a40b1de9c335d57189b67b5175f65cb9.ppt
- Количество слайдов: 57
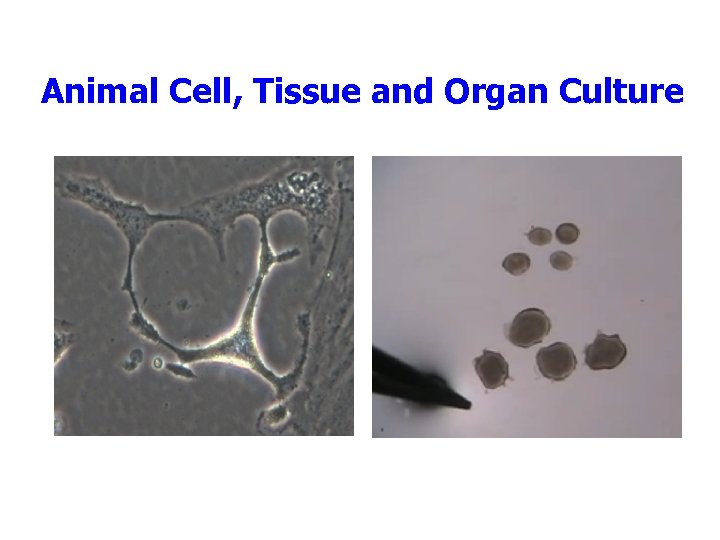 Animal Cell, Tissue and Organ Culture
Animal Cell, Tissue and Organ Culture
 What is cell culture? v Cell culture refers to the removal of cells from a mammal or an animal, and their subsequent growth in a favorable artificial environment. v The cells may be removed from the tissue directly and disaggregated by enzymatic or mechanical means before cultivation, or they may be derived from a cell line or cell strain that has already been established. v Basically, mammalian/animal cell culture techniques are similar to those employed for bacteria, fungi, and yeast, although there are some characteristic differences. v In general, mammalian cells are more delicate, vulnerable to mechanical damage, present lower growth rates, and require more complex culture media and special substrates.
What is cell culture? v Cell culture refers to the removal of cells from a mammal or an animal, and their subsequent growth in a favorable artificial environment. v The cells may be removed from the tissue directly and disaggregated by enzymatic or mechanical means before cultivation, or they may be derived from a cell line or cell strain that has already been established. v Basically, mammalian/animal cell culture techniques are similar to those employed for bacteria, fungi, and yeast, although there are some characteristic differences. v In general, mammalian cells are more delicate, vulnerable to mechanical damage, present lower growth rates, and require more complex culture media and special substrates.
 Cell Culture in vitro - A brief history Ø 1885: Roux maintained embryonic chick cells alive in saline solution for short lengths of time. Ø 1912: Alexis Carrel cultured connective tissue and showed heart muscle tissue contractility over 2 -3 months. Ø 1943: Earle et al. produced continuous rat cell line. Ø 1962: Buonassisi et al. Published methods for maintaining differentiated cells of tumour origin. Ø 1970: Gordon Sato et al. published the specific growth factor and media requirements for many cell types. Ø 1979: Bottenstein and Sato defined a serum-free medium for neural cells. Ø 1980: Chinese Hamster Ovary (CHO) cell lines were developed. Recombinant erythropoietin was produced on CHO cell by AMGEN (USA).
Cell Culture in vitro - A brief history Ø 1885: Roux maintained embryonic chick cells alive in saline solution for short lengths of time. Ø 1912: Alexis Carrel cultured connective tissue and showed heart muscle tissue contractility over 2 -3 months. Ø 1943: Earle et al. produced continuous rat cell line. Ø 1962: Buonassisi et al. Published methods for maintaining differentiated cells of tumour origin. Ø 1970: Gordon Sato et al. published the specific growth factor and media requirements for many cell types. Ø 1979: Bottenstein and Sato defined a serum-free medium for neural cells. Ø 1980: Chinese Hamster Ovary (CHO) cell lines were developed. Recombinant erythropoietin was produced on CHO cell by AMGEN (USA).
 Acquiring cell cultures or cell lines Ø Any laboratory may establish their own culture from primary cells, or may choose to buy established cell cultures from commercial or non-profit suppliers (i. e. , cell banks). Ø Various cell lines including those from insects, humans, mice, rats, and other mammals are commercially available to purchase. Ø Reputable suppliers provide high quality cell lines that are carefully tested for their integrity and to ensure that the culture is free from any contaminants. Ø Regardless of their source, it is advised to make sure that all new cell lines are tested for mycoplasma contamination before use them for any experiment.
Acquiring cell cultures or cell lines Ø Any laboratory may establish their own culture from primary cells, or may choose to buy established cell cultures from commercial or non-profit suppliers (i. e. , cell banks). Ø Various cell lines including those from insects, humans, mice, rats, and other mammals are commercially available to purchase. Ø Reputable suppliers provide high quality cell lines that are carefully tested for their integrity and to ensure that the culture is free from any contaminants. Ø Regardless of their source, it is advised to make sure that all new cell lines are tested for mycoplasma contamination before use them for any experiment.
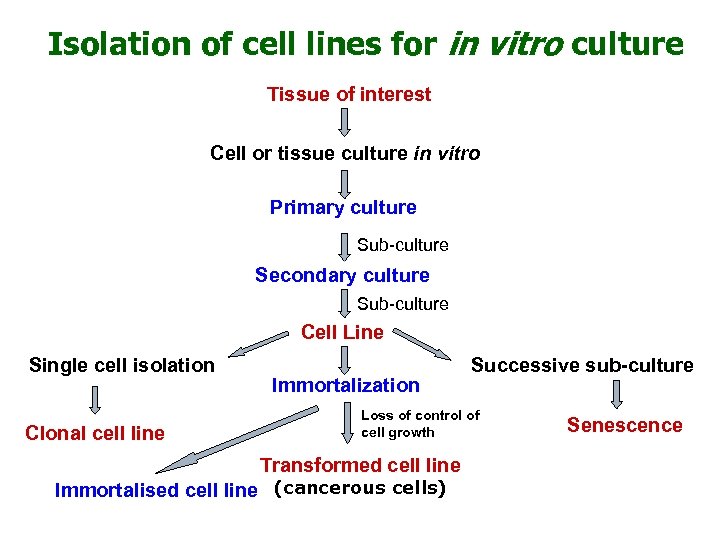 Isolation of cell lines for in vitro culture Tissue of interest Cell or tissue culture in vitro Primary culture Sub-culture Secondary culture Sub-culture Cell Line Single cell isolation Clonal cell line Immortalization Successive sub-culture Loss of control of cell growth Transformed cell line Immortalised cell line (cancerous cells) Senescence
Isolation of cell lines for in vitro culture Tissue of interest Cell or tissue culture in vitro Primary culture Sub-culture Secondary culture Sub-culture Cell Line Single cell isolation Clonal cell line Immortalization Successive sub-culture Loss of control of cell growth Transformed cell line Immortalised cell line (cancerous cells) Senescence
 Finite vs Continuous Cell Line Ø Normal cells usually divide only a limited number of times before losing their ability to proliferate, which is a genetically determined event known as senescence; these cell lines are known as finite. Ø However, some cell lines become immortal through a process called transformation, which can occur spontaneously or can be chemically or virally induced. When a finite cell line undergoes transformation and acquires the ability to divide indefinitely, it becomes a continuous cell line.
Finite vs Continuous Cell Line Ø Normal cells usually divide only a limited number of times before losing their ability to proliferate, which is a genetically determined event known as senescence; these cell lines are known as finite. Ø However, some cell lines become immortal through a process called transformation, which can occur spontaneously or can be chemically or virally induced. When a finite cell line undergoes transformation and acquires the ability to divide indefinitely, it becomes a continuous cell line.
 Primary cell cultures or cell lines Ø Primary culture refers to the stage of the culture after the cells are isolated from the tissue and proliferated under the appropriate conditions until they occupy all of the available substrate (i. e. , reach confluence) and retains differentiated phenotype. - At this stage, the cells have to be sub-cultured (i. e. , passaged) by transferring them to a new vessel with fresh growth medium to provide more room for continued growth. Ø Cell lines derived from primary cultures have a limited life span (i. e. , they are finite) and as they are passaged, cells with the highest growth capacity predominate, resulting in a degree of genotypic and phenotypic uniformity in the population.
Primary cell cultures or cell lines Ø Primary culture refers to the stage of the culture after the cells are isolated from the tissue and proliferated under the appropriate conditions until they occupy all of the available substrate (i. e. , reach confluence) and retains differentiated phenotype. - At this stage, the cells have to be sub-cultured (i. e. , passaged) by transferring them to a new vessel with fresh growth medium to provide more room for continued growth. Ø Cell lines derived from primary cultures have a limited life span (i. e. , they are finite) and as they are passaged, cells with the highest growth capacity predominate, resulting in a degree of genotypic and phenotypic uniformity in the population.
 Secondary cultures • Derived from a primary cell culture and isolated by selection or cloning. • Becoming a more homogeneous cell population. • Finite life span in vitro. • Retain differentiated phenotype. • Mainly anchorage dependant. • Exhibit contact inhibition. Ø Contact inhibition is a growth mechanism. In most cases when two cells collide they attempt to move in a different direction to avoid future collisions.
Secondary cultures • Derived from a primary cell culture and isolated by selection or cloning. • Becoming a more homogeneous cell population. • Finite life span in vitro. • Retain differentiated phenotype. • Mainly anchorage dependant. • Exhibit contact inhibition. Ø Contact inhibition is a growth mechanism. In most cases when two cells collide they attempt to move in a different direction to avoid future collisions.
 Contact inhibition of growth Normal somatic cells Ø Normal somatic cells when grown in culture will become growth inhibited when they encounter another cell. The cells in our bodies are governed by growth control mechanisms and cellular senescence (aging). Cell aging puts a limit on the number of times a cell can divide: the more a cell has divided, the less likely it will be to divide again. Growth mechanisms are in place to stop or continue cell growth depending on the conditions. Cancerous Cells Ø Cancerous cells typically lose this property and thus grow in an uncontrolled manner even when in contact with neighboring cells. They aren't motivated to change direction upon contact, so they pile up and grow over each other.
