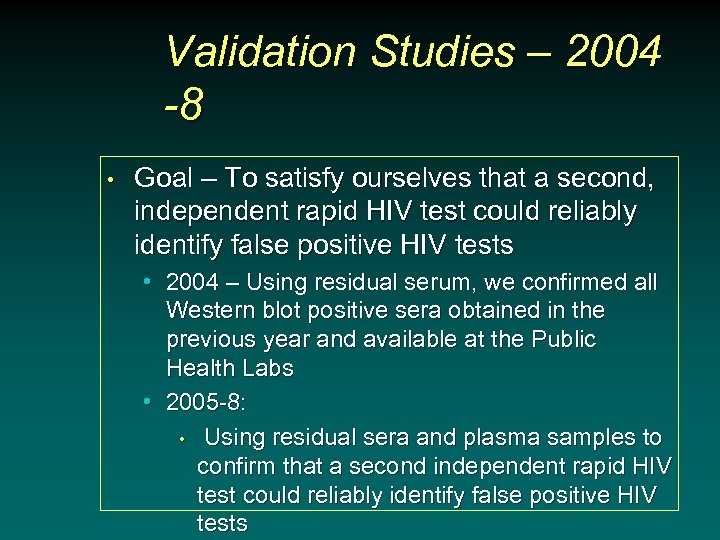 Validation Studies – 2004 -8 • Goal – To satisfy ourselves that a second, independent rapid HIV test could reliably identify false positive HIV tests • 2004 – Using residual serum, we confirmed all Western blot positive sera obtained in the previous year and available at the Public Health Labs • 2005 -8: • Using residual sera and plasma samples to confirm that a second independent rapid HIV test could reliably identify false positive HIV tests
Validation Studies – 2004 -8 • Goal – To satisfy ourselves that a second, independent rapid HIV test could reliably identify false positive HIV tests • 2004 – Using residual serum, we confirmed all Western blot positive sera obtained in the previous year and available at the Public Health Labs • 2005 -8: • Using residual sera and plasma samples to confirm that a second independent rapid HIV test could reliably identify false positive HIV tests
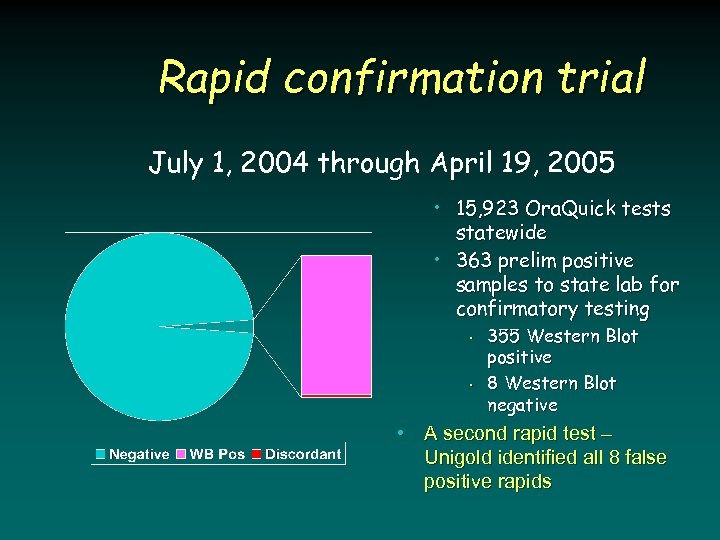 Rapid confirmation trial July 1, 2004 through April 19, 2005 • 15, 923 Ora. Quick tests statewide • 363 prelim positive samples to state lab for confirmatory testing • • 355 Western Blot positive 8 Western Blot negative • A second rapid test – Unigold identified all 8 false positive rapids
