999248a915d8559015c71dafce9a6c71.ppt
- Количество слайдов: 37
 Announcements, Agenda Week 3 • Reading for today: Ch. 1, 2 in Hibbs, Zucker 2006 • Start up your computers – you will need them for some in -class exercises. • Open today’s Power point slides and Internet Explorer I. Lecture: Intro to Confocal, optics II. Paper discussion: Zucker 2006 III. TBA: Collect Zseries of Artemia samples IV. Assignment due Jan. 29
Announcements, Agenda Week 3 • Reading for today: Ch. 1, 2 in Hibbs, Zucker 2006 • Start up your computers – you will need them for some in -class exercises. • Open today’s Power point slides and Internet Explorer I. Lecture: Intro to Confocal, optics II. Paper discussion: Zucker 2006 III. TBA: Collect Zseries of Artemia samples IV. Assignment due Jan. 29
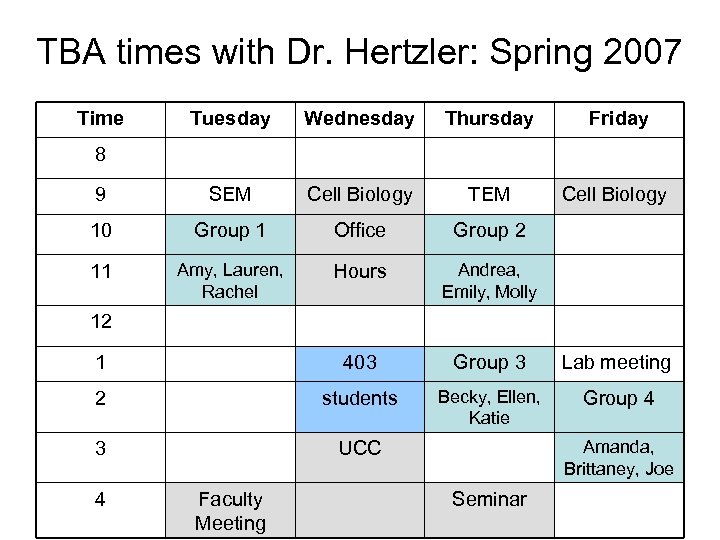 TBA times with Dr. Hertzler: Spring 2007 Time Tuesday Wednesday Thursday Friday 9 SEM Cell Biology TEM Cell Biology 10 Group 1 Office Group 2 11 Amy, Lauren, Rachel Hours Andrea, Emily, Molly 1 403 Group 3 Lab meeting 2 students Becky, Ellen, Katie Group 4 3 UCC 8 12 4 Faculty Meeting Amanda, Brittaney, Joe Seminar
TBA times with Dr. Hertzler: Spring 2007 Time Tuesday Wednesday Thursday Friday 9 SEM Cell Biology TEM Cell Biology 10 Group 1 Office Group 2 11 Amy, Lauren, Rachel Hours Andrea, Emily, Molly 1 403 Group 3 Lab meeting 2 students Becky, Ellen, Katie Group 4 3 UCC 8 12 4 Faculty Meeting Amanda, Brittaney, Joe Seminar
 Outline: Understanding Microscopy A. Introduction to Confocal Microscopy 1. Confocal versus conventional (widefield) fluorescence 2. Optical sectioning 3. Imaging modes and applications 4. Advantages, limitations of confocal B. Essential Optics 1. Wave/particle nature of Light 2. Diffraction 3. Numerical aperture 4. Lateral resolution 5. Axial resolution Useful resource: Molecular Expression Microscopy Primer: • http: //micro. magnet. fsu. edu/primer/index. html
Outline: Understanding Microscopy A. Introduction to Confocal Microscopy 1. Confocal versus conventional (widefield) fluorescence 2. Optical sectioning 3. Imaging modes and applications 4. Advantages, limitations of confocal B. Essential Optics 1. Wave/particle nature of Light 2. Diffraction 3. Numerical aperture 4. Lateral resolution 5. Axial resolution Useful resource: Molecular Expression Microscopy Primer: • http: //micro. magnet. fsu. edu/primer/index. html
 Laser Scanning Confocal Microscope Components Scan Head Microscope Controller box Computer, display Laser
Laser Scanning Confocal Microscope Components Scan Head Microscope Controller box Computer, display Laser
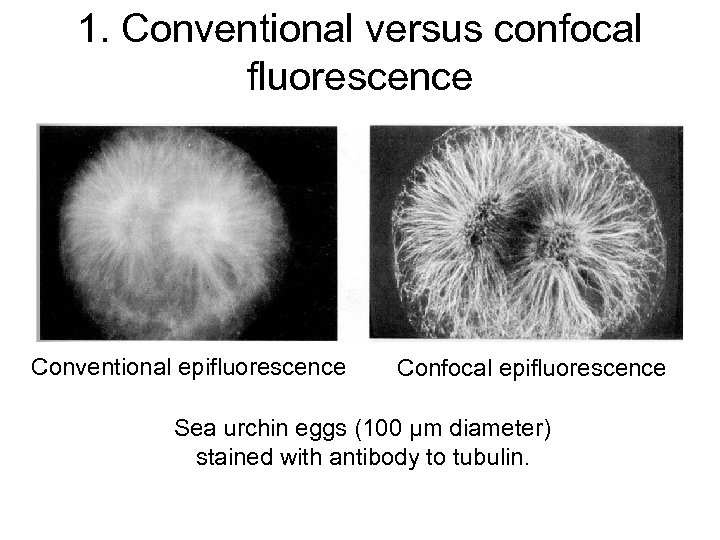 1. Conventional versus confocal fluorescence Conventional epifluorescence Confocal epifluorescence Sea urchin eggs (100 μm diameter) stained with antibody to tubulin.
1. Conventional versus confocal fluorescence Conventional epifluorescence Confocal epifluorescence Sea urchin eggs (100 μm diameter) stained with antibody to tubulin.