Contact inhibition of growth Normal somatic cells Ø Normal somatic cells when grown in culture will become growth inhibited when they encounter another cell. The cells in our bodies are governed by growth control mechanisms and cellular senescence (aging). Cell aging puts a limit on the number of times a cell can divide: the more a cell has divided, the less likely it will be to divide again. Growth mechanisms are in place to stop or continue cell growth depending on the conditions. Cancerous Cells Ø Cancerous cells typically lose this property and thus grow in an uncontrolled manner even when in contact with neighboring cells. They aren't motivated to change direction upon contact, so they pile up and grow over each other.
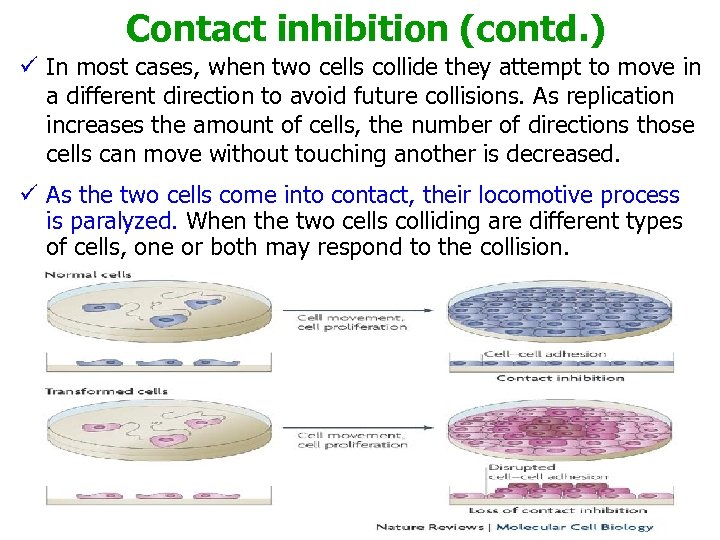 Contact inhibition (contd. ) ü In most cases, when two cells collide they attempt to move in a different direction to avoid future collisions. As replication increases the amount of cells, the number of directions those cells can move without touching another is decreased. ü As the two cells come into contact, their locomotive process is paralyzed. When the two cells colliding are different types of cells, one or both may respond to the collision.
Contact inhibition (contd. ) ü In most cases, when two cells collide they attempt to move in a different direction to avoid future collisions. As replication increases the amount of cells, the number of directions those cells can move without touching another is decreased. ü As the two cells come into contact, their locomotive process is paralyzed. When the two cells colliding are different types of cells, one or both may respond to the collision.
 Continuous cultures Ø Derived from a primary or secondary culture. Ø Immortalised: - Spontaneously (e. g. : spontaneous genetic mutation). - By transformation vectors (e. g. : viruses &/or plasmids). Ø Serially propagated culture shows an increased growth rate and shows homogeneous cell population. Ø Loss of anchorage dependency and contact inhibition. Ø Infinite life span in vitro. Ø Differentiated phenotype: very little retained with transformed cell lines (cancerous cells).
Continuous cultures Ø Derived from a primary or secondary culture. Ø Immortalised: - Spontaneously (e. g. : spontaneous genetic mutation). - By transformation vectors (e. g. : viruses &/or plasmids). Ø Serially propagated culture shows an increased growth rate and shows homogeneous cell population. Ø Loss of anchorage dependency and contact inhibition. Ø Infinite life span in vitro. Ø Differentiated phenotype: very little retained with transformed cell lines (cancerous cells).
 Cell lines used for in vitro culture are two types 1. Anchorage dependant - Most animal derived cells. - Adhere to bottom of a flask and form a monolayer. - Eventually cover entire surface of substratum. - Proliferation then stops. - Need to subculture cells to fresh medium. - Proliferation can begin again. 2. Anchorage independent - Cells associated with body fluid (eg. blood cells). - Grown in suspension. - Will eventually need sub-culturing.
Cell lines used for in vitro culture are two types 1. Anchorage dependant - Most animal derived cells. - Adhere to bottom of a flask and form a monolayer. - Eventually cover entire surface of substratum. - Proliferation then stops. - Need to subculture cells to fresh medium. - Proliferation can begin again. 2. Anchorage independent - Cells associated with body fluid (eg. blood cells). - Grown in suspension. - Will eventually need sub-culturing.
 Mammalian cell morphology Most mammalian cells in culture can be divided into three basic categories based on their morphology: 1. Fibroblastic (or fibroblast-like) cells are bipolar or multipolar, have elongated shapes and grow attached to the surface of growth container. 2. Epithelial-like cells are polygonal in shape with more regular dimensions and grow attached to the surface of growth container in discrete patches. 3. Lymphoblast-like cells are spherical in shape and usually grown in suspension without attaching to a surface. Fibroblastic cells Epithelial-like cells Lymphoblast-like cells
Mammalian cell morphology Most mammalian cells in culture can be divided into three basic categories based on their morphology: 1. Fibroblastic (or fibroblast-like) cells are bipolar or multipolar, have elongated shapes and grow attached to the surface of growth container. 2. Epithelial-like cells are polygonal in shape with more regular dimensions and grow attached to the surface of growth container in discrete patches. 3. Lymphoblast-like cells are spherical in shape and usually grown in suspension without attaching to a surface. Fibroblastic cells Epithelial-like cells Lymphoblast-like cells
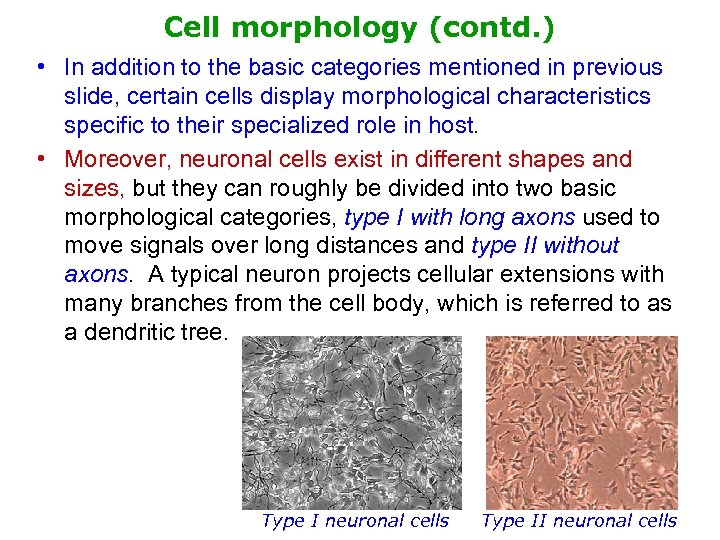 Cell morphology (contd. ) • In addition to the basic categories mentioned in previous slide, certain cells display morphological characteristics specific to their specialized role in host. • Moreover, neuronal cells exist in different shapes and sizes, but they can roughly be divided into two basic morphological categories, type I with long axons used to move signals over long distances and type II without axons. A typical neuron projects cellular extensions with many branches from the cell body, which is referred to as a dendritic tree. Type I neuronal cells Type II neuronal cells
Cell morphology (contd. ) • In addition to the basic categories mentioned in previous slide, certain cells display morphological characteristics specific to their specialized role in host. • Moreover, neuronal cells exist in different shapes and sizes, but they can roughly be divided into two basic morphological categories, type I with long axons used to move signals over long distances and type II without axons. A typical neuron projects cellular extensions with many branches from the cell body, which is referred to as a dendritic tree. Type I neuronal cells Type II neuronal cells
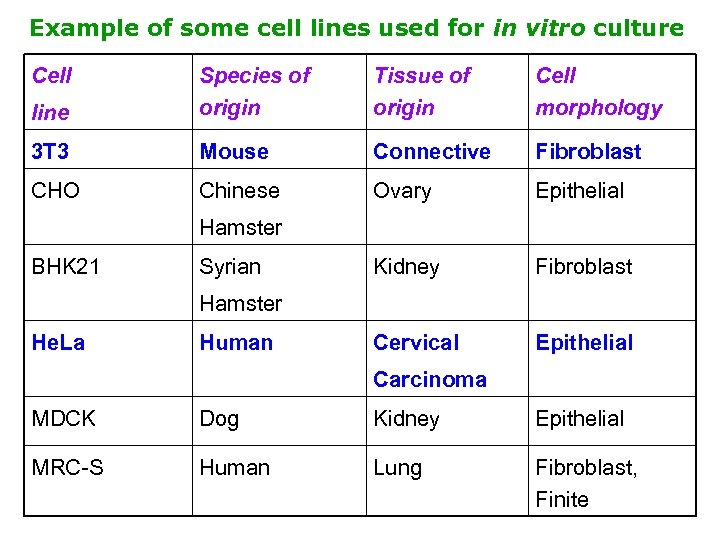 Example of some cell lines used for in vitro culture Cell line Species of origin Tissue of origin Cell morphology 3 T 3 Mouse Connective Fibroblast CHO Chinese Ovary Epithelial Kidney Fibroblast Cervical Epithelial Hamster BHK 21 Syrian Hamster He. La Human Carcinoma MDCK Dog Kidney Epithelial MRC-S Human Lung Fibroblast, Finite
Example of some cell lines used for in vitro culture Cell line Species of origin Tissue of origin Cell morphology 3 T 3 Mouse Connective Fibroblast CHO Chinese Ovary Epithelial Kidney Fibroblast Cervical Epithelial Hamster BHK 21 Syrian Hamster He. La Human Carcinoma MDCK Dog Kidney Epithelial MRC-S Human Lung Fibroblast, Finite
 Applications of Mammalian Cell Culture Ø Cell culture is one of the major tools used in cellular and molecular biology, providing excellent model systems for studying the normal physiology and biochemistry of cells (e. g. , metabolic studies, aging), the effects of drugs and toxic compounds on the cells, and mutagenesis as well as carcinogenesis. Ø It is also used in drug screening and development, and large scale manufacturing of biological compounds (e. g. , vaccines, therapeutic proteins). Ø The major advantage of using cell culture for any of these applications is the consistency and reproducibility of results that can be obtained from using a batch of clonal cells.
Applications of Mammalian Cell Culture Ø Cell culture is one of the major tools used in cellular and molecular biology, providing excellent model systems for studying the normal physiology and biochemistry of cells (e. g. , metabolic studies, aging), the effects of drugs and toxic compounds on the cells, and mutagenesis as well as carcinogenesis. Ø It is also used in drug screening and development, and large scale manufacturing of biological compounds (e. g. , vaccines, therapeutic proteins). Ø The major advantage of using cell culture for any of these applications is the consistency and reproducibility of results that can be obtained from using a batch of clonal cells.