Rapid confirmation trial July 1, 2004 through April 19, 2005 • 15, 923 Ora. Quick tests statewide • 363 prelim positive samples to state lab for confirmatory testing • • 355 Western Blot positive 8 Western Blot negative • A second rapid test – Unigold identified all 8 false positive rapids
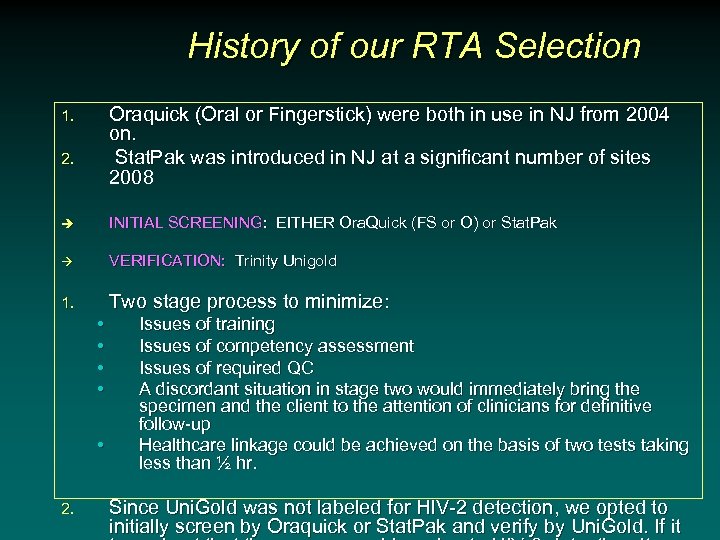 History of our RTA Selection Oraquick (Oral or Fingerstick) were both in use in NJ from 2004 on. Stat. Pak was introduced in NJ at a significant number of sites 2008 1. 2. è INITIAL SCREENING: EITHER Ora. Quick (FS or O) or Stat. Pak VERIFICATION: Trinity Unigold 1. Two stage process to minimize: • • • 2. Issues of training Issues of competency assessment Issues of required QC A discordant situation in stage two would immediately bring the specimen and the client to the attention of clinicians for definitive follow-up Healthcare linkage could be achieved on the basis of two tests taking less than ½ hr. Since Uni. Gold was not labeled for HIV-2 detection, we opted to initially screen by Oraquick or Stat. Pak and verify by Uni. Gold. If it
History of our RTA Selection Oraquick (Oral or Fingerstick) were both in use in NJ from 2004 on. Stat. Pak was introduced in NJ at a significant number of sites 2008 1. 2. è INITIAL SCREENING: EITHER Ora. Quick (FS or O) or Stat. Pak VERIFICATION: Trinity Unigold 1. Two stage process to minimize: • • • 2. Issues of training Issues of competency assessment Issues of required QC A discordant situation in stage two would immediately bring the specimen