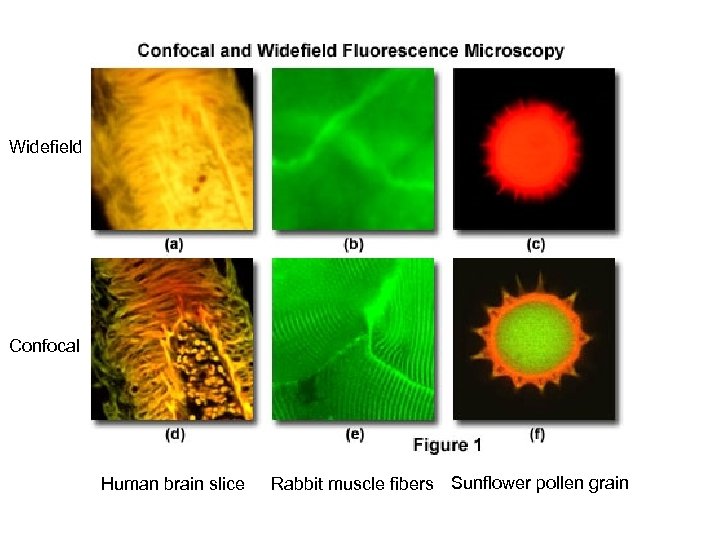 Widefield Confocal Human brain slice Rabbit muscle fibers Sunflower pollen grain
Widefield Confocal Human brain slice Rabbit muscle fibers Sunflower pollen grain
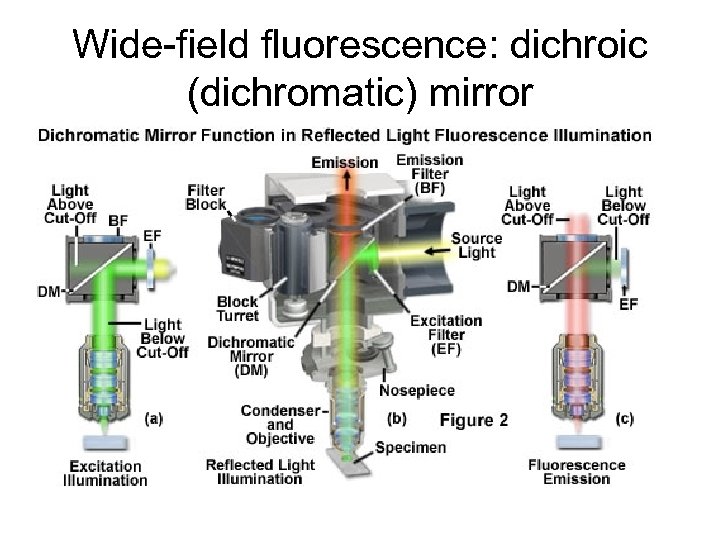 Wide-field fluorescence: dichroic (dichromatic) mirror
Wide-field fluorescence: dichroic (dichromatic) mirror
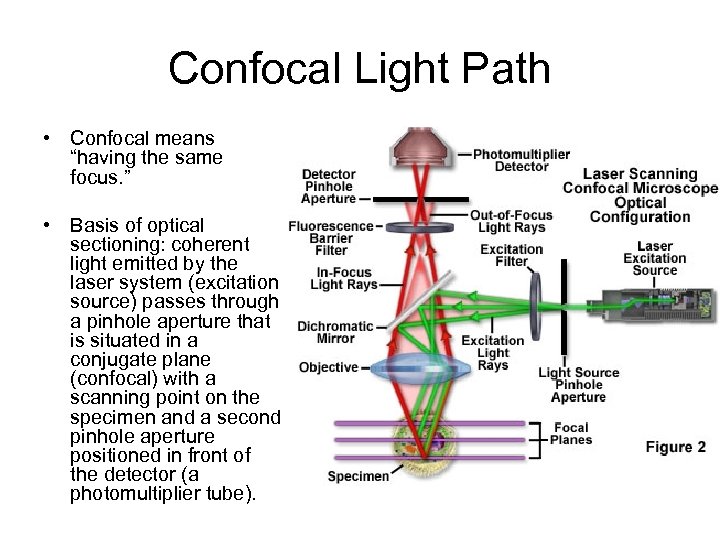 Confocal Light Path • Confocal means “having the same focus. ” • Basis of optical sectioning: coherent light emitted by the laser system (excitation source) passes through a pinhole aperture that is situated in a conjugate plane (confocal) with a scanning point on the specimen and a second pinhole aperture positioned in front of the detector (a photomultiplier tube).
Confocal Light Path • Confocal means “having the same focus. ” • Basis of optical sectioning: coherent light emitted by the laser system (excitation source) passes through a pinhole aperture that is situated in a conjugate plane (confocal) with a scanning point on the specimen and a second pinhole aperture positioned in front of the detector (a photomultiplier tube).
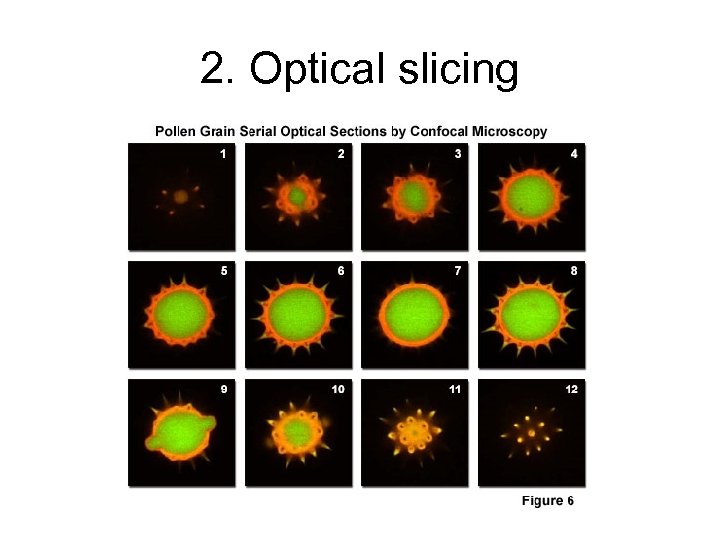 2. Optical slicing
2. Optical slicing
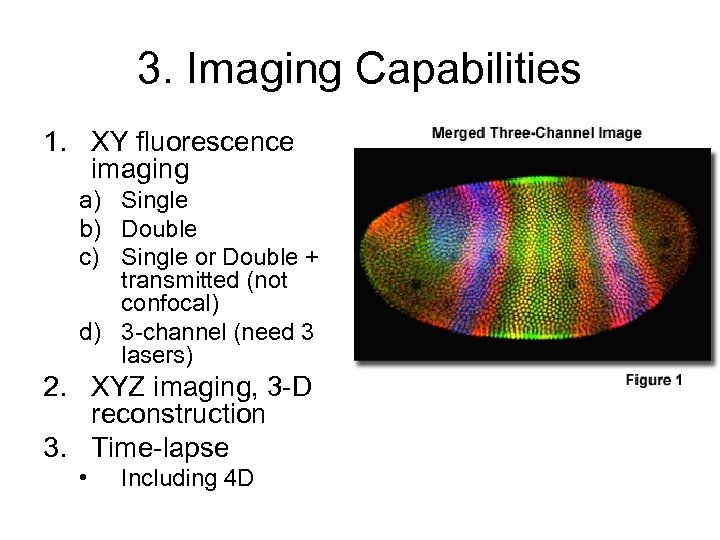 3. Imaging Capabilities 1. XY fluorescence imaging a) Single b) Double c) Single or Double + transmitted (not confocal) d) 3 -channel (need 3 lasers) 2. XYZ imaging, 3 -D reconstruction 3. Time-lapse • Including 4 D
3. Imaging Capabilities 1. XY fluorescence imaging a) Single b) Double c) Single or Double + transmitted (not confocal) d) 3 -channel (need 3 lasers) 2. XYZ imaging, 3 -D reconstruction 3. Time-lapse • Including 4 D
 Applications • • • Immunolabelling Organelle ID Protein trafficking Locating genes on chromosomes Analysis of molecular mobility Multiple labeling Live cell imaging Transmission imaging Measurement of subcellular functions and ion concentrations
Applications • • • Immunolabelling Organelle ID Protein trafficking Locating genes on chromosomes Analysis of molecular mobility Multiple labeling Live cell imaging Transmission imaging Measurement of subcellular functions and ion concentrations