 What do the cells need to grow? Culture conditions vary widely for each cell type, but the artificial environment in which the cells are cultured invariably consists of a suitable vessel containing a substrate or medium that - supplies the essential nutrients (amino acids, carbohydrates, vitamins, minerals), growth factors, hormones, and gases (O 2, CO 2), and - regulates the physicochemical environment (p. H, osmotic pressure, temperature). Anchorage dependent must be cultured while attached to a solid or semi-solid substrate (adherent or monolayer culture), while others can be grown floating in the culture medium (suspension culture). Sterility (aseptic technique, antibiotics and antimycotics) - Mycoplasma contamination must be tested.
What do the cells need to grow? Culture conditions vary widely for each cell type, but the artificial environment in which the cells are cultured invariably consists of a suitable vessel containing a substrate or medium that - supplies the essential nutrients (amino acids, carbohydrates, vitamins, minerals), growth factors, hormones, and gases (O 2, CO 2), and - regulates the physicochemical environment (p. H, osmotic pressure, temperature). Anchorage dependent must be cultured while attached to a solid or semi-solid substrate (adherent or monolayer culture), while others can be grown floating in the culture medium (suspension culture). Sterility (aseptic technique, antibiotics and antimycotics) - Mycoplasma contamination must be tested.
 Humid CO 2 incubator v A good CO 2 incubator is expensive. v A controlled atmosphere is achieved by using a humidifying tray and controlling the CO 2 tension with a CO 2 -monitoring device, which draws air from the incubator into a sample chamber, determines the concentration of CO 2, and injects pure CO 2 into the incubator to make up any deficiency. v The incubator should be large enough (ranging 50 -200 liters, and have forced air circulation, temperature control and a safety thermostat to protect from overheating or poor heating.
Humid CO 2 incubator v A good CO 2 incubator is expensive. v A controlled atmosphere is achieved by using a humidifying tray and controlling the CO 2 tension with a CO 2 -monitoring device, which draws air from the incubator into a sample chamber, determines the concentration of CO 2, and injects pure CO 2 into the incubator to make up any deficiency. v The incubator should be large enough (ranging 50 -200 liters, and have forced air circulation, temperature control and a safety thermostat to protect from overheating or poor heating.
 Nutrients (culture media) Basal Media are used to maintain p. H and osmolarity (260320 m. Osm/L) and provide nutrients and energy source. The components of basal media are as follows. Inorganic Salts • Maintain osmolarity. • Regulate membrane potential (Na+, K+, Ca 2+). • Provide ions for cell attachment and enzyme cofactors. p. H Indicator - Phenol Red • Optimum cell growth occurs at approx. p. H 7. 4 • Phenol red is used to monitor the changes from red to yellow Buffers (Bicarbonate and HEPES) • Bicarbonate buffered media requires CO 2 atmosphere. But, HEPES does not require CO 2.
Nutrients (culture media) Basal Media are used to maintain p. H and osmolarity (260320 m. Osm/L) and provide nutrients and energy source. The components of basal media are as follows. Inorganic Salts • Maintain osmolarity. • Regulate membrane potential (Na+, K+, Ca 2+). • Provide ions for cell attachment and enzyme cofactors. p. H Indicator - Phenol Red • Optimum cell growth occurs at approx. p. H 7. 4 • Phenol red is used to monitor the changes from red to yellow Buffers (Bicarbonate and HEPES) • Bicarbonate buffered media requires CO 2 atmosphere. But, HEPES does not require CO 2.
 Nutrients (contd. ) Keto acids (oxalacetate and pyruvate) - Intermediate in Glycolysis/Krebs cycle - Keto acids added to the media as additional energy source - Maintain maximum cell metabolism Carbohydrates - Energy source - Glucose and galactose - Low (1 g/L) and high (4. 5 g/L) concentrations of sugars in basal media Vitamins - Precursors for numerous co-factors - B group vitamins necessary for cell growth and proliferation - Common vitamins found in basal media are riboflavin, thiamine and biotin Trace Elements - Zinc, copper, selenium and tricarboxylic acid intermediates
Nutrients (contd. ) Keto acids (oxalacetate and pyruvate) - Intermediate in Glycolysis/Krebs cycle - Keto acids added to the media as additional energy source - Maintain maximum cell metabolism Carbohydrates - Energy source - Glucose and galactose - Low (1 g/L) and high (4. 5 g/L) concentrations of sugars in basal media Vitamins - Precursors for numerous co-factors - B group vitamins necessary for cell growth and proliferation - Common vitamins found in basal media are riboflavin, thiamine and biotin Trace Elements - Zinc, copper, selenium and tricarboxylic acid intermediates
 Supplements to basal media L-glutamine - Essential amino acid (not synthesised by the cell) - Energy source (citric acid cycle), used in protein synthesis - Unstable in liquid media - added as a supplement Non-essential amino acids (NEAA) - Energy source, used in protein synthesis - May reduce metabolic burden on cells Growth Factors and Hormones (e. g. : insulin) - Stimulate glucose transport and utilization - Uptake of amino acids - Maintenance of differentiation Antibiotics and Antimycotics - Penicillin, streptomycin, gentamicin, amphotericin B - Reduce the risk of bacterial and fungal contamination - Cells can become antibiotic resistant - changing phenotype - Preferably avoided in long term culture
Supplements to basal media L-glutamine - Essential amino acid (not synthesised by the cell) - Energy source (citric acid cycle), used in protein synthesis - Unstable in liquid media - added as a supplement Non-essential amino acids (NEAA) - Energy source, used in protein synthesis - May reduce metabolic burden on cells Growth Factors and Hormones (e. g. : insulin) - Stimulate glucose transport and utilization - Uptake of amino acids - Maintenance of differentiation Antibiotics and Antimycotics - Penicillin, streptomycin, gentamicin, amphotericin B - Reduce the risk of bacterial and fungal contamination - Cells can become antibiotic resistant - changing phenotype - Preferably avoided in long term culture
 Supplements to basal media (contd. ) Foetal Calf/Bovine Serum (FCS & FBS) - Growth factors and hormones - Aids cell attachment - Binds and neutralise toxins - Long history of use Heat Inactivation (560 C for 30 mins) - why? - Destruction of immunoglobulins - Destruction of some viruses (also gamma irradiated serum) Ø Care! Overdoing heat inactivation can damage growth factors, hormones & vitamins and affect cell growth.
Supplements to basal media (contd. ) Foetal Calf/Bovine Serum (FCS & FBS) - Growth factors and hormones - Aids cell attachment - Binds and neutralise toxins - Long history of use Heat Inactivation (560 C for 30 mins) - why? - Destruction of immunoglobulins - Destruction of some viruses (also gamma irradiated serum) Ø Care! Overdoing heat inactivation can damage growth factors, hormones & vitamins and affect cell growth.
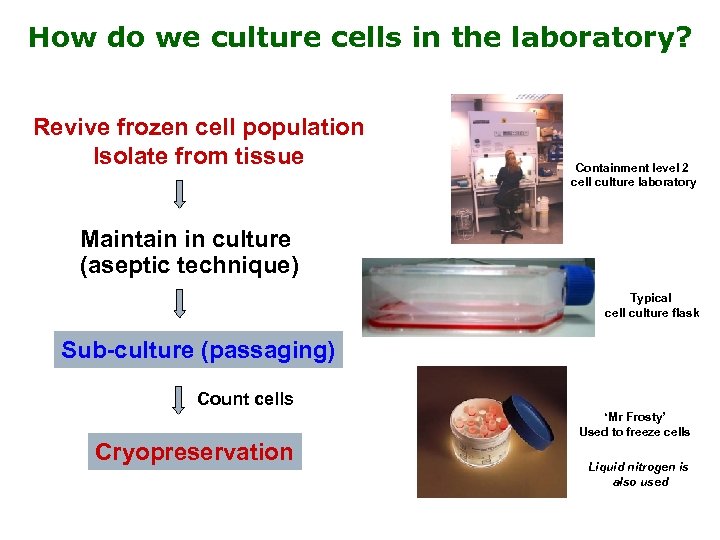 How do we culture cells in the laboratory? Revive frozen cell population Isolate from tissue Containment level 2 cell culture laboratory Maintain in culture (aseptic technique) Typical cell culture flask Sub-culture (passaging) Count cells ‘Mr Frosty’ Used to freeze cells Cryopreservation Liquid nitrogen is also used
How do we culture cells in the laboratory? Revive frozen cell population Isolate from tissue Containment level 2 cell culture laboratory Maintain in culture (aseptic technique) Typical cell culture flask Sub-culture (passaging) Count cells ‘Mr Frosty’ Used to freeze cells Cryopreservation Liquid nitrogen is also used