and the client to the attention of clinicians for definitive follow-up Healthcare linkage could be achieved on the basis of two tests taking less than ½ hr. Since Uni. Gold was not labeled for HIV-2 detection, we opted to initially screen by Oraquick or Stat. Pak and verify by Uni. Gold. If it
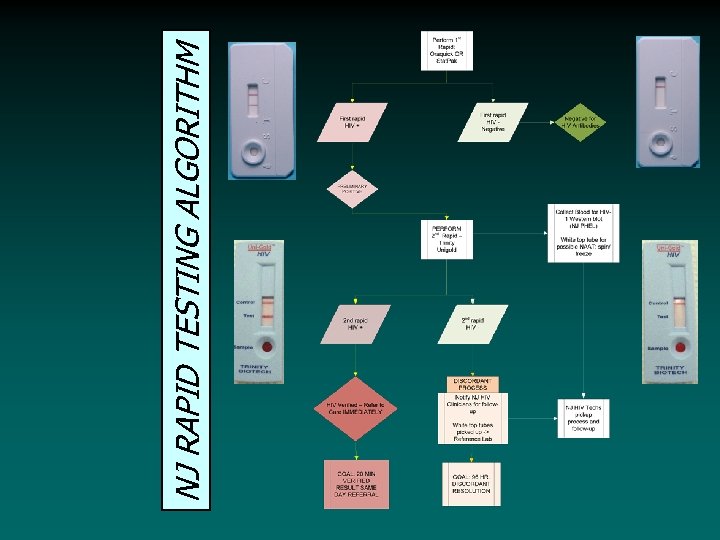 NJ RAPID TESTING ALGORITHM
NJ RAPID TESTING ALGORITHM
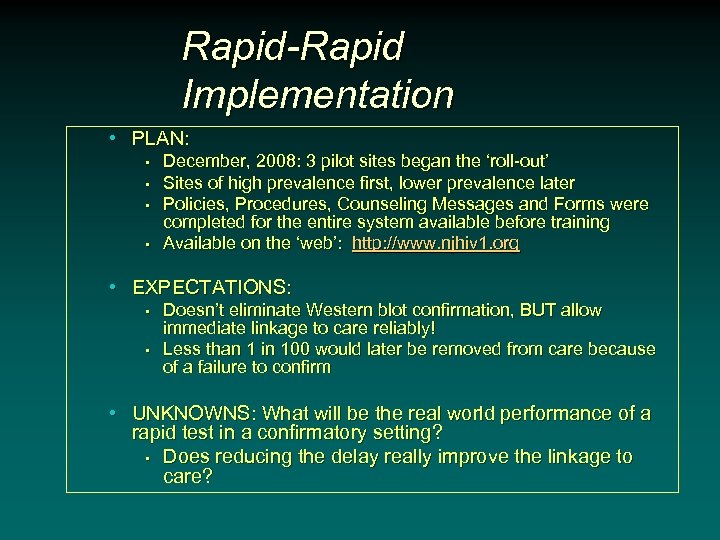 Rapid-Rapid Implementation • PLAN: • • December, 2008: 3 pilot sites began the ‘roll-out’ Sites of high prevalence first, lower prevalence later Policies, Procedures, Counseling Messages and Forms were completed for the entire system available before training Available on the ‘web’: http: //www. njhiv 1. org • EXPECTATIONS: • • Doesn’t eliminate Western blot confirmation, BUT allow immediate linkage to care reliably! Less than 1 in 100 would later be removed from care because of a failure to confirm • UNKNOWNS: What will be the real world performance of a rapid test in a confirmatory setting? • Does reducing the delay really improve the linkage to care?
Rapid-Rapid Implementation • PLAN: • • December, 2008: 3 pilot sites began the ‘roll-out’ Sites of high prevalence first, lower prevalence later Policies, Procedures, Counseling Messages and Forms were completed for the entire system available before training Available on the ‘web’: http: //www. njhiv 