 4. Advantages, limitations of confocal microscopy • Optical sectioning ability – Can image cells/tissues internally • 3 D reconstruction – Improved spatial relationships of structures • Excellent resolution – Close to theoretical limit of LM: 0. 2 μm • Improved multiple labeling – Since specific wavelengths of light used by lasers • Very high sensitivity – Capable of collecting single fluorescent molecule • Easy manipulation and merging of images – Since they are digital • Computer controlled – Complex settings can be programmed and recalled. • Expensive to buy and maintain. – $250, 000 + • Difficult to operate. – Fixed material easy, live difficult. • Fluorescent tag usually required. – May be bulky or toxic • Objects smaller than 0. 2 not resolved – Need to use EM. • Damaging high intensity laser – Need to minimize exposure, especially in live cells. • Digital images are easily mishandled. – Honesty in imaging very important.
4. Advantages, limitations of confocal microscopy • Optical sectioning ability – Can image cells/tissues internally • 3 D reconstruction – Improved spatial relationships of structures • Excellent resolution – Close to theoretical limit of LM: 0. 2 μm • Improved multiple labeling – Since specific wavelengths of light used by lasers • Very high sensitivity – Capable of collecting single fluorescent molecule • Easy manipulation and merging of images – Since they are digital • Computer controlled – Complex settings can be programmed and recalled. • Expensive to buy and maintain. – $250, 000 + • Difficult to operate. – Fixed material easy, live difficult. • Fluorescent tag usually required. – May be bulky or toxic • Objects smaller than 0. 2 not resolved – Need to use EM. • Damaging high intensity laser – Need to minimize exposure, especially in live cells. • Digital images are easily mishandled. – Honesty in imaging very important.
 B. Basic Optics 1. The nature of light • Light behaves as both a particle and a wave. • Can bounce (reflect) and bend (diffract or refract) • Has wave properties – Amplitude – Wavelength: visible is between 400 -700 nm • White light carries all visible wavelengths – Frequency – Direction of travel – Direction of vibration
B. Basic Optics 1. The nature of light • Light behaves as both a particle and a wave. • Can bounce (reflect) and bend (diffract or refract) • Has wave properties – Amplitude – Wavelength: visible is between 400 -700 nm • White light carries all visible wavelengths – Frequency – Direction of travel – Direction of vibration
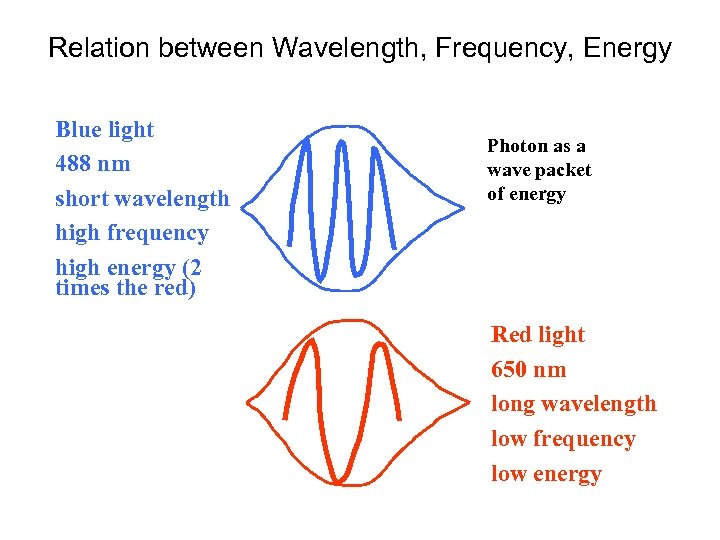 Relation between Wavelength, Frequency, Energy Blue light 488 nm short wavelength high frequency high energy (2 times the red) Photon as a wave packet of energy Red light 650 nm long wavelength low frequency low energy
Relation between Wavelength, Frequency, Energy Blue light 488 nm short wavelength high frequency high energy (2 times the red) Photon as a wave packet of energy Red light 650 nm long wavelength low frequency low energy