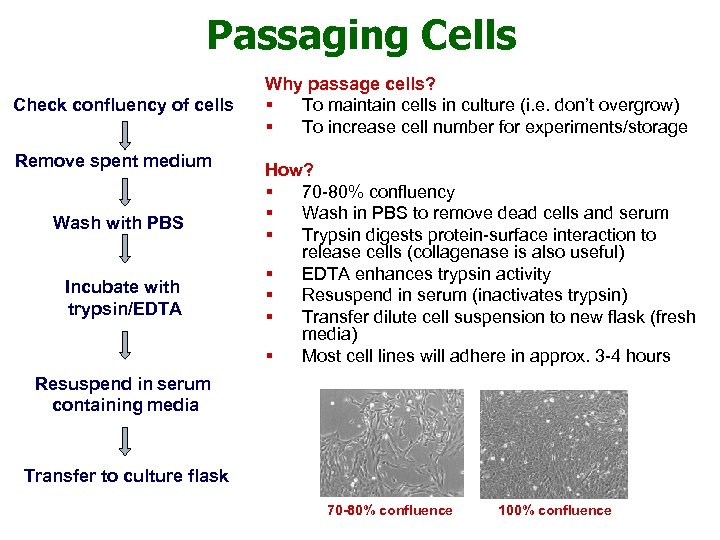 Passaging Cells Check confluency of cells Remove spent medium Wash with PBS Incubate with trypsin/EDTA Why passage cells? § To maintain cells in culture (i. e. don’t overgrow) § To increase cell number for experiments/storage How? § 70 -80% confluency § Wash in PBS to remove dead cells and serum § Trypsin digests protein-surface interaction to release cells (collagenase is also useful) § EDTA enhances trypsin activity § Resuspend in serum (inactivates trypsin) § Transfer dilute cell suspension to new flask (fresh media) § Most cell lines will adhere in approx. 3 -4 hours Resuspend in serum containing media Transfer to culture flask 70 -80% confluence 100% confluence
Passaging Cells Check confluency of cells Remove spent medium Wash with PBS Incubate with trypsin/EDTA Why passage cells? § To maintain cells in culture (i. e. don’t overgrow) § To increase cell number for experiments/storage How? § 70 -80% confluency § Wash in PBS to remove dead cells and serum § Trypsin digests protein-surface interaction to release cells (collagenase is also useful) § EDTA enhances trypsin activity § Resuspend in serum (inactivates trypsin) § Transfer dilute cell suspension to new flask (fresh media) § Most cell lines will adhere in approx. 3 -4 hours Resuspend in serum containing media Transfer to culture flask 70 -80% confluence 100% confluence
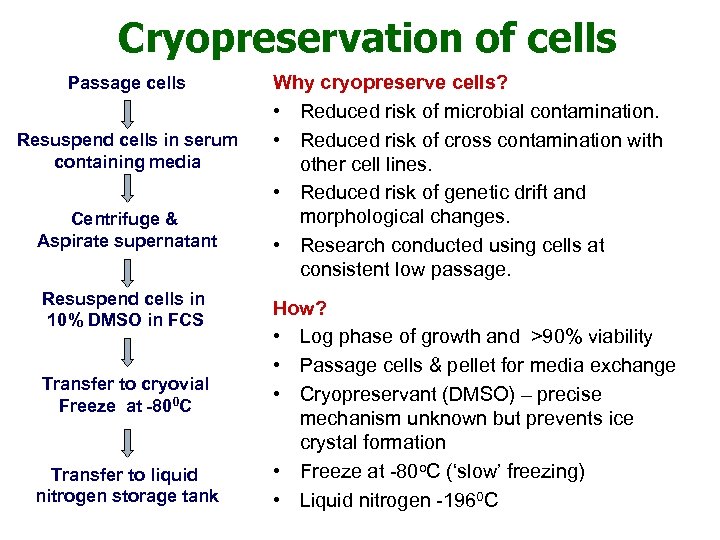 Cryopreservation of cells Passage cells Resuspend cells in serum containing media Centrifuge & Aspirate supernatant Resuspend cells in 10% DMSO in FCS Transfer to cryovial Freeze at -800 C Transfer to liquid nitrogen storage tank Why cryopreserve cells? • Reduced risk of microbial contamination. • Reduced risk of cross contamination with other cell lines. • Reduced risk of genetic drift and morphological changes. • Research conducted using cells at consistent low passage. How? • Log phase of growth and >90% viability • Passage cells & pellet for media exchange • Cryopreservant (DMSO) – precise mechanism unknown but prevents ice crystal formation • Freeze at -80 o. C (‘slow’ freezing) • Liquid nitrogen -1960 C
Cryopreservation of cells Passage cells Resuspend cells in serum containing media Centrifuge & Aspirate supernatant Resuspend cells in 10% DMSO in FCS Transfer to cryovial Freeze at -800 C Transfer to liquid nitrogen storage tank Why cryopreserve cells? • Reduced risk of microbial contamination. • Reduced risk of cross contamination with other cell lines. • Reduced risk of genetic drift and morphological changes. • Research conducted using cells at consistent low passage. How? • Log phase of growth and >90% viability • Passage cells & pellet for media exchange • Cryopreservant (DMSO) – precise mechanism unknown but prevents ice crystal formation • Freeze at -80 o. C (‘slow’ freezing) • Liquid nitrogen -1960 C
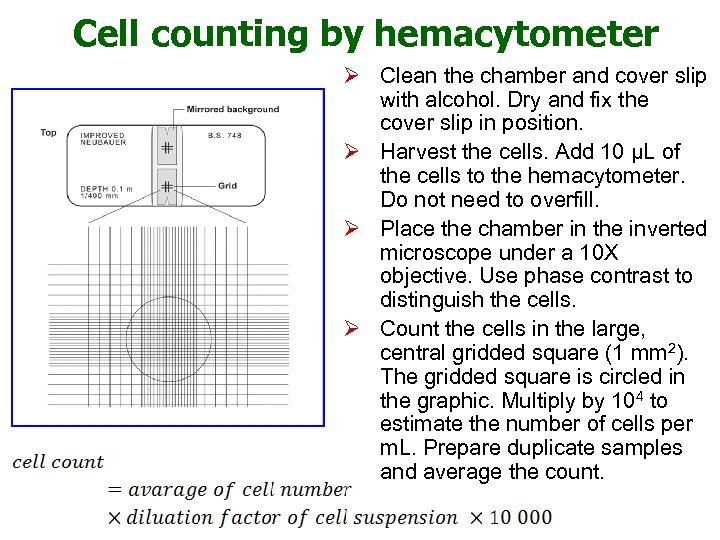 Cell counting by hemacytometer Ø Clean the chamber and cover slip with alcohol. Dry and fix the cover slip in position. Ø Harvest the cells. Add 10 μL of the cells to the hemacytometer. Do not need to overfill. Ø Place the chamber in the inverted microscope under a 10 X objective. Use phase contrast to distinguish the cells. Ø Count the cells in the large, central gridded square (1 mm 2). The gridded square is circled in the graphic. Multiply by 104 to estimate the number of cells per m. L. Prepare duplicate samples and average the count.
Cell counting by hemacytometer Ø Clean the chamber and cover slip with alcohol. Dry and fix the cover slip in position. Ø Harvest the cells. Add 10 μL of the cells to the hemacytometer. Do not need to overfill. Ø Place the chamber in the inverted microscope under a 10 X objective. Use phase contrast to distinguish the cells. Ø Count the cells in the large, central gridded square (1 mm 2). The gridded square is circled in the graphic. Multiply by 104 to estimate the number of cells per m. L. Prepare duplicate samples and average the count.
 Automated cell counter Cellometer lets you: • View cell morphology, for visual confirmation after cell counting. • Take advantage of 300+ cell types and easy, wizard-based parameter set-up. • Save sample images with results securely on your computer, plus autosave results on the network for added convenience and data protection.
Automated cell counter Cellometer lets you: • View cell morphology, for visual confirmation after cell counting. • Take advantage of 300+ cell types and easy, wizard-based parameter set-up. • Save sample images with results securely on your computer, plus autosave results on the network for added convenience and data protection.
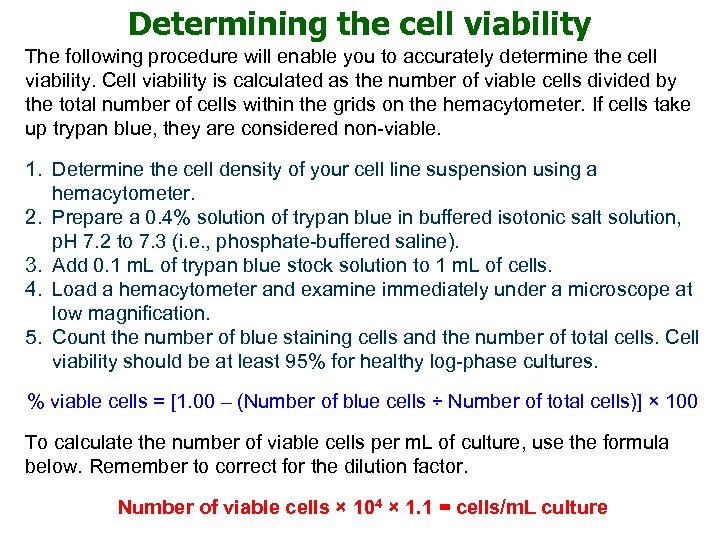 Determining the cell viability The following procedure will enable you to accurately determine the cell viability. Cell viability is calculated as the number of viable cells divided by the total number of cells within the grids on the hemacytometer. If cells take up trypan blue, they are considered non-viable. 1. Determine the cell density of your cell line suspension using a hemacytometer. 2. Prepare a 0. 4% solution of trypan blue in buffered isotonic salt solution, p. H 7. 2 to 7. 3 (i. e. , phosphate-buffered saline). 3. Add 0. 1 m. L of trypan blue stock solution to 1 m. L of cells. 4. Load a hemacytometer and examine immediately under a microscope at low magnification. 5. Count the number of blue staining cells and the number of total cells. Cell viability should be at least 95% for healthy log-phase cultures. % viable cells = [1. 00 – (Number of blue cells ÷ Number of total cells)] × 100 To calculate the number of viable cells per m. L of culture, use the formula below. Remember to correct for the dilution factor. Number of viable cells × 104 × 1. 1 = cells/m. L culture
Determining the cell viability The following procedure will enable you to accurately determine the cell viability. Cell viability is calculated as the number of viable cells divided by the total number of cells within the grids on the hemacytometer. If cells take up trypan blue, they are considered non-viable. 1. Determine the cell density of your cell line suspension using a hemacytometer. 2. Prepare a 0. 4% solution of trypan blue in buffered isotonic salt solution, p. H 7. 2 to 7. 3 (i. e. , phosphate-buffered saline). 3. Add 0. 1 m. L of trypan blue stock solution to 1 m. L of cells. 4. Load a hemacytometer and examine immediately under a microscope at low magnification. 5. Count the number of blue staining cells and the number of total cells. Cell viability should be at least 95% for healthy log-phase cultures. % viable cells = [1. 00 – (Number of blue cells ÷ Number of total cells)] × 100 To calculate the number of viable cells per m. L of culture, use the formula below. Remember to correct for the dilution factor. Number of viable cells × 104 × 1. 1 = cells/m. L culture
 The ideal growth curve for cells in culture
The ideal growth curve for cells in culture
 Organ culture Ø Not whole but pieces of organs can be cultured on artificial medium. For organ culture, care should be taken to handle in such a way that tissue should not be damaged. Ø The culture media on which organ is cultured are the same as described for cell and tissue culture. However, it is more easy to culture embryonic organs than adult animals. Ø Methods of culturing embryonic organ and adult organs differ. Due to the requirement of high O 2 amount (~95%), special serum-free media (e. g. T 8) and special apparatus (Towell’s Type II culture chamber) are used for adult organ culture. Ø The embryonic organs can be cultured either on agar or in liquid media.
Organ culture Ø Not whole but pieces of organs can be cultured on artificial medium. For organ culture, care should be taken to handle in such a way that tissue should not be damaged. Ø The culture media on which organ is cultured are the same as described for cell and tissue culture. However, it is more easy to culture embryonic organs than adult animals. Ø Methods of culturing embryonic organ and adult organs differ. Due to the requirement of high O 2 amount (~95%), special serum-free media (e. g. T 8) and special apparatus (Towell’s Type II culture chamber) are used for adult organ culture. Ø The embryonic organs can be cultured either on agar or in liquid media.