1. org • EXPECTATIONS: • • Doesn’t eliminate Western blot confirmation, BUT allow immediate linkage to care reliably! Less than 1 in 100 would later be removed from care because of a failure to confirm • UNKNOWNS: What will be the real world performance of a rapid test in a confirmatory setting? • Does reducing the delay really improve the linkage to care?
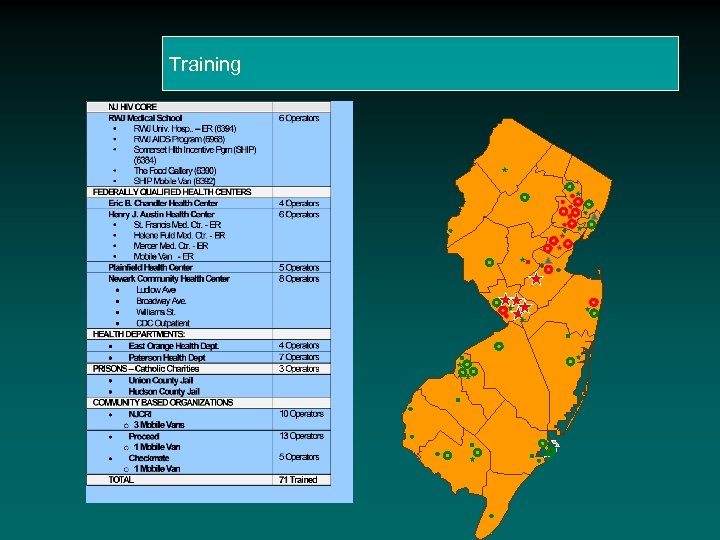 Training
Training
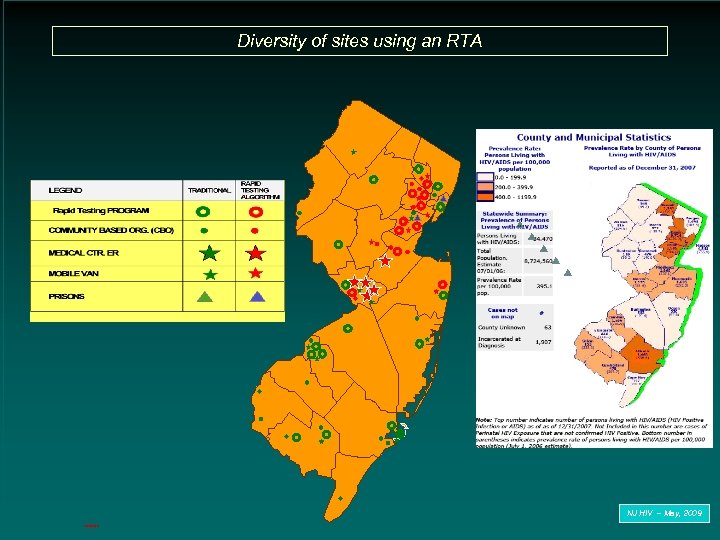 Diversity of sites using an RTA NJ HIV – May, 2009 3/20/2018
Diversity of sites using an RTA NJ HIV – May, 2009 3/20/2018
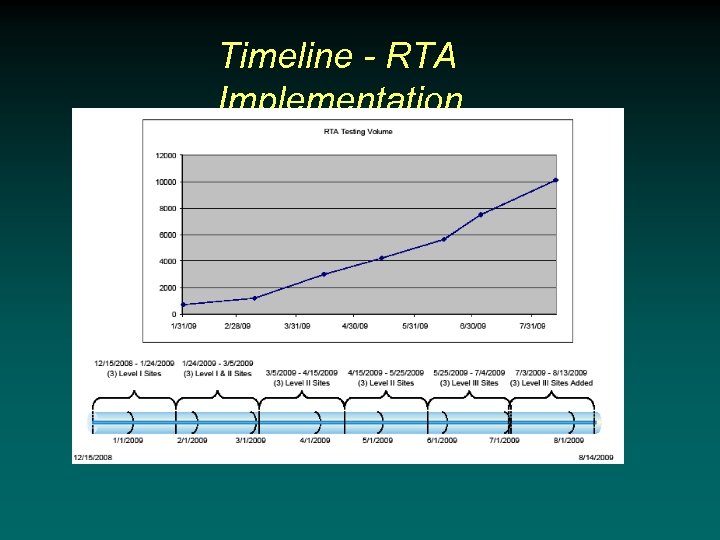 Timeline - RTA Implementation
Timeline - RTA Implementation
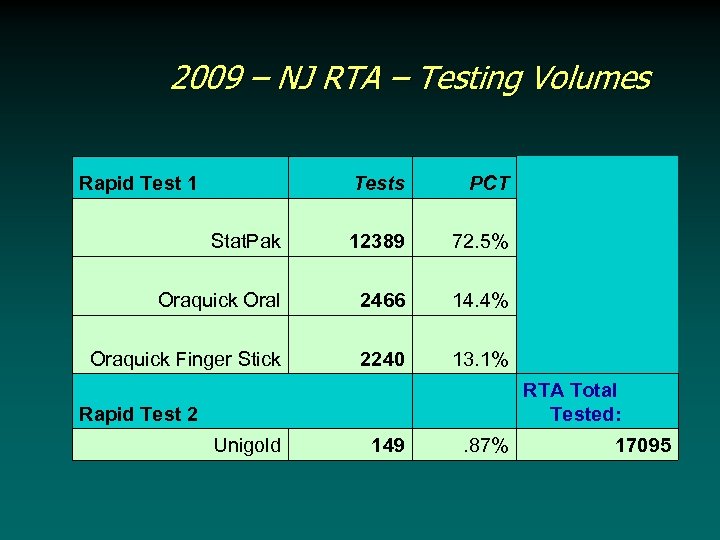 2009 – NJ RTA – Testing Volumes Rapid Test 1 Tests PCT Stat. Pak 12389 72. 5% Oraquick Oral 2466 14. 4% Oraquick Finger Stick 2240 13. 1% Rapid Test 2 RTA Total Tested: Unigold 149 . 87% 17095
2009 – NJ RTA – Testing Volumes Rapid Test 1 Tests PCT Stat. Pak 12389 72. 5% Oraquick Oral 2466 14. 4% Oraquick Finger Stick 2240 13. 1% Rapid Test 2 RTA Total Tested: Unigold 149 . 87% 17095
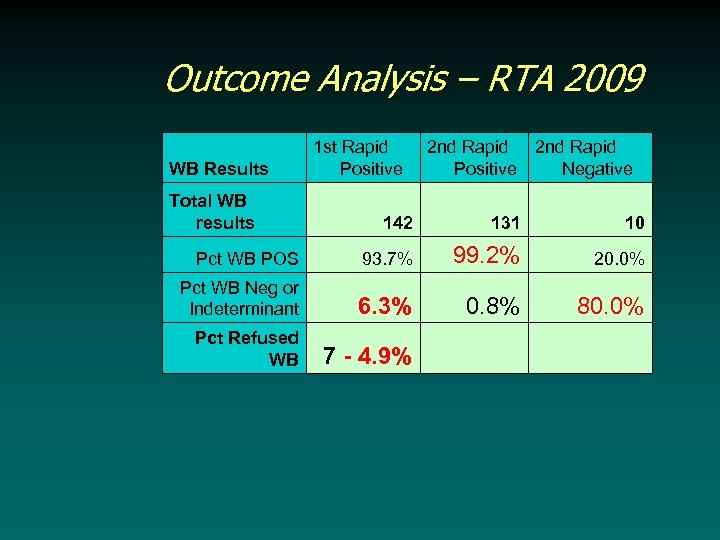 Outcome Analysis – RTA 2009 WB Results 1 st Rapid Positive Total WB results 2 nd Rapid Negative 142 Pct WB Neg or Indeterminant 131 10 93. 7% Pct WB POS Pct Refused WB 2 nd Rapid Positive 99. 2% 20. 0% 6. 3% 0. 8% 80. 0% 7 - 4. 9%