 Light-Matter Interactions • Absorption • Reflection • Refraction: bending of light as it passes, at an angle, from one material to another • Diffraction: bending of light as it passes an edge • Fluorescence: spontaneous emission of light after excitation • Polarization • Dispersion
Light-Matter Interactions • Absorption • Reflection • Refraction: bending of light as it passes, at an angle, from one material to another • Diffraction: bending of light as it passes an edge • Fluorescence: spontaneous emission of light after excitation • Polarization • Dispersion
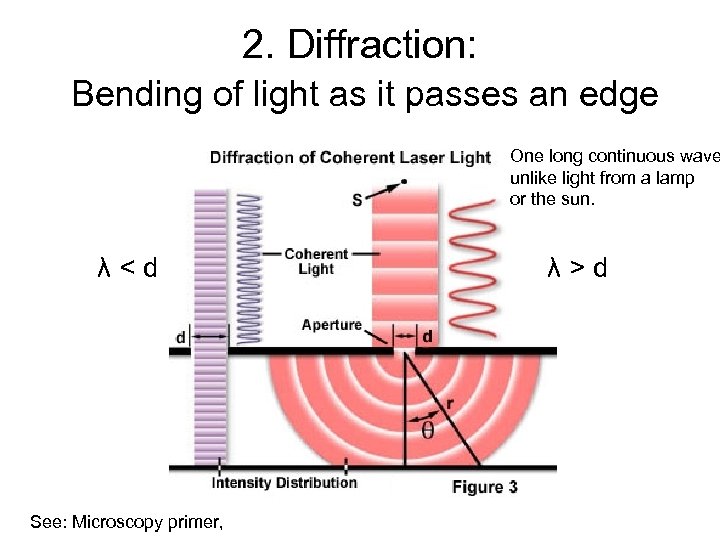 2. Diffraction: Bending of light as it passes an edge One long continuous wave unlike light from a lamp or the sun. λ
2. Diffraction: Bending of light as it passes an edge One long continuous wave unlike light from a lamp or the sun. λ
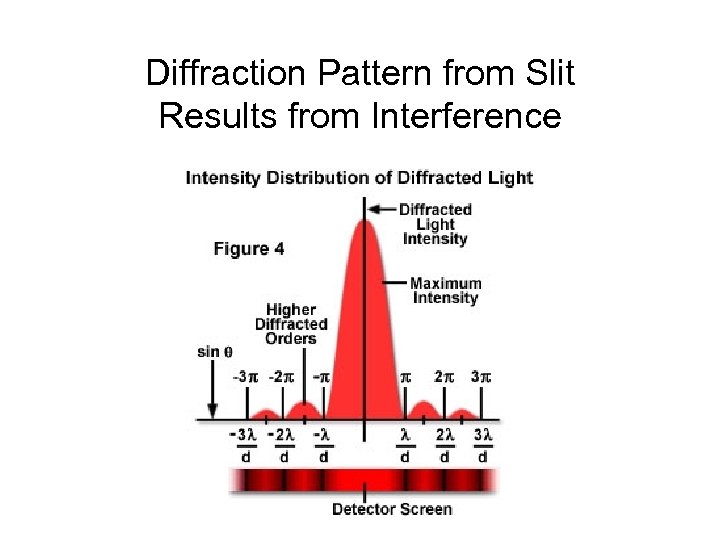 Diffraction Pattern from Slit Results from Interference
Diffraction Pattern from Slit Results from Interference
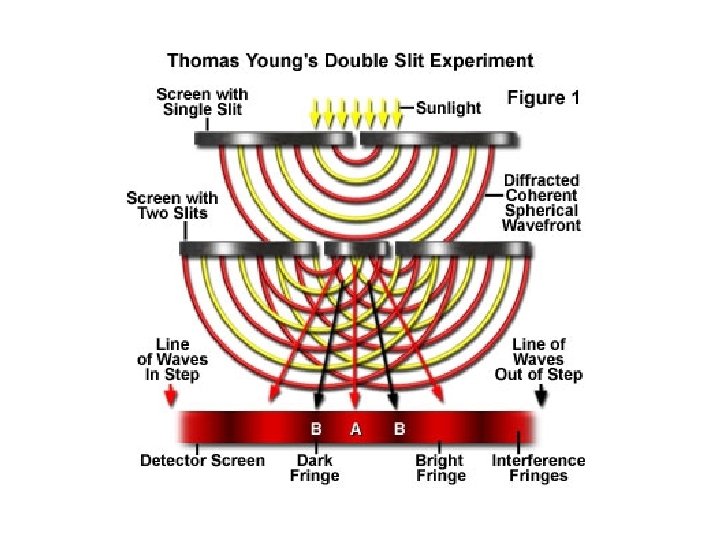
 Java Tutorial: Diffraction Patterns • http: //micro. magnet. fsu. edu/primer/java/diff raction/basicdiffraction/index. html • How does the width of the central maximum vary with the wavelength?
Java Tutorial: Diffraction Patterns • http: //micro. magnet. fsu. edu/primer/java/diff raction/basicdiffraction/index. html • How does the width of the central maximum vary with the wavelength?
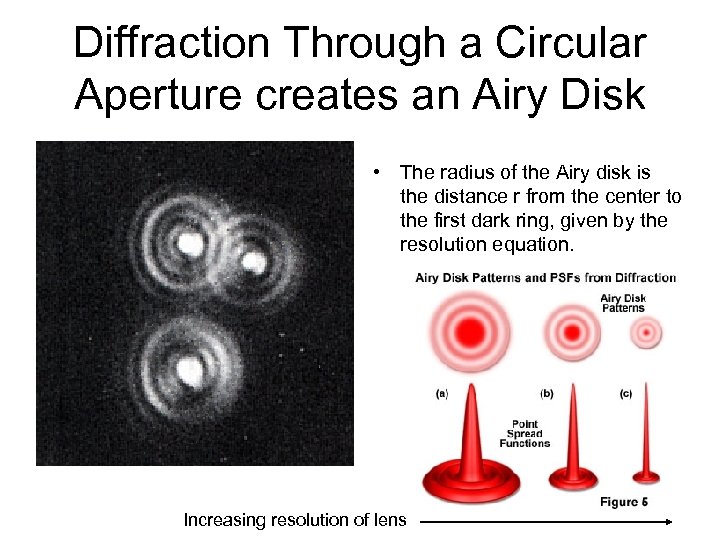 Diffraction Through a Circular Aperture creates an Airy Disk • The radius of the Airy disk is the distance r from the center to the first dark ring, given by the resolution equation. Increasing resolution of lens
Diffraction Through a Circular Aperture creates an Airy Disk • The radius of the Airy disk is the distance r from the center to the first dark ring, given by the resolution equation. Increasing resolution of lens
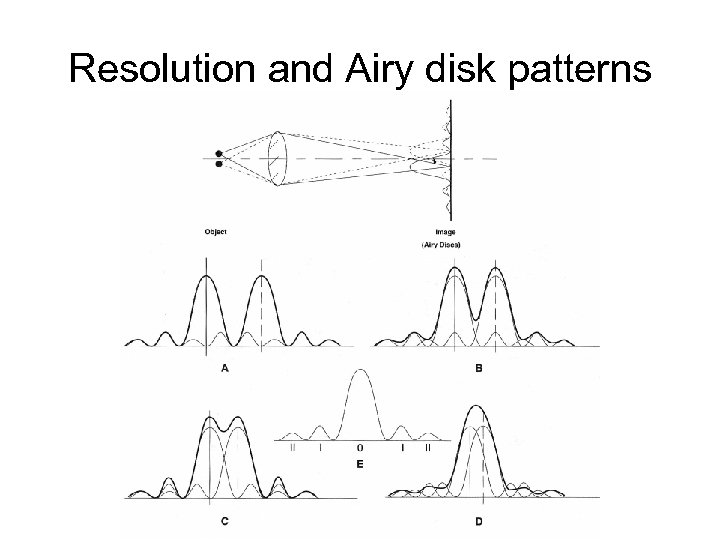 Resolution and Airy disk patterns
Resolution and Airy disk patterns
 Java Tutorial: Airy Pattern Basics • http: //micro. magnet. fsu. edu/primer/java/imagefor mation/airydiskbasics/index. html – How does resolution vary with wavelength and numerical aperture? • http: //micro. magnet. fsu. edu/primer/java/imagefor mation/airyna/index. html – What is the effect of higher NA? • http: //micro. magnet. fsu. edu/primer/java/imagefor mation/rayleighdisks/index. html – What is the Rayleigh criterion?