 Contamination Ø A cell culture contaminant can be defined as some element in the culture system that is undesirable because of its possible adverse effects on either the system or its use. Contamination must be avoided because Ø They compete for nutrients with host cells and secrete acidic or alkaline by-products that ceases the growth of the host cells. Ø Degraded arginine & purine inhibits the synthesis of histone and nucleic acid. Ø They also produces H 2 O 2 which is directly toxic to cells Two types of contaminants 1. Chemicals - difficult to detect caused by impurities in media, sera, water, endotoxins, plasticizers, metal ions and traces of detergent that are invisible. 2. Biological contaminants
Contamination Ø A cell culture contaminant can be defined as some element in the culture system that is undesirable because of its possible adverse effects on either the system or its use. Contamination must be avoided because Ø They compete for nutrients with host cells and secrete acidic or alkaline by-products that ceases the growth of the host cells. Ø Degraded arginine & purine inhibits the synthesis of histone and nucleic acid. Ø They also produces H 2 O 2 which is directly toxic to cells Two types of contaminants 1. Chemicals - difficult to detect caused by impurities in media, sera, water, endotoxins, plasticizers, metal ions and traces of detergent that are invisible. 2. Biological contaminants
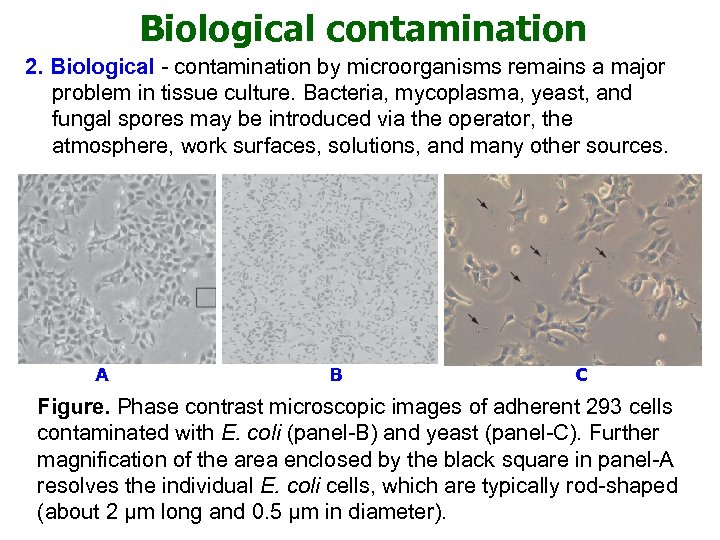 Biological contamination 2. Biological - contamination by microorganisms remains a major problem in tissue culture. Bacteria, mycoplasma, yeast, and fungal spores may be introduced via the operator, the atmosphere, work surfaces, solutions, and many other sources. A B C Figure. Phase contrast microscopic images of adherent 293 cells contaminated with E. coli (panel-B) and yeast (panel-C). Further magnification of the area enclosed by the black square in panel-A resolves the individual E. coli cells, which are typically rod-shaped (about 2 μm long and 0. 5 μm in diameter).
Biological contamination 2. Biological - contamination by microorganisms remains a major problem in tissue culture. Bacteria, mycoplasma, yeast, and fungal spores may be introduced via the operator, the atmosphere, work surfaces, solutions, and many other sources. A B C Figure. Phase contrast microscopic images of adherent 293 cells contaminated with E. coli (panel-B) and yeast (panel-C). Further magnification of the area enclosed by the black square in panel-A resolves the individual E. coli cells, which are typically rod-shaped (about 2 μm long and 0. 5 μm in diameter).
 Biosafety Levels According to the United States guidelines prepared by the Centers for Disease Control (CDC), the National Institutes of Health (NIH), and the Department of Health and Human Services (DHHS), there are four ascending levels of containment, referred to as biosafety levels 1 through 4. Biosafety Level 1 (BSL-1) BSL-1 is the basic level of protection common to most research and clinical laboratories, and is appropriate for agents that are not known to cause disease in normal, healthy humans. Biosafety Level 2 (BSL-2) BSL-2 is appropriate for moderate-risk agents known to cause human disease of varying severity by ingestion or through percutaneous or mucous membrane exposure. Most cell culture labs should be at least BSL-2, but the exact requirements depend upon the cell line used and the type of work conducted. Biosafety Level 3 (BSL-3) BSL-3 is appropriate for indigenous or exotic agents with a known potential for aerosol transmission, and for agents that may cause serious and potentially lethal infections. Biosafety Level 4 (BSL-4) BSL-4 is appropriate for exotic agents that pose a high individual risk of lifethreatening disease by infectious aerosols and for which no treatment is available. These agents are restricted to high containment laboratories.
Biosafety Levels According to the United States guidelines prepared by the Centers for Disease Control (CDC), the National Institutes of Health (NIH), and the Department of Health and Human Services (DHHS), there are four ascending levels of containment, referred to as biosafety levels 1 through 4. Biosafety Level 1 (BSL-1) BSL-1 is the basic level of protection common to most research and clinical laboratories, and is appropriate for agents that are not known to cause disease in normal, healthy humans. Biosafety Level 2 (BSL-2) BSL-2 is appropriate for moderate-risk agents known to cause human disease of varying severity by ingestion or through percutaneous or mucous membrane exposure. Most cell culture labs should be at least BSL-2, but the exact requirements depend upon the cell line used and the type of work conducted. Biosafety Level 3 (BSL-3) BSL-3 is appropriate for indigenous or exotic agents with a known potential for aerosol transmission, and for agents that may cause serious and potentially lethal infections. Biosafety Level 4 (BSL-4) BSL-4 is appropriate for exotic agents that pose a high individual risk of lifethreatening disease by infectious aerosols and for which no treatment is available. These agents are restricted to high containment laboratories.
 How can cell culture contamination be controlled? Ø Watch the video on tissue culture and safety practice.
How can cell culture contamination be controlled? Ø Watch the video on tissue culture and safety practice.
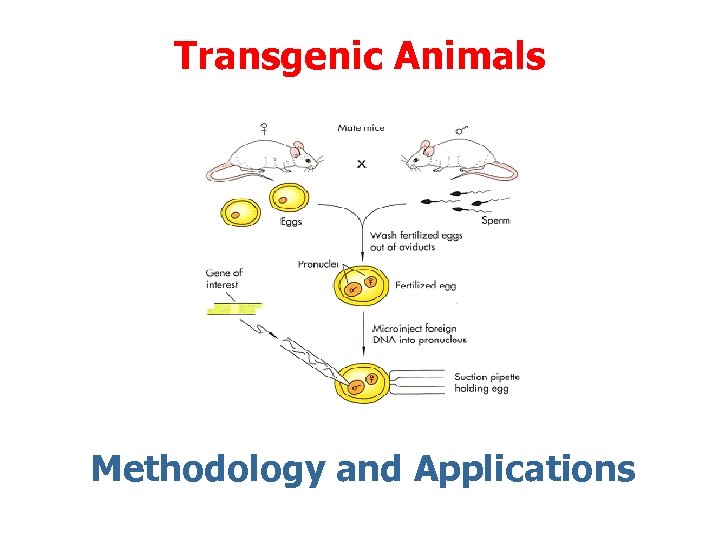 Transgenic Animals Methodology and Applications
Transgenic Animals Methodology and Applications
 Transgenic mice Ø Hundreds of different genes have been introduced into various mouse strains. These studies have contributed to an understanding of gene regulation, tumor development, immunolo-gical specificity, molecular genetics of development, and many other biological processees of interest. Ø Transgenic mice have also played a role in examining the feasibility of the industrial production of human therapeutic drugs by domesticated animals and in the creation of transgenic strains that act as biomedical models for various human genetic diseases.
Transgenic mice Ø Hundreds of different genes have been introduced into various mouse strains. These studies have contributed to an understanding of gene regulation, tumor development, immunolo-gical specificity, molecular genetics of development, and many other biological processees of interest. Ø Transgenic mice have also played a role in examining the feasibility of the industrial production of human therapeutic drugs by domesticated animals and in the creation of transgenic strains that act as biomedical models for various human genetic diseases.
 Transgenic mice: methodology Ø DNA can be introduced into mice by 1. retroviral vectors that infect the cells of an earlystage embryo prior to implantation into a respective female, 2. microinjection into the enlarged sperm nucleus (male pronucleus) of a fertilized egg, or 3. introduction of genetically engineered embryonic stem cells into an early-stage developing embryo before implantation into a respective female.
Transgenic mice: methodology Ø DNA can be introduced into mice by 1. retroviral vectors that infect the cells of an earlystage embryo prior to implantation into a respective female, 2. microinjection into the enlarged sperm nucleus (male pronucleus) of a fertilized egg, or 3. introduction of genetically engineered embryonic stem cells into an early-stage developing embryo before implantation into a respective female.
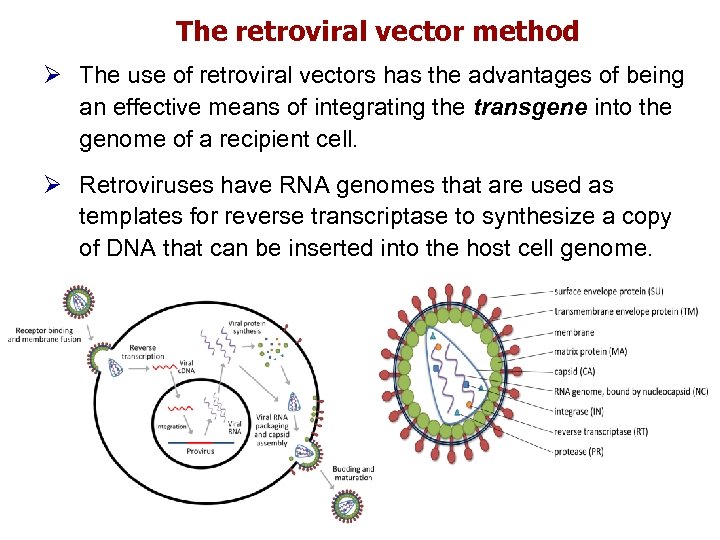 The retroviral vector method Ø The use of retroviral vectors has the advantages of being an effective means of integrating the transgene into the genome of a recipient cell. Ø Retroviruses have RNA genomes that are used as templates for reverse transcriptase to synthesize a copy of DNA that can be inserted into the host cell genome.