Outcome Analysis – RTA 2009 WB Results 1 st Rapid Positive Total WB results 2 nd Rapid Negative 142 Pct WB Neg or Indeterminant 131 10 93. 7% Pct WB POS Pct Refused WB 2 nd Rapid Positive 99. 2% 20. 0% 6. 3% 0. 8% 80. 0% 7 - 4. 9%
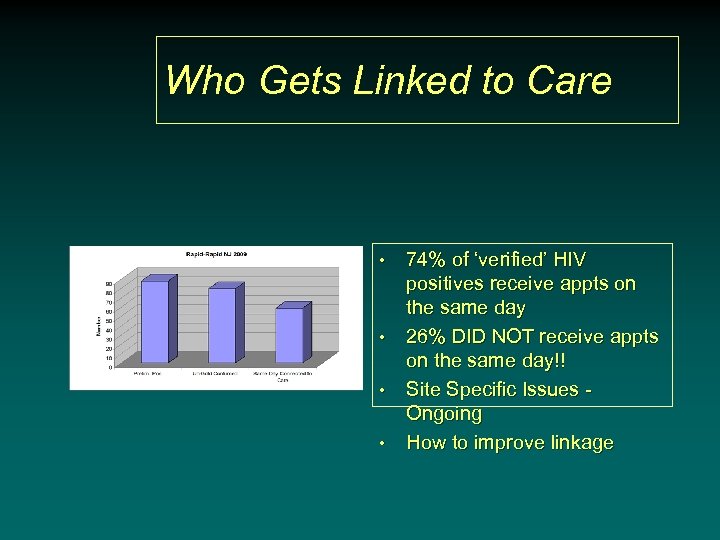 Who Gets Linked to Care • • 74% of ‘verified’ HIV positives receive appts on the same day 26% DID NOT receive appts on the same day!! Site Specific Issues - Ongoing How to improve linkage
Who Gets Linked to Care • • 74% of ‘verified’ HIV positives receive appts on the same day 26% DID NOT receive appts on the same day!! Site Specific Issues - Ongoing How to improve linkage
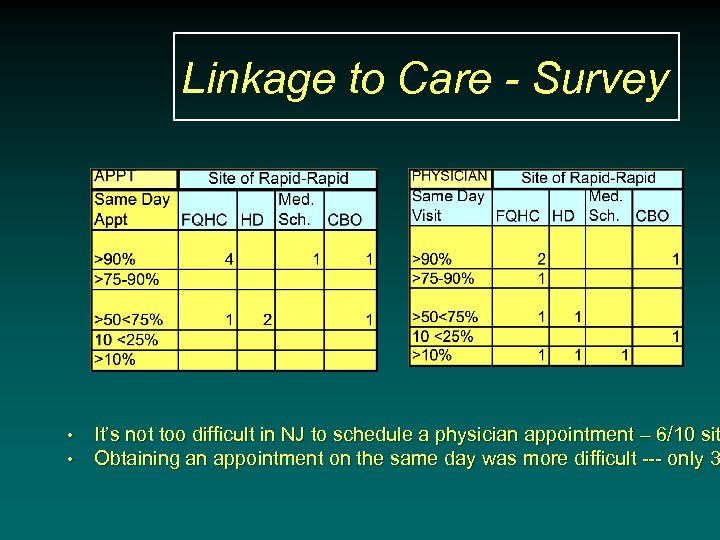 Linkage to Care - Survey • • It’s not too difficult in NJ to schedule a physician appointment – 6/10 sit Obtaining an appointment on the same day was more difficult --- only 3
Linkage to Care - Survey • • It’s not too difficult in NJ to schedule a physician appointment – 6/10 sit Obtaining an appointment on the same day was more difficult --- only 3
 The Next Phase • Expand Rapid-Rapid Testing • Seeking non-RWJ sites to implement Rapid-Rapid. • Goal: Linkage to care on the day HIV result is verified. • Possible Elimination of the Confirmatory Western blot • Current surveillance definition requires IFA, Western blot or RNA testing – a CDC taskforce is addressing this issue. – it matters because funding is influenced!!