Java Tutorial: Airy Pattern Basics • http: //micro. magnet. fsu. edu/primer/java/imagefor mation/airydiskbasics/index. html – How does resolution vary with wavelength and numerical aperture? • http: //micro. magnet. fsu. edu/primer/java/imagefor mation/airyna/index. html – What is the effect of higher NA? • http: //micro. magnet. fsu. edu/primer/java/imagefor mation/rayleighdisks/index. html – What is the Rayleigh criterion?
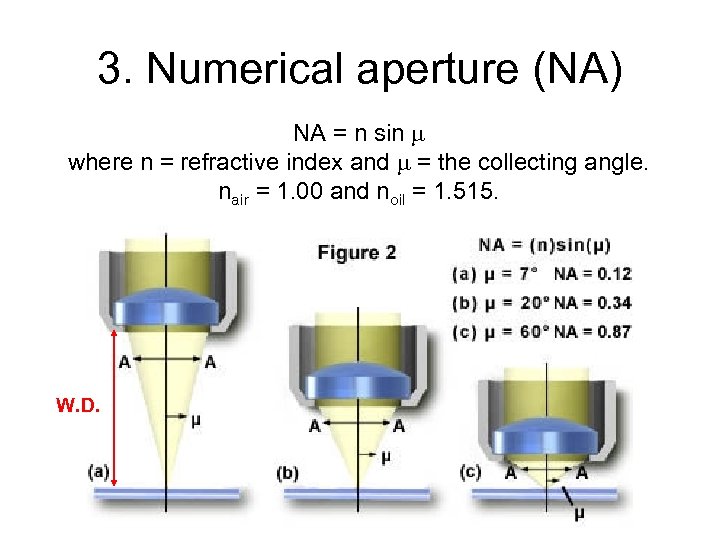 3. Numerical aperture (NA) NA = n sin where n = refractive index and = the collecting angle. nair = 1. 00 and noil = 1. 515. W. D.
3. Numerical aperture (NA) NA = n sin where n = refractive index and = the collecting angle. nair = 1. 00 and noil = 1. 515. W. D.
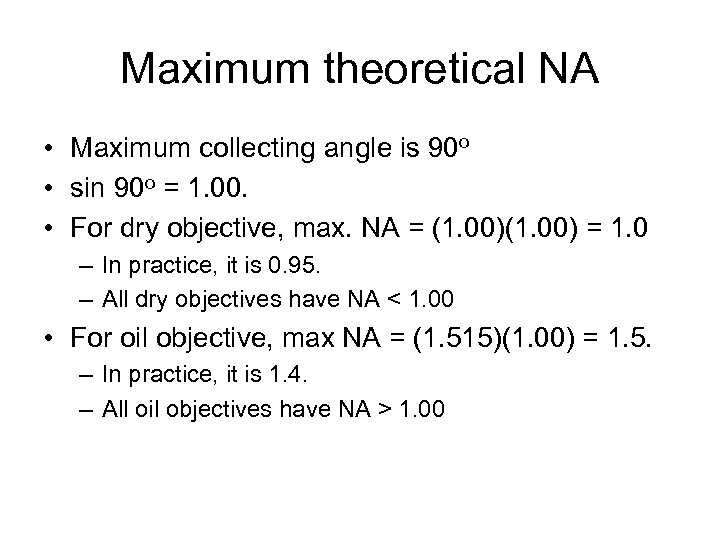 Maximum theoretical NA • Maximum collecting angle is 90 o • sin 90 o = 1. 00. • For dry objective, max. NA = (1. 00) = 1. 0 – In practice, it is 0. 95. – All dry objectives have NA < 1. 00 • For oil objective, max NA = (1. 515)(1. 00) = 1. 5. – In practice, it is 1. 4. – All oil objectives have NA > 1. 00
Maximum theoretical NA • Maximum collecting angle is 90 o • sin 90 o = 1. 00. • For dry objective, max. NA = (1. 00) = 1. 0 – In practice, it is 0. 95. – All dry objectives have NA < 1. 00 • For oil objective, max NA = (1. 515)(1. 00) = 1. 5. – In practice, it is 1. 4. – All oil objectives have NA > 1. 00
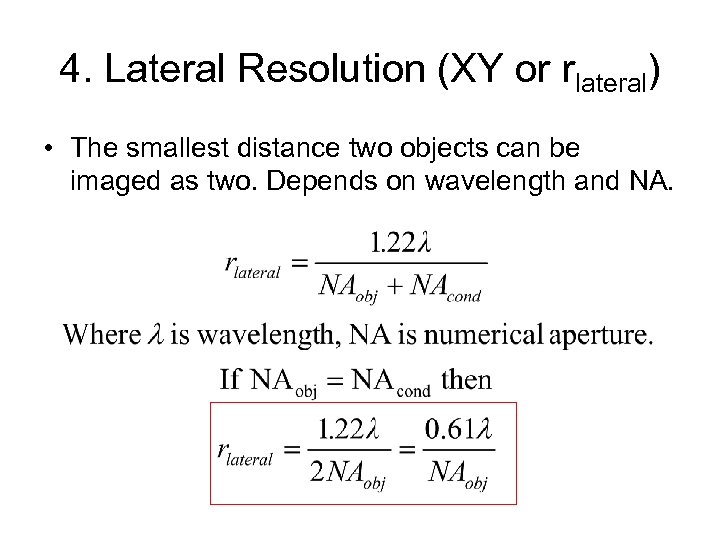 4. Lateral Resolution (XY or rlateral) • The smallest distance two objects can be imaged as two. Depends on wavelength and NA.