The retroviral vector method Ø The use of retroviral vectors has the advantages of being an effective means of integrating the transgene into the genome of a recipient cell. Ø Retroviruses have RNA genomes that are used as templates for reverse transcriptase to synthesize a copy of DNA that can be inserted into the host cell genome.
 The retroviral vector method (contd. ) There are some drawbacks to the use of retroviral vectors. Ø Vector derived from this viruses can transfer only small pieces (~8 kb) of DNA. Ø Although these vectors are designed to be replication defective, the genome of the retroviral strain (helper virus) that is needed to create large quantities of the vector DNA can be integrated into the same nucleus as the transgene. Ø It is absolutely necessary that there should not be any retroviral contamination for applications in which either a commercial product is to be synthesized by the transgenic organism or the transgenic organism is to be used as food. Ø In addition, transgene introduced on some retroviral vectors are silenced in mouse embryos.
The retroviral vector method (contd. ) There are some drawbacks to the use of retroviral vectors. Ø Vector derived from this viruses can transfer only small pieces (~8 kb) of DNA. Ø Although these vectors are designed to be replication defective, the genome of the retroviral strain (helper virus) that is needed to create large quantities of the vector DNA can be integrated into the same nucleus as the transgene. Ø It is absolutely necessary that there should not be any retroviral contamination for applications in which either a commercial product is to be synthesized by the transgenic organism or the transgenic organism is to be used as food. Ø In addition, transgene introduced on some retroviral vectors are silenced in mouse embryos.
 The lentiviral vector system The lentivirus vector system is similar to other retroviral vector systems and Ø is capable of delivering large segments of DNA into host genome, Ø is stable for relatively long periods, Ø has low immunogenicity, and Ø can infect both dividing and non-dividing cells.
The lentiviral vector system The lentivirus vector system is similar to other retroviral vector systems and Ø is capable of delivering large segments of DNA into host genome, Ø is stable for relatively long periods, Ø has low immunogenicity, and Ø can infect both dividing and non-dividing cells.
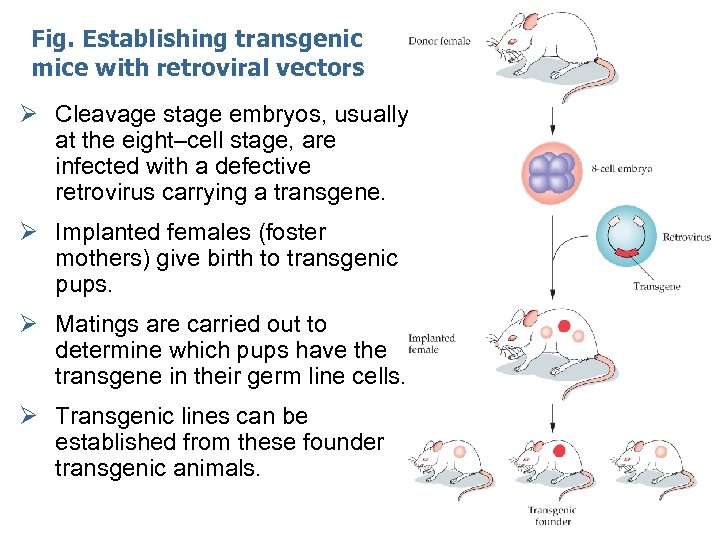 Fig. Establishing transgenic mice with retroviral vectors Ø Cleavage stage embryos, usually at the eight–cell stage, are infected with a defective retrovirus carrying a transgene. Ø Implanted females (foster mothers) give birth to transgenic pups. Ø Matings are carried out to determine which pups have the transgene in their germ line cells. Ø Transgenic lines can be established from these founder transgenic animals.
Fig. Establishing transgenic mice with retroviral vectors Ø Cleavage stage embryos, usually at the eight–cell stage, are infected with a defective retrovirus carrying a transgene. Ø Implanted females (foster mothers) give birth to transgenic pups. Ø Matings are carried out to determine which pups have the transgene in their germ line cells. Ø Transgenic lines can be established from these founder transgenic animals.
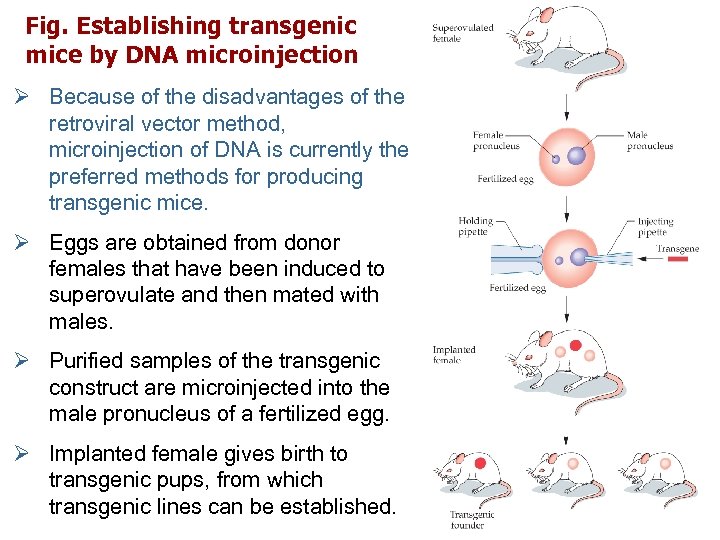 Fig. Establishing transgenic mice by DNA microinjection Ø Because of the disadvantages of the retroviral vector method, microinjection of DNA is currently the preferred methods for producing transgenic mice. Ø Eggs are obtained from donor females that have been induced to superovulate and then mated with males. Ø Purified samples of the transgenic construct are microinjected into the male pronucleus of a fertilized egg. Ø Implanted female gives birth to transgenic pups, from which transgenic lines can be established.
Fig. Establishing transgenic mice by DNA microinjection Ø Because of the disadvantages of the retroviral vector method, microinjection of DNA is currently the preferred methods for producing transgenic mice. Ø Eggs are obtained from donor females that have been induced to superovulate and then mated with males. Ø Purified samples of the transgenic construct are microinjected into the male pronucleus of a fertilized egg. Ø Implanted female gives birth to transgenic pups, from which transgenic lines can be established.
 How to create superovulated female mouse? Ø The number of available fertilized eggs that are to be inoculated by microinjection is increased by stimulating donor females to superovulate. Ø Female mice are given an initial injection of pregnant mare’s serum and another injection of human chorionic gonadoprotein after about 48 hours. Ø The superovulated female mice produces about 35 eggs instead of the normal 5 to 10 that are subsequently mated so that eggs become fertilized. Ø Microinjection of the fertilized eggs has to be performed immediately after their collection from oviducts.
How to create superovulated female mouse? Ø The number of available fertilized eggs that are to be inoculated by microinjection is increased by stimulating donor females to superovulate. Ø Female mice are given an initial injection of pregnant mare’s serum and another injection of human chorionic gonadoprotein after about 48 hours. Ø The superovulated female mice produces about 35 eggs instead of the normal 5 to 10 that are subsequently mated so that eggs become fertilized. Ø Microinjection of the fertilized eggs has to be performed immediately after their collection from oviducts.
 Fig. Establishing transgenic mice by DNA microinjection (contd. ) Ø In DNA microinjection method, the injected DNA integrates at random sites within the genome, and often multiple copies of the injected DNA are incorporated in one site. Ø Therefore, not all of the transgenic pups will have the appropriate characteristic. Ø Need to check mouse pups for DNA (by PCR or Southerns), RNA (by northerns or RT-PCR), and protein (by western or by some specific assay method). Ø In some individuals, the transgene may not be expressed because of the site of integration, and in others, the copy number may be excessive and may lead to overexpression, which disrupts the normal physiology of the animal.
Fig. Establishing transgenic mice by DNA microinjection (contd. ) Ø In DNA microinjection method, the injected DNA integrates at random sites within the genome, and often multiple copies of the injected DNA are incorporated in one site. Ø Therefore, not all of the transgenic pups will have the appropriate characteristic. Ø Need to check mouse pups for DNA (by PCR or Southerns), RNA (by northerns or RT-PCR), and protein (by western or by some specific assay method). Ø In some individuals, the transgene may not be expressed because of the site of integration, and in others, the copy number may be excessive and may lead to overexpression, which disrupts the normal physiology of the animal.
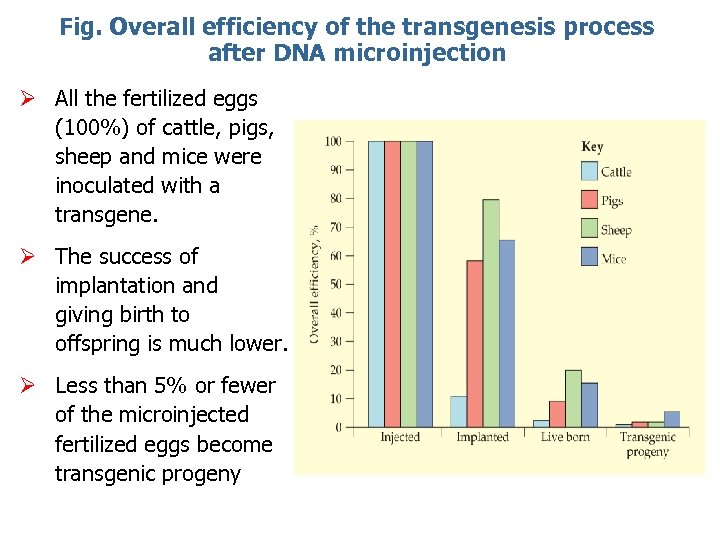 Fig. Overall efficiency of the transgenesis process after DNA microinjection Ø All the fertilized eggs (100%) of cattle, pigs, sheep and mice were inoculated with a transgene. Ø The success of implantation and giving birth to offspring is much lower. Ø Less than 5% or fewer of the microinjected fertilized eggs become transgenic progeny
Fig. Overall efficiency of the transgenesis process after DNA microinjection Ø All the fertilized eggs (100%) of cattle, pigs, sheep and mice were inoculated with a transgene. Ø The success of implantation and giving birth to offspring is much lower. Ø Less than 5% or fewer of the microinjected fertilized eggs become transgenic progeny
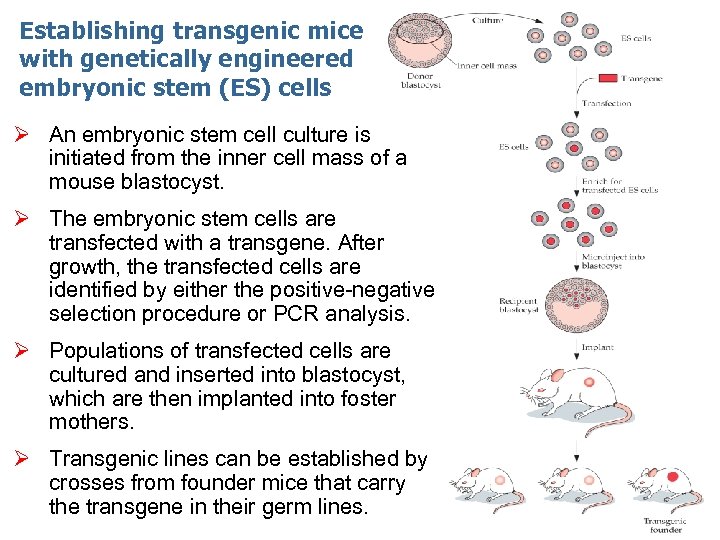 Establishing transgenic mice with genetically engineered embryonic stem (ES) cells Ø An embryonic stem cell culture is initiated from the inner cell mass of a mouse blastocyst. Ø The embryonic stem cells are transfected with a transgene. After growth, the transfected cells are identified by either the positive-negative selection procedure or PCR analysis. Ø Populations of transfected cells are cultured and inserted into blastocyst, which are then implanted into foster mothers. Ø Transgenic lines can be established by crosses from founder mice that carry the transgene in their germ lines.