The Next Phase • Expand Rapid-Rapid Testing • Seeking non-RWJ sites to implement Rapid-Rapid. • Goal: Linkage to care on the day HIV result is verified. • Possible Elimination of the Confirmatory Western blot • Current surveillance definition requires IFA, Western blot or RNA testing – a CDC taskforce is addressing this issue. – it matters because funding is influenced!!
 Rapid HIV Testing in NJ Future Directions
Rapid HIV Testing in NJ Future Directions
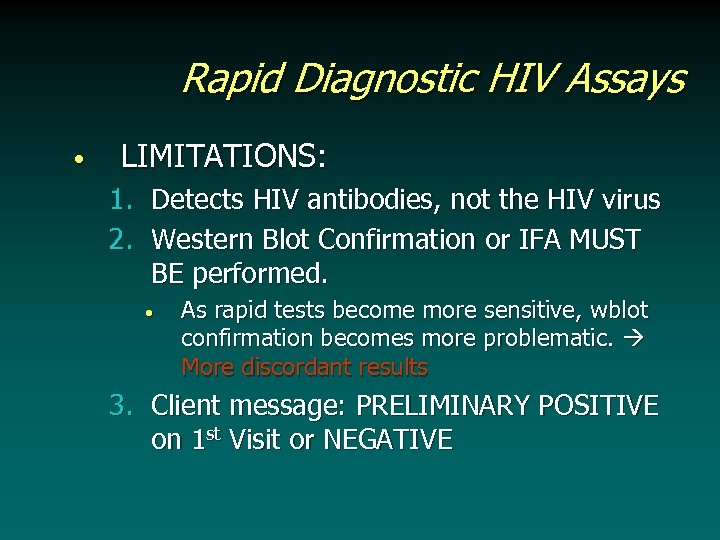 Rapid Diagnostic HIV Assays • LIMITATIONS: 1. Detects HIV antibodies, not the HIV virus 2. Western Blot Confirmation or IFA MUST BE performed. • As rapid tests become more sensitive, wblot confirmation becomes more problematic. More discordant results 3. Client message: PRELIMINARY POSITIVE on 1 st Visit or NEGATIVE
Rapid Diagnostic HIV Assays • LIMITATIONS: 1. Detects HIV antibodies, not the HIV virus 2. Western Blot Confirmation or IFA MUST BE performed. • As rapid tests become more sensitive, wblot confirmation becomes more problematic. More discordant results 3. Client message: PRELIMINARY POSITIVE on 1 st Visit or NEGATIVE
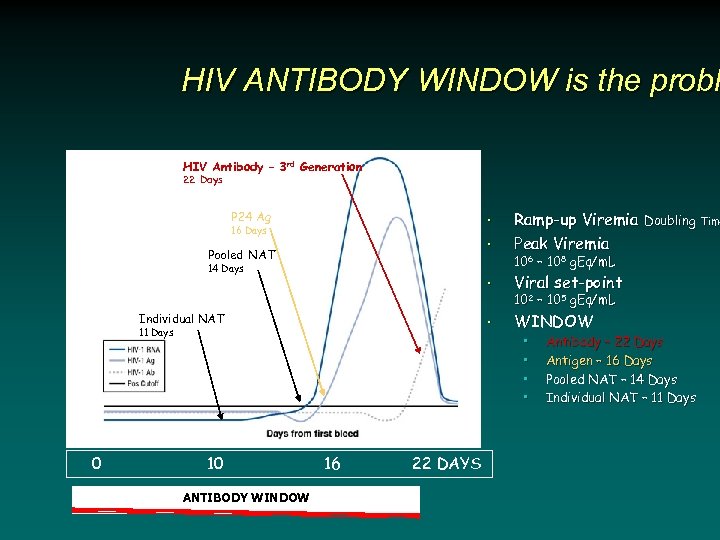 HIV ANTIBODY WINDOW is the probl HIV Antibody – 3 rd Generation 22 Days • Viral set-point • WINDOW • 16 Days Pooled NAT 14 Days Individual NAT 11 Days 0 Ramp-up Viremia Doubling Time Peak Viremia • P 24 Ag 106 – 108 g. Eq/m. L 102 – 105 g. Eq/m. L • • 10 ANTIBODY WINDOW 16 22 DAYS Antibody – 22 Days Antigen – 16 Days Pooled NAT – 14 Days Individual NAT – 11 Days
HIV ANTIBODY WINDOW is the probl HIV Antibody – 3 rd Generation 22 Days • Viral set-point • WINDOW • 16 Days Pooled NAT 14 Days Individual NAT 11 Days 0 Ramp-up Viremia Doubling Time Peak Viremia • P 24 Ag 106 – 108 g. Eq/m. L 102 – 105 g. Eq/m. L • • 10 ANTIBODY WINDOW 16 22 DAYS Antibody – 22 Days Antigen – 16 Days Pooled NAT – 14 Days Individual NAT – 11 Days
 Opportunity Summary 1. 2. 3. ~ 55, 000 new HIV infections per year in the US Reaching and testing those at risk • ~ 25% of the 850, 000 - 950, 000 HIV+ people in the United States are unaware of their status • ~ 30% or more who test positive for HIV by conventional testing do not receive their results!! Stop the cycle by interfering with transmission • • • 4. More than 50% of transmission occurs in the earliest stages of an HIV infection! If we detect infections at the earliest stages possibility of interrupting the cycle of transmission. Once the antibody appears, infectivity is diminishing How to detect early infections in a simpler, more economical manner