4. Lateral Resolution (XY or rlateral) • The smallest distance two objects can be imaged as two. Depends on wavelength and NA.
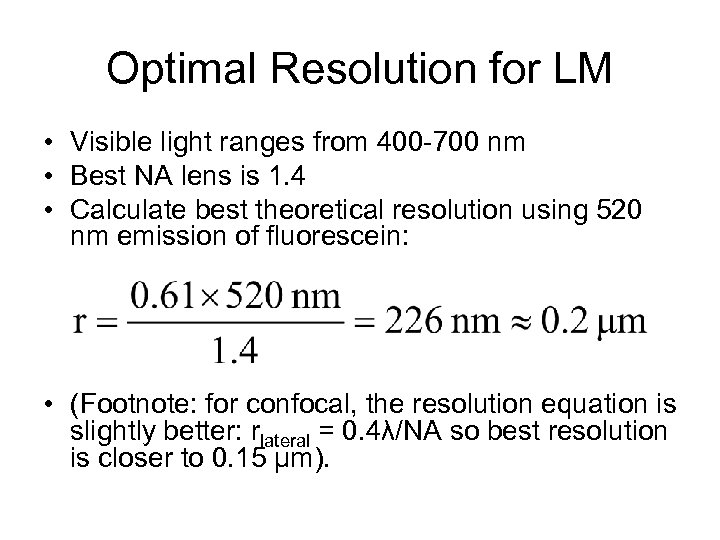 Optimal Resolution for LM • Visible light ranges from 400 -700 nm • Best NA lens is 1. 4 • Calculate best theoretical resolution using 520 nm emission of fluorescein: • (Footnote: for confocal, the resolution equation is slightly better: rlateral = 0. 4λ/NA so best resolution is closer to 0. 15 μm).
Optimal Resolution for LM • Visible light ranges from 400 -700 nm • Best NA lens is 1. 4 • Calculate best theoretical resolution using 520 nm emission of fluorescein: • (Footnote: for confocal, the resolution equation is slightly better: rlateral = 0. 4λ/NA so best resolution is closer to 0. 15 μm).
 XY under- and over-sampling • Optimal zoom settings (for full xy resolution) for 512 X 512 pixel box are given for various lenses on p. 126. – You don’t need to operate at these settings unless you want to push the resolution limit. • Rules of thumb for 1024 X 1024 box: – 60 X 1. 4 NA: 4 X max zoom – 40 X 0. 75 NA: 5 X max zoom – 20 X 0. 7 NA: 6 X max zoom • Zooming higher than this creates empty magnification.
XY under- and over-sampling • Optimal zoom settings (for full xy resolution) for 512 X 512 pixel box are given for various lenses on p. 126. – You don’t need to operate at these settings unless you want to push the resolution limit. • Rules of thumb for 1024 X 1024 box: – 60 X 1. 4 NA: 4 X max zoom – 40 X 0. 75 NA: 5 X max zoom – 20 X 0. 7 NA: 6 X max zoom • Zooming higher than this creates empty magnification.
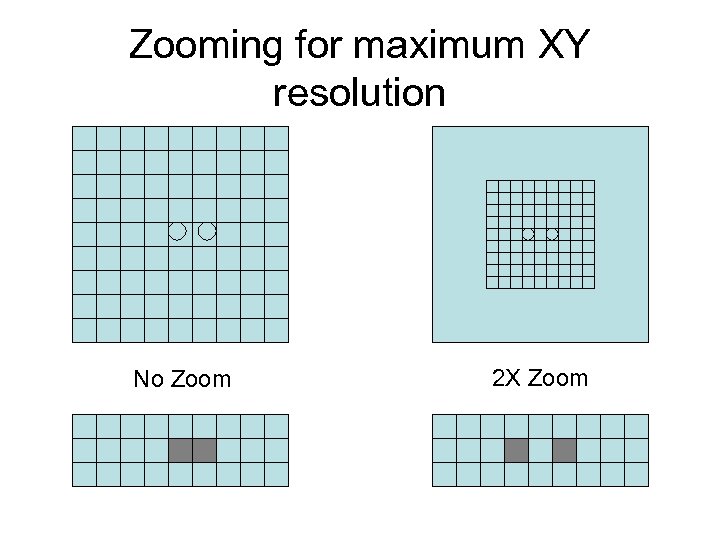 Zooming for maximum XY resolution No Zoom 2 X Zoom
Zooming for maximum XY resolution No Zoom 2 X Zoom
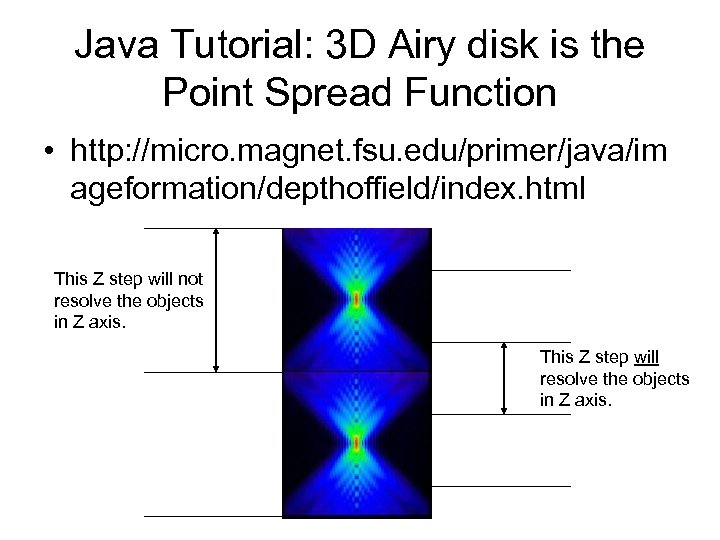 Java Tutorial: 3 D Airy disk is the Point Spread Function • http: //micro. magnet. fsu. edu/primer/java/im ageformation/depthoffield/index. html This Z step will not resolve the objects in Z axis. This Z step will resolve the objects in Z axis.