Establishing transgenic mice with genetically engineered embryonic stem (ES) cells Ø An embryonic stem cell culture is initiated from the inner cell mass of a mouse blastocyst. Ø The embryonic stem cells are transfected with a transgene. After growth, the transfected cells are identified by either the positive-negative selection procedure or PCR analysis. Ø Populations of transfected cells are cultured and inserted into blastocyst, which are then implanted into foster mothers. Ø Transgenic lines can be established by crosses from founder mice that carry the transgene in their germ lines.
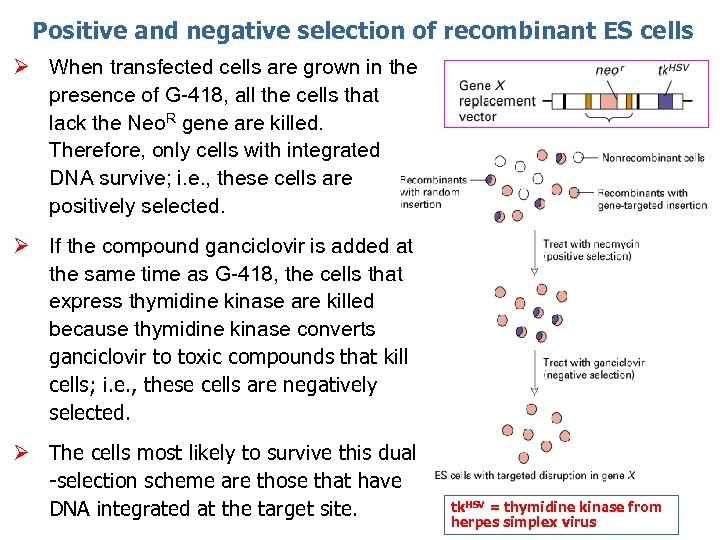 Positive and negative selection of recombinant ES cells Ø When transfected cells are grown in the presence of G-418, all the cells that lack the Neo. R gene are killed. Therefore, only cells with integrated DNA survive; i. e. , these cells are positively selected. Ø If the compound ganciclovir is added at the same time as G-418, the cells that express thymidine kinase are killed because thymidine kinase converts ganciclovir to toxic compounds that kill cells; i. e. , these cells are negatively selected. Ø The cells most likely to survive this dual -selection scheme are those that have DNA integrated at the target site. tk. HSV = thymidine kinase from herpes simplex virus
Positive and negative selection of recombinant ES cells Ø When transfected cells are grown in the presence of G-418, all the cells that lack the Neo. R gene are killed. Therefore, only cells with integrated DNA survive; i. e. , these cells are positively selected. Ø If the compound ganciclovir is added at the same time as G-418, the cells that express thymidine kinase are killed because thymidine kinase converts ganciclovir to toxic compounds that kill cells; i. e. , these cells are negatively selected. Ø The cells most likely to survive this dual -selection scheme are those that have DNA integrated at the target site. tk. HSV = thymidine kinase from herpes simplex virus
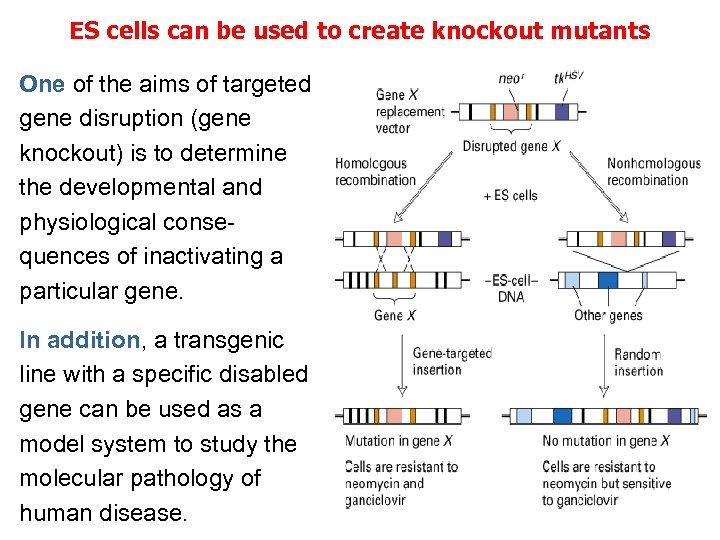 ES cells can be used to create knockout mutants One of the aims of targeted gene disruption (gene knockout) is to determine the developmental and physiological consequences of inactivating a particular gene. In addition, a transgenic line with a specific disabled gene can be used as a model system to study the molecular pathology of human disease.
ES cells can be used to create knockout mutants One of the aims of targeted gene disruption (gene knockout) is to determine the developmental and physiological consequences of inactivating a particular gene. In addition, a transgenic line with a specific disabled gene can be used as a model system to study the molecular pathology of human disease.
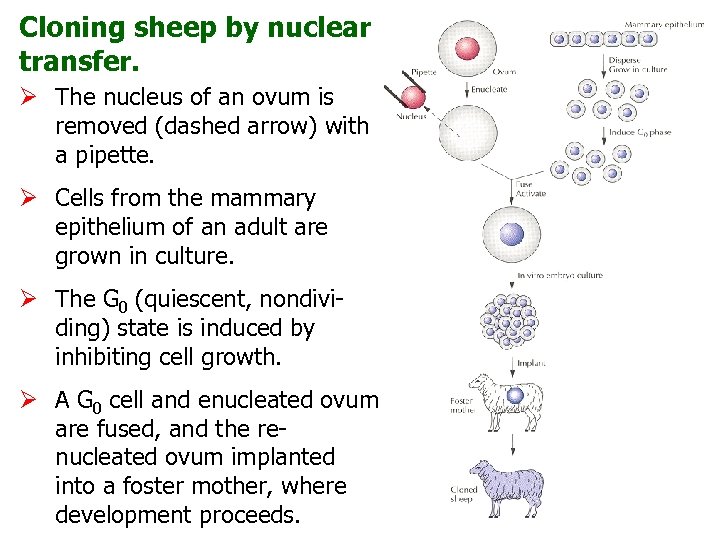 Cloning sheep by nuclear transfer. Ø The nucleus of an ovum is removed (dashed arrow) with a pipette. Ø Cells from the mammary epithelium of an adult are grown in culture. Ø The G 0 (quiescent, nondividing) state is induced by inhibiting cell growth. Ø A G 0 cell and enucleated ovum are fused, and the renucleated ovum implanted into a foster mother, where development proceeds.
Cloning sheep by nuclear transfer. Ø The nucleus of an ovum is removed (dashed arrow) with a pipette. Ø Cells from the mammary epithelium of an adult are grown in culture. Ø The G 0 (quiescent, nondividing) state is induced by inhibiting cell growth. Ø A G 0 cell and enucleated ovum are fused, and the renucleated ovum implanted into a foster mother, where development proceeds.
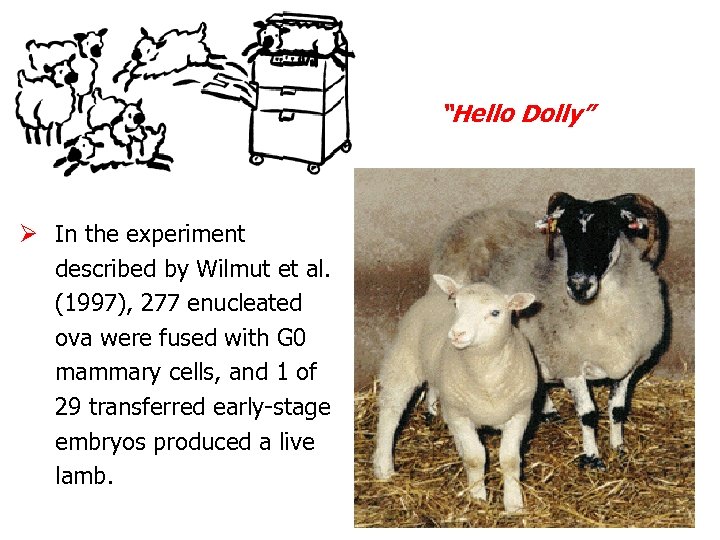 “Hello Dolly” Ø In the experiment described by Wilmut et al. (1997), 277 enucleated ova were fused with G 0 mammary cells, and 1 of 29 transferred early-stage embryos produced a live lamb.
“Hello Dolly” Ø In the experiment described by Wilmut et al. (1997), 277 enucleated ova were fused with G 0 mammary cells, and 1 of 29 transferred early-stage embryos produced a live lamb.
 And now there is pet cloning for a “small” fee. Nine-week-old "Little Nicky" peers out from her carrying case in Texas. Little Nicky, a cloned cat, was sold to its new owner by ”Genetic Savings and Clone” for $50, 000 in December 2004. Ø August 07, 2008 Bernann Mc. Kinney with one of the 5 puppies cloned from Booger, her late pet pit bull. It cost her $50, 000. When Booger was diagnosed with cancer, a grief-stricken Mc. Kinney sought to have him cloned - first by the now-defunct “Genetic Savings and Clone”, and then by South Korean company “RNL Bio”.
And now there is pet cloning for a “small” fee. Nine-week-old "Little Nicky" peers out from her carrying case in Texas. Little Nicky, a cloned cat, was sold to its new owner by ”Genetic Savings and Clone” for $50, 000 in December 2004. Ø August 07, 2008 Bernann Mc. Kinney with one of the 5 puppies cloned from Booger, her late pet pit bull. It cost her $50, 000. When Booger was diagnosed with cancer, a grief-stricken Mc. Kinney sought to have him cloned - first by the now-defunct “Genetic Savings and Clone”, and then by South Korean company “RNL Bio”.
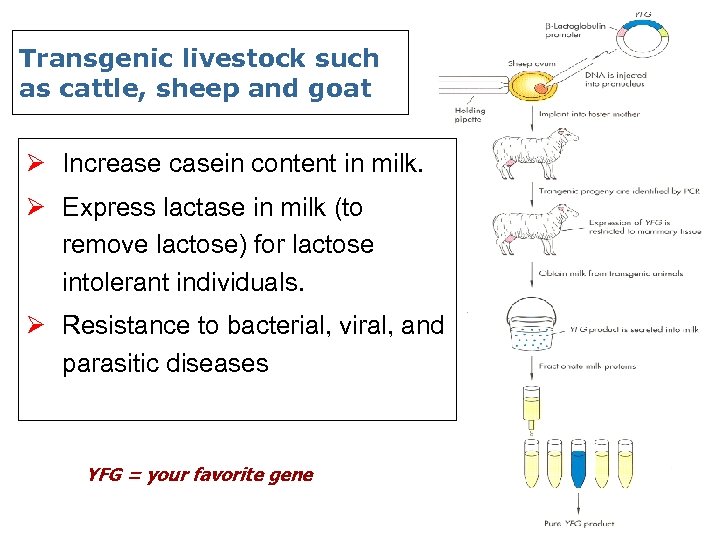 Transgenic livestock such as cattle, sheep and goat Ø Increase casein content in milk. Ø Express lactase in milk (to remove lactose) for lactose intolerant individuals. Ø Resistance to bacterial, viral, and parasitic diseases YFG = your favorite gene
Transgenic livestock such as cattle, sheep and goat Ø Increase casein content in milk. Ø Express lactase in milk (to remove lactose) for lactose intolerant individuals. Ø Resistance to bacterial, viral, and parasitic diseases YFG = your favorite gene
 Some human proteins expressed in the mammary glands of transgenic animals Ø Antithrombin III (the first transgenic animal drug, an anticlotting protein, approved by the FDA in 2009). Ø Erythropoietin (used to treat anaemia resulting from cancer and chemotherapy). Ø Human growth hormones such as h. GH and somatotropin (used to treat human growth deficiency in children and chronic renal insufficiency) Ø Monoclonal antibodies such as anti-lipopolysaccharide (used to treat sepsis). Ø Plasminogen activator such as urokinase type plasminogen activator (used to treat acute myocardial infraction and acute stroke).
Some human proteins expressed in the mammary glands of transgenic animals Ø Antithrombin III (the first transgenic animal drug, an anticlotting protein, approved by the FDA in 2009). Ø Erythropoietin (used to treat anaemia resulting from cancer and chemotherapy). Ø Human growth hormones such as h. GH and somatotropin (used to treat human growth deficiency in children and chronic renal insufficiency) Ø Monoclonal antibodies such as anti-lipopolysaccharide (used to treat sepsis). Ø Plasminogen activator such as urokinase type plasminogen activator (used to treat acute myocardial infraction and acute stroke).
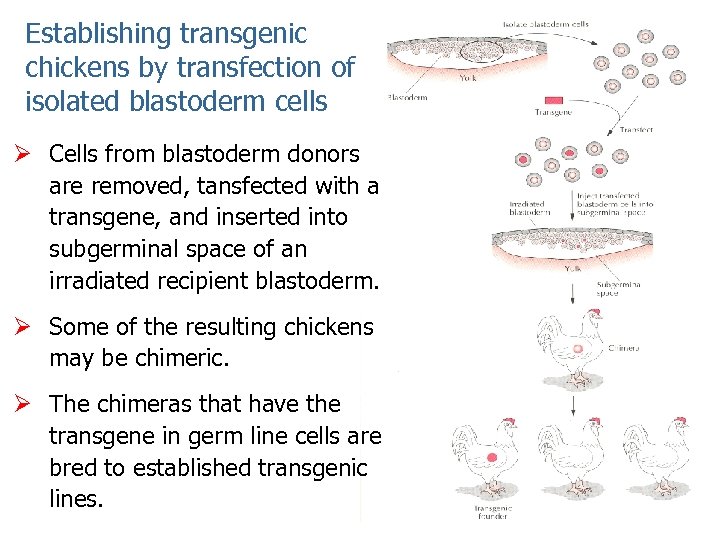 Establishing transgenic chickens by transfection of isolated blastoderm cells Ø Cells from blastoderm donors are removed, tansfected with a transgene, and inserted into subgerminal space of an irradiated recipient blastoderm. Ø Some of the resulting chickens may be chimeric. Ø The chimeras that have the transgene in germ line cells are bred to established transgenic lines.
Establishing transgenic chickens by transfection of isolated blastoderm cells Ø Cells from blastoderm donors are removed, tansfected with a transgene, and inserted into subgerminal space of an irradiated recipient blastoderm. Ø Some of the resulting chickens may be chimeric. Ø The chimeras that have the transgene in germ line cells are bred to established transgenic lines.
 Why transgenic chickens? Ø Resistance to viral, bacterial, and coccidial diseases. Ø Better feed efficiency. Ø Lower fat and cholesterol levels in eggs. Ø Better meat quality. Ø Eggs with pharmaceutical proteins in them.
Why transgenic chickens? Ø Resistance to viral, bacterial, and coccidial diseases. Ø Better feed efficiency. Ø Lower fat and cholesterol levels in eggs. Ø Better meat quality. Ø Eggs with pharmaceutical proteins in them.
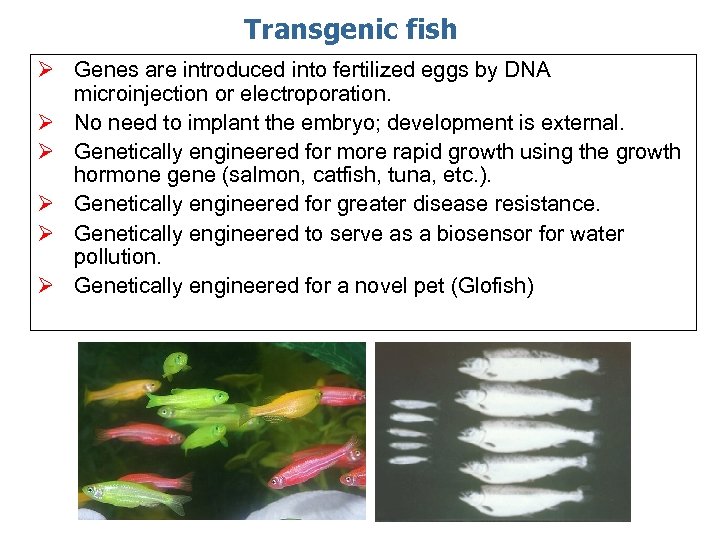 Transgenic fish Ø Genes are introduced into fertilized eggs by DNA microinjection or electroporation. Ø No need to implant the embryo; development is external. Ø Genetically engineered for more rapid growth using the growth hormone gene (salmon, catfish, tuna, etc. ). Ø Genetically engineered for greater disease resistance. Ø Genetically engineered to serve as a biosensor for water pollution. Ø Genetically engineered for a novel pet (Glofish)
Transgenic fish Ø Genes are introduced into fertilized eggs by DNA microinjection or electroporation. Ø No need to implant the embryo; development is external. Ø Genetically engineered for more rapid growth using the growth hormone gene (salmon, catfish, tuna, etc. ). Ø Genetically engineered for greater disease resistance. Ø Genetically engineered to serve as a biosensor for water pollution. Ø Genetically engineered for a novel pet (Glofish)
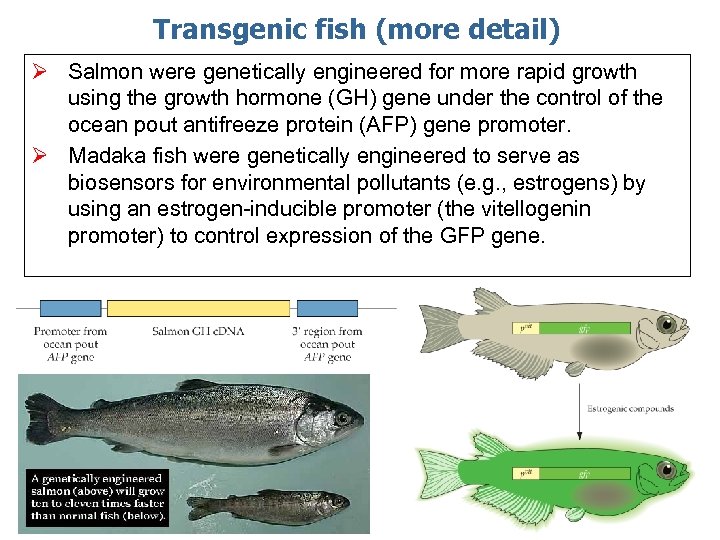 Transgenic fish (more detail) Ø Salmon were genetically engineered for more rapid growth using the growth hormone (GH) gene under the control of the ocean pout antifreeze protein (AFP) gene promoter. Ø Madaka fish were genetically engineered to serve as biosensors for environmental pollutants (e. g. , estrogens) by using an estrogen-inducible promoter (the vitellogenin promoter) to control expression of the GFP gene.
Transgenic fish (more detail) Ø Salmon were genetically engineered for more rapid growth using the growth hormone (GH) gene under the control of the ocean pout antifreeze protein (AFP) gene promoter. Ø Madaka fish were genetically engineered to serve as biosensors for environmental pollutants (e. g. , estrogens) by using an estrogen-inducible promoter (the vitellogenin promoter) to control expression of the GFP gene.