Opportunity Summary 1. 2. 3. ~ 55, 000 new HIV infections per year in the US Reaching and testing those at risk • ~ 25% of the 850, 000 - 950, 000 HIV+ people in the United States are unaware of their status • ~ 30% or more who test positive for HIV by conventional testing do not receive their results!! Stop the cycle by interfering with transmission • • • 4. More than 50% of transmission occurs in the earliest stages of an HIV infection! If we detect infections at the earliest stages possibility of interrupting the cycle of transmission. Once the antibody appears, infectivity is diminishing How to detect early infections in a simpler, more economical manner
 Natural History - HIV Infection Couthino et al. , Bulletin of Mathematical Biology 2001
Natural History - HIV Infection Couthino et al. , Bulletin of Mathematical Biology 2001
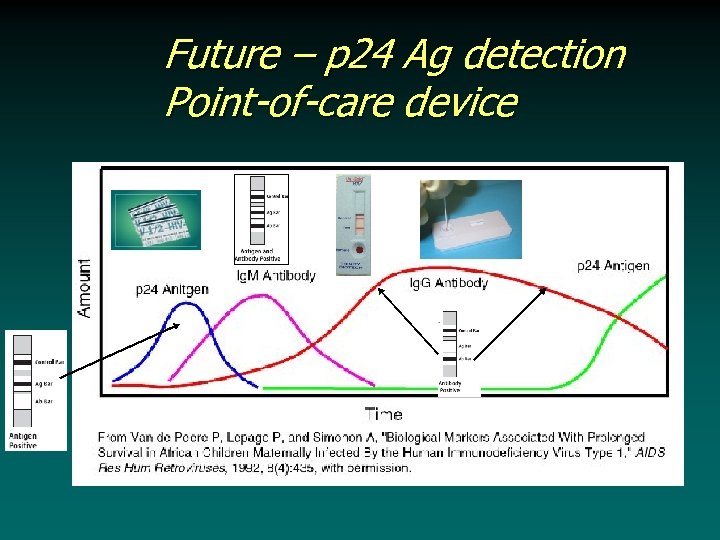 Future – p 24 Ag detection Point-of-care device
Future – p 24 Ag detection Point-of-care device
 Detecting HIV virus before HIV antibody appears • Pooled NAT on antibody negative blood • Blood donor facilities use to protect blood recipients since the late 1990’s. • Concept – If you’re in the window phase, you have no antibody, you may have no p 24 Ag, but you still have the virus • As of 2001, 100% of the US blood supply was tested by pooled NAT. Yield: 8 HIV antibody negative infected units in 23 million tested units. 2 p 24 Ag+ units also detected. (~1: 3, 292, 400) • Between 2003 -7 discussions in the HIV community regarding the use of pooled NAT in high risk individuals. • Expensive • Cases eventually demonstrate antibody, so… • Why bother? • Crucial bit of information missing to justify pooled
Detecting HIV virus before HIV antibody appears • Pooled NAT on antibody negative blood • Blood donor facilities use to protect blood recipients since the late 1990’s. • Concept – If you’re in the window phase, you have no antibody, you may have no p 24 Ag, but you still have the virus • As of 2001, 100% of the US blood supply was tested by pooled NAT. Yield: 8 HIV antibody negative infected units in 23 million tested units. 2 p 24 Ag+ units also detected. (~1: 3, 292, 400) • Between 2003 -7 discussions in the HIV community regarding the use of pooled NAT in high risk individuals. • Expensive • Cases eventually demonstrate antibody, so… • Why bother? • Crucial bit of information missing to justify pooled
 The missing link • More than 50% of transmission occurs in the earliest stages of an HIV infection! • If we detect infections at the earliest stages, there is the possibility of interrupting the cycle of transmission. • Once the antibody appears, infectivity is already diminishing
The missing link • More than 50% of transmission occurs in the earliest stages of an HIV infection! • If we detect infections at the earliest stages, there is the possibility of interrupting the cycle of transmission. • Once the antibody appears, infectivity is already diminishing
 The Question • If we have the capacity to check p 24 Ag with a rapid test and it narrows the window for detection by 6 days is that good enough? • We have implemented pooled NAT testing from antibody negative blood at high prevalence sites where individuals who are recently infected might logically go, if they were feeling poorly. • University Hospital • St. Michael’s • In San Francisco, last year they identified 39 individuals with Acute HIV infection, but the majority WOULD have been identified with access to p 24 Ag testing! • What about New Jersey?
The Question • If we have the capacity to check p 24 Ag with a rapid test and it narrows the window for detection by 6 days is that good enough? • We have implemented pooled NAT testing from antibody negative blood at high prevalence sites where individuals who are recently infected might logically go, if they were feeling poorly. • University Hospital • St. Michael’s • In San Francisco, last year they identified 39 individuals with Acute HIV infection, but the majority WOULD have been identified with access to p 24 Ag testing! • What about New Jersey?
 New Jersey HIV We’ll let you know… Next year! And Most Importantly Thanks for all you do!
New Jersey HIV We’ll let you know… Next year! And Most Importantly Thanks for all you do!
 Thanks To: RWJMS • Evan Cadoff, MD • Eugene Martin, Ph. D. • Gratian Salaru, MD • • • Joanne Corbo, MBA, MT (ASCP) Claudia Carron, MSN, RN Franchesca Jackson, BS (Biology) Nisha Intwala, BS, MT (ASCP) Aida Gilanchi, BS, MT Mary Ann Garrihy, BS, MT (ASCP) Patricia Riberio, BS, MT (ASCP) Lisa May Karen Williams All site coordinators and counselors throughout New Jersey NJDHSS/DHAS • Sindy Paul, MD, MPH* • Linda Berezny, RN • Maureen Wolski, BS • Aye Maung NJDHSS/PHEL
Thanks To: RWJMS • Evan Cadoff, MD • Eugene Martin, Ph. D. • Gratian Salaru, MD • • • Joanne Corbo, MBA, MT (ASCP) Claudia Carron, MSN, RN Franchesca Jackson, BS (Biology) Nisha Intwala, BS, MT (ASCP) Aida Gilanchi, BS, MT Mary Ann Garrihy, BS, MT (ASCP) Patricia Riberio, BS, MT (ASCP) Lisa May Karen Williams All site coordinators and counselors throughout New Jersey NJDHSS/DHAS • Sindy Paul, MD, MPH* • Linda Berezny, RN • Maureen Wolski, BS • Aye Maung NJDHSS/PHEL