Java Tutorial: 3 D Airy disk is the Point Spread Function • http: //micro. magnet. fsu. edu/primer/java/im ageformation/depthoffield/index. html This Z step will not resolve the objects in Z axis. This Z step will resolve the objects in Z axis.
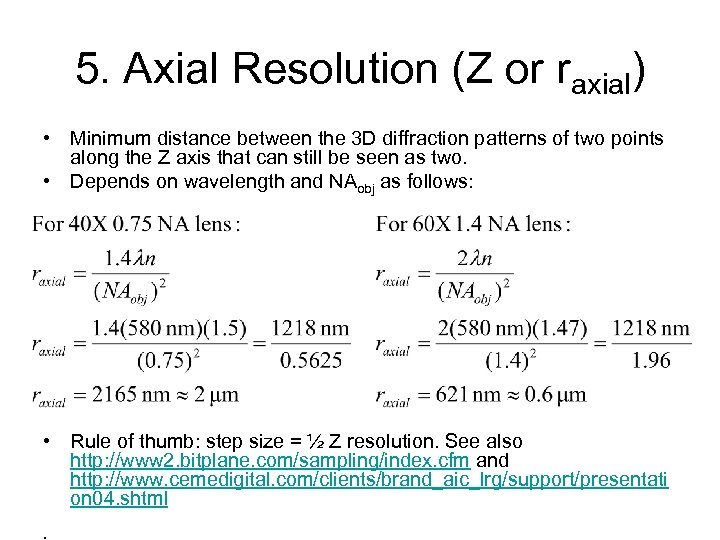 5. Axial Resolution (Z or raxial) • Minimum distance between the 3 D diffraction patterns of two points along the Z axis that can still be seen as two. • Depends on wavelength and NAobj as follows: • Rule of thumb: step size = ½ Z resolution. See also http: //www 2. bitplane. com/sampling/index. cfm and http: //www. cemedigital. com/clients/brand_aic_lrg/support/presentati on 04. shtml
5. Axial Resolution (Z or raxial) • Minimum distance between the 3 D diffraction patterns of two points along the Z axis that can still be seen as two. • Depends on wavelength and NAobj as follows: • Rule of thumb: step size = ½ Z resolution. See also http: //www 2. bitplane. com/sampling/index. cfm and http: //www. cemedigital. com/clients/brand_aic_lrg/support/presentati on 04. shtml
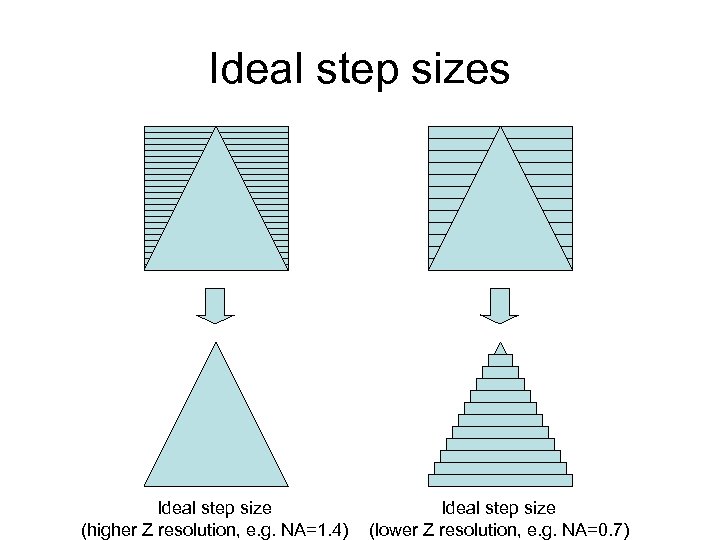 Ideal step sizes Ideal step size (higher Z resolution, e. g. NA=1. 4) Ideal step size (lower Z resolution, e. g. NA=0. 7)
Ideal step sizes Ideal step size (higher Z resolution, e. g. NA=1. 4) Ideal step size (lower Z resolution, e. g. NA=0. 7)
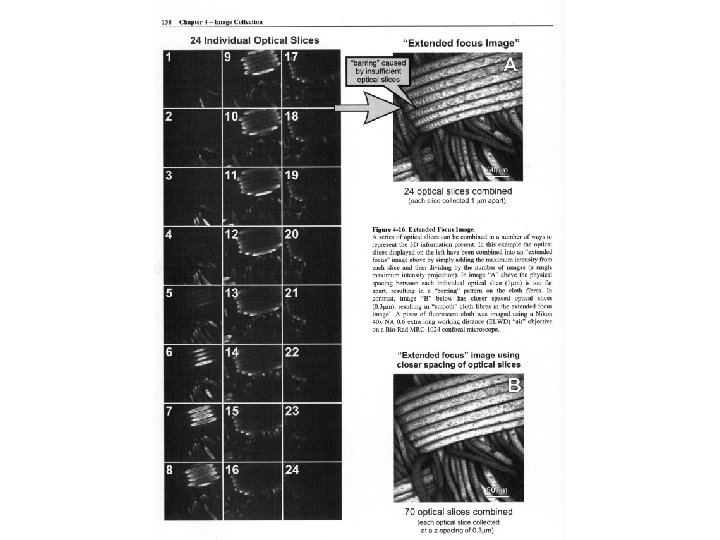
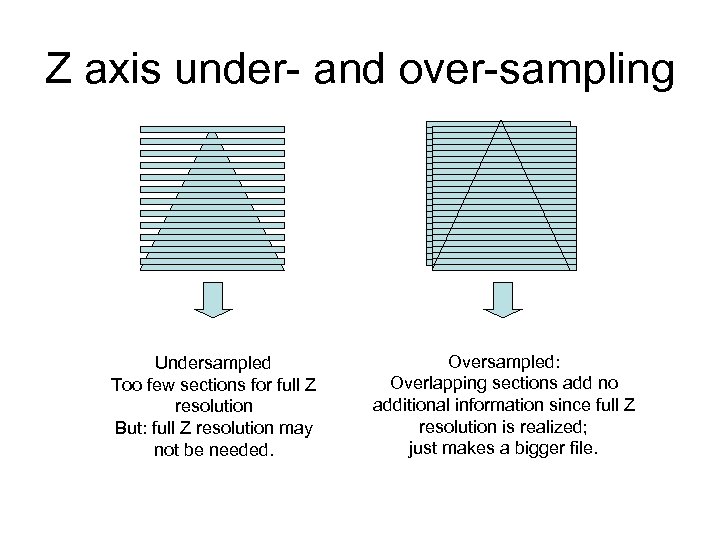 Z axis under- and over-sampling Undersampled Too few sections for full Z resolution But: full Z resolution may not be needed. Oversampled: Overlapping sections add no additional information since full Z resolution is realized; just makes a bigger file.
Z axis under- and over-sampling Undersampled Too few sections for full Z resolution But: full Z resolution may not be needed. Oversampled: Overlapping sections add no additional information since full Z resolution is realized; just makes a bigger file.
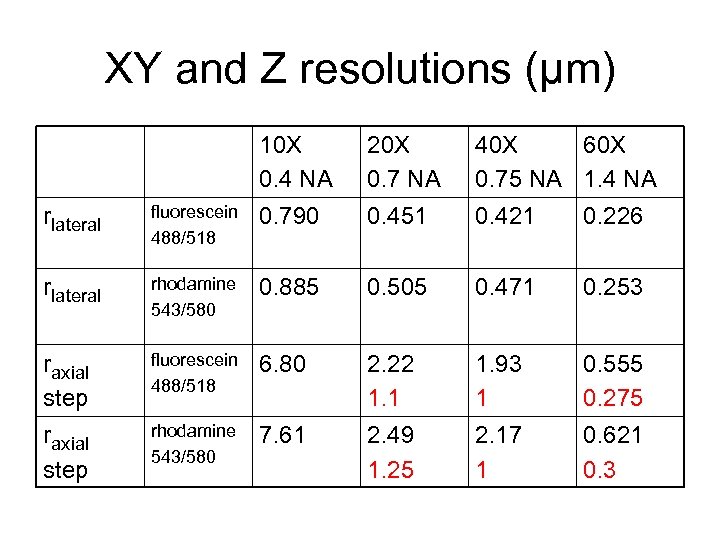 XY and Z resolutions (μm) 10 X 0. 4 NA 20 X 0. 7 NA 40 X 60 X 0. 75 NA 1. 4 NA rlateral fluorescein 488/518 0. 790 0. 451 0. 421 0. 226 rlateral rhodamine 543/580 0. 885 0. 505 0. 471 0. 253 raxial step fluorescein 488/518 6. 80 2. 22 1. 1 1. 93 1 0. 555 0. 275 raxial step rhodamine 543/580 7. 61 2. 49 1. 25 2. 17 1 0. 621 0. 3
XY and Z resolutions (μm) 10 X 0. 4 NA 20 X 0. 7 NA 40 X 60 X 0. 75 NA 1. 4 NA rlateral fluorescein 488/518 0. 790 0. 451 0. 421 0. 226 rlateral rhodamine 543/580 0. 885 0. 505 0. 471 0. 253 raxial step fluorescein 488/518 6. 80 2. 22 1. 1 1. 93 1 0. 555 0. 275 raxial step rhodamine 543/580 7. 61 2. 49 1. 25 2. 17 1 0. 621 0. 3
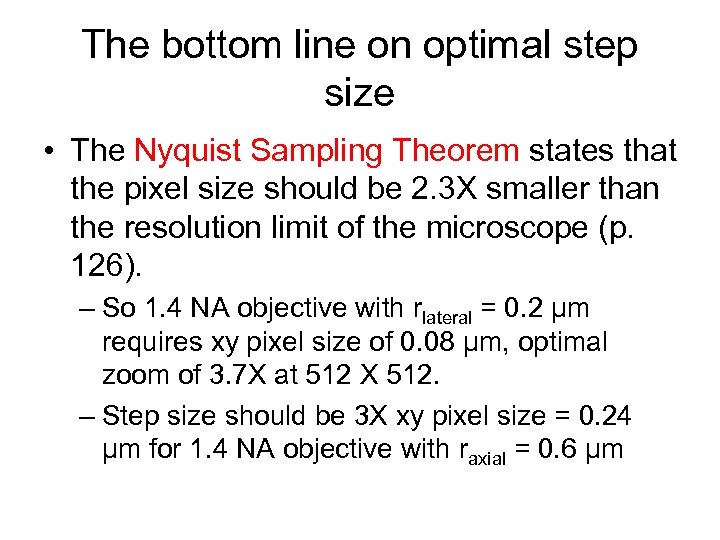 The bottom line on optimal step size • The Nyquist Sampling Theorem states that the pixel size should be 2. 3 X smaller than the resolution limit of the microscope (p. 126). – So 1. 4 NA objective with rlateral = 0. 2 μm requires xy pixel size of 0. 08 μm, optimal zoom of 3. 7 X at 512 X 512. – Step size should be 3 X xy pixel size = 0. 24 μm for 1. 4 NA objective with raxial = 0. 6 μm
The bottom line on optimal step size • The Nyquist Sampling Theorem states that the pixel size should be 2. 3 X smaller than the resolution limit of the microscope (p. 126). – So 1. 4 NA objective with rlateral = 0. 2 μm requires xy pixel size of 0. 08 μm, optimal zoom of 3. 7 X at 512 X 512. – Step size should be 3 X xy pixel size = 0. 24 μm for 1. 4 NA objective with raxial = 0. 6 μm
 Week 3 TBA • Assignment (each person): – – – Collect Z-series of one of your Artemia samples, using the 20 X lens and a step size of 1 or 2 um. Display the sections in tile mode. Save (as a normal TIFFs) extended focus images in black and white, showing (a) every section of the Zseries, (b) the top 1/3, (c) the middle 1/3, and d) the bottom 1/3. • • – Always include a scale bar on your images. Save in the BIO 553 file on the imaging computer. Turn in a description of your images using the form available on Blackboard.
Week 3 TBA • Assignment (each person): – – – Collect Z-series of one of your Artemia samples, using the 20 X lens and a step size of 1 or 2 um. Display the sections in tile mode. Save (as a normal TIFFs) extended focus images in black and white, showing (a) every section of the Zseries, (b) the top 1/3, (c) the middle 1/3, and d) the bottom 1/3. • • – Always include a scale bar on your images. Save in the BIO 553 file on the imaging computer. Turn in a description of your images using the form available on Blackboard.
 Paper discussion • • • Today, Jan. 22: Zucker 2006 (Hertzler) Jan. 29: (Hertzler) Feb. 5: Feb. 12: Feb. 19: Feb. 26:
Paper discussion • • • Today, Jan. 22: Zucker 2006 (Hertzler) Jan. 29: (Hertzler) Feb. 5: Feb. 12: Feb. 19: Feb. 26:


