c86b34d05b6459631be137557427c3ec.ppt
- Количество слайдов: 30

ANCO ASH 2005 Review Acute Leukemias Feb 22, 2006 Charles Linker MD

Abstract # 43 Mini-allo for AML Herr et al EBMT Review
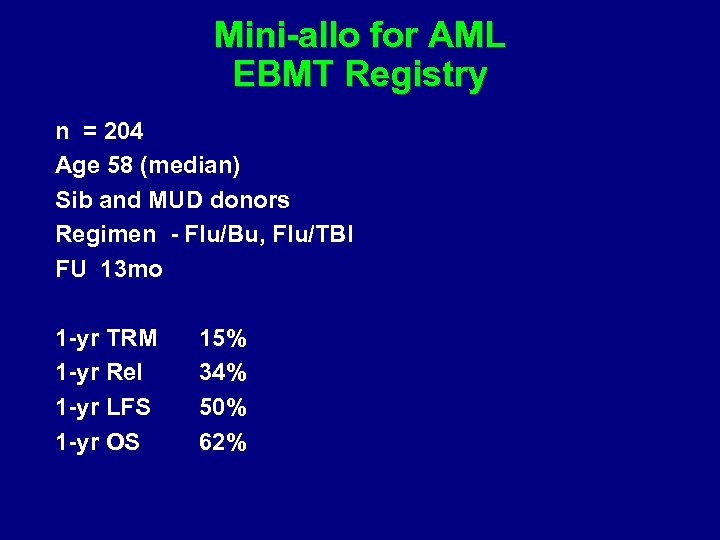
Mini-allo for AML EBMT Registry n = 204 Age 58 (median) Sib and MUD donors Regimen - Flu/Bu, Flu/TBI FU 13 mo 1 -yr TRM 1 -yr Rel 1 -yr LFS 1 -yr OS 15% 34% 50% 62%

Abstract # 47 Mini-allo for AML Shimoni et al Tel Hashomer, Israel
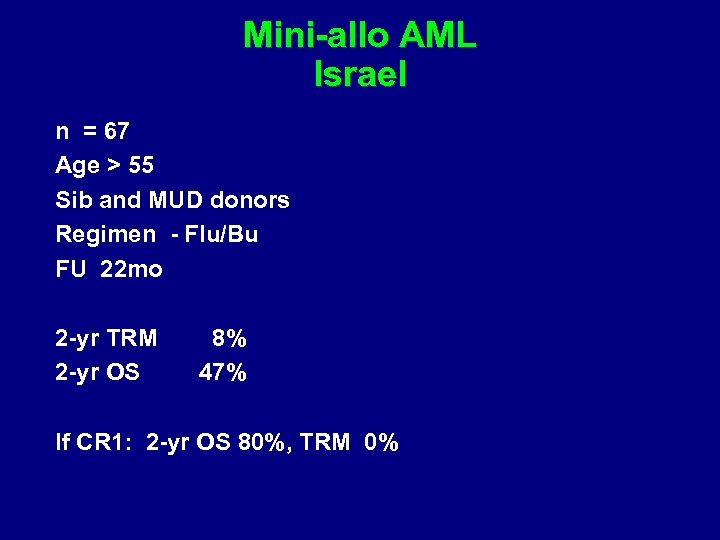
Mini-allo AML Israel n = 67 Age > 55 Sib and MUD donors Regimen - Flu/Bu FU 22 mo 2 -yr TRM 2 -yr OS 8% 47% If CR 1: 2 -yr OS 80%, TRM 0%

CALGB 100103 Phase II Study of mini-allo for AML CR 1, age > 60 Study Chair: Steve Devine CTN co-chair: Sergio Giralt

CALGB 100103 Background - 1 • • • Poor results of chemotherapy No signs of progress in chemotherapy New approaches are warranted
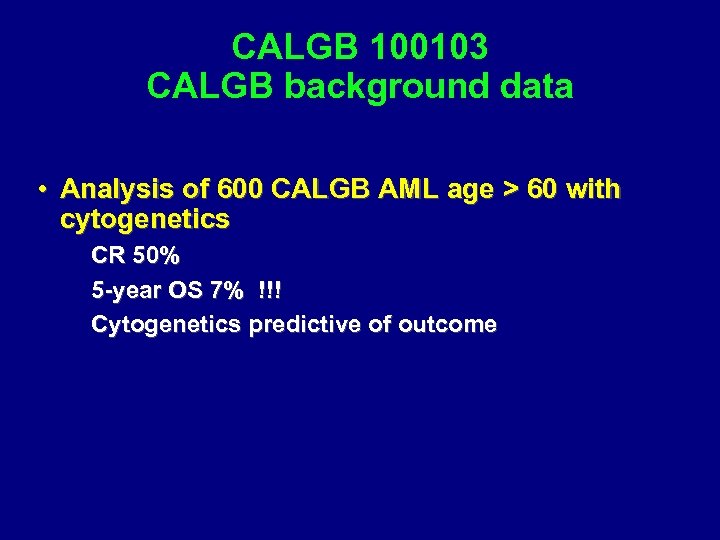
CALGB 100103 CALGB background data • Analysis of 600 CALGB AML age > 60 with cytogenetics CR 50% 5 -year OS 7% !!! Cytogenetics predictive of outcome
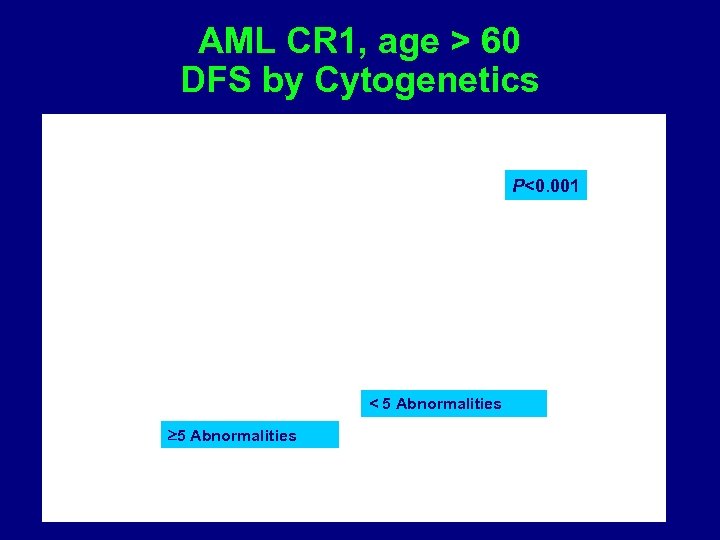
AML CR 1, age > 60 DFS by Cytogenetics P<0. 001 < 5 Abnormalities

AML CR 1, age > 60 OS by Cytogenetics

AML CR 1, age > 60 OS by Age

CALGB 100103 Background - 2 • Results in best group are still poor (n = 276) CR 1 Age 60 -75 Receive first consolidation on randomized trial • 2 -year DFS 24% • 3 -year DFS 17%

CALGB 100103 Eligibility - 1 • AML CR 1 Prior MDS, t-AML allowed < 2 cycles induction < 2 courses consolidation < 6 months in CR 1 exclude APL, prior MPD • Age 60 -74 • Matched sibling or 10/10 MUD donor
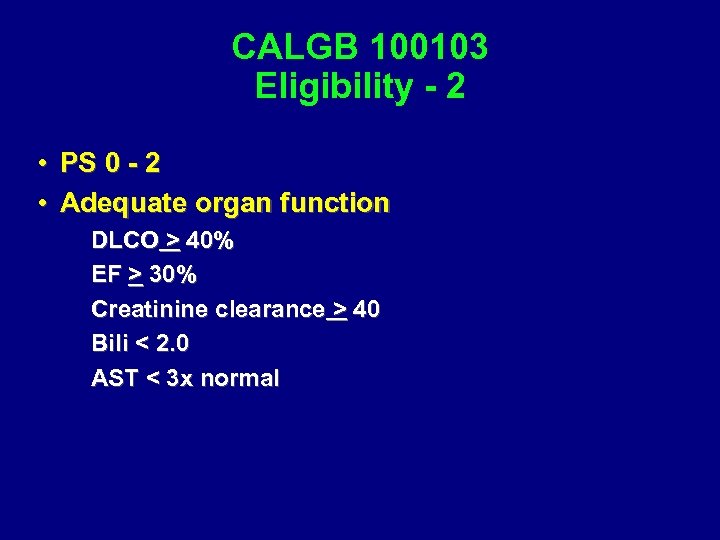
CALGB 100103 Eligibility - 2 • PS 0 - 2 • Adequate organ function DLCO > 40% EF > 30% Creatinine clearance > 40 Bili < 2. 0 AST < 3 x normal
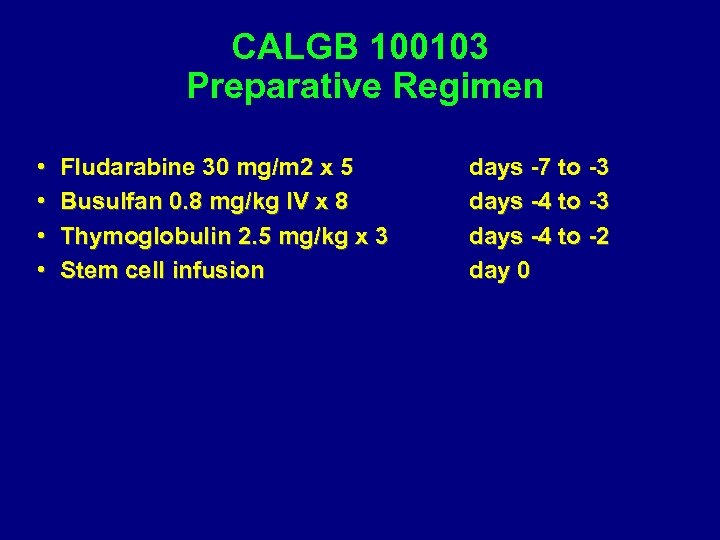
CALGB 100103 Preparative Regimen • • Fludarabine 30 mg/m 2 x 5 Busulfan 0. 8 mg/kg IV x 8 Thymoglobulin 2. 5 mg/kg x 3 Stem cell infusion days -7 to -3 days -4 to -2 day 0

CALGB 100103 GVH Prophylaxis • Tacrolimus • MTX 5 mg/m 2 • Taper tac day - 2 to +90 days, +1, 3, 6, 11 day +90 to +150/+180

CALGB 100103 Statistics • Primary objective 2 -year DFS > 35% 90% power to exclude DFS < 20% • Accrual goal = 61 • Stopping rules for TRM Assume true TRM 20% Unacceptable TRM 40%

CALGB 100103 • Currently active in CALGB sib donors only • Amendment in process Add CTN Add MUD

Mini-allo for AML, age > 60 • • • Currently treatments work poorly Mini-allo is feasible Several pilot studies show DFS > 40% Deserves testing in Group setting CALGB 100103 is last chance for USA study

Abstract # 146 Ph+ ALL Dellanoy et al GRALL, France
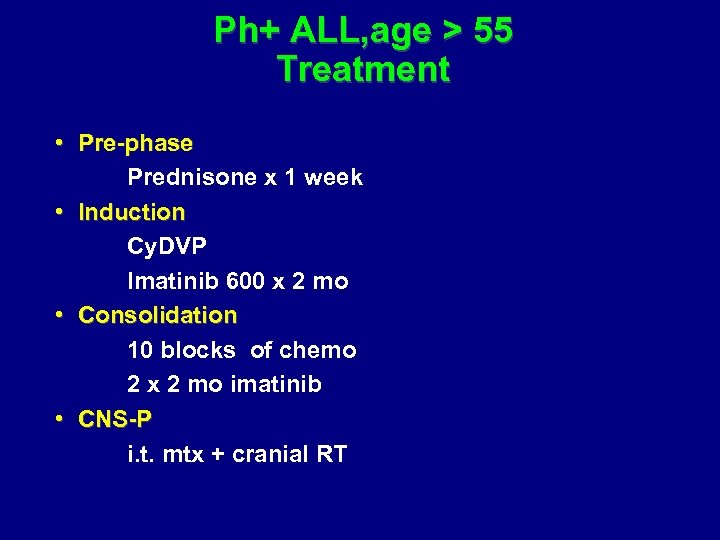
Ph+ ALL, age > 55 Treatment • Pre-phase Prednisone x 1 week • Induction Cy. DVP Imatinib 600 x 2 mo • Consolidation 10 blocks of chemo 2 x 2 mo imatinib • CNS-P i. t. mtx + cranial RT

Ph+ ALL, age > 55 Results N = 30 Age 66 (58 - 78) FU 15 mo CR 20/29 ( vs 6/21 historical control) 1 -yr OS 71% (11% control) 1 -yr EFS 57% (5% control)

ASCT for high-risk ALL Protocol 9501 & SOC
Protocol 9501 SOC for Ph+ Effect of Imatinib
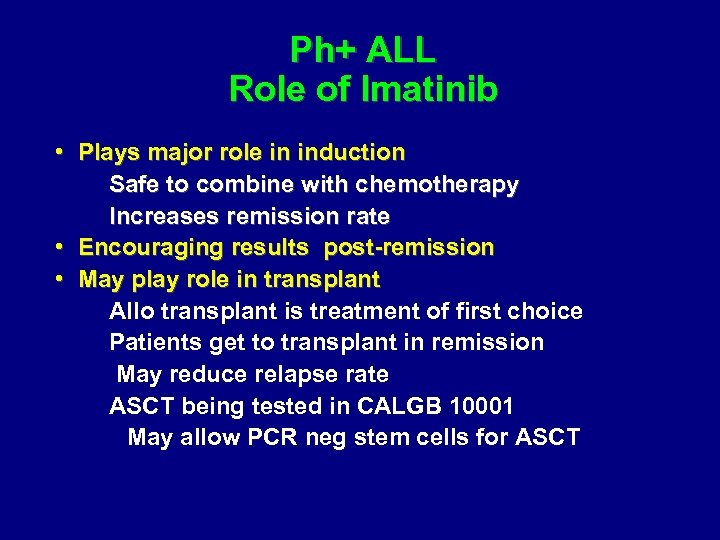
Ph+ ALL Role of Imatinib • Plays major role in induction Safe to combine with chemotherapy Increases remission rate • Encouraging results post-remission • May play role in transplant Allo transplant is treatment of first choice Patients get to transplant in remission May reduce relapse rate ASCT being tested in CALGB 10001 May allow PCR neg stem cells for ASCT

Abstract # 150 Nelarabine for T-ALL Goekbuget et al GMALL, Germany

CALGB 19801 De Angelo et al ASH #743 (2002) • Eligibility T-ALL or T-LL Relapse or refractory • Treatment Nelarabine (GW 506 U) 1. 5 g/m 2 days 1, 3, 5 q 3 weeks x 2 cycles Responders may get additional 2 cycles • Results 10/38 CR (26%) MDCR 10 mo 1 -yr DFS 40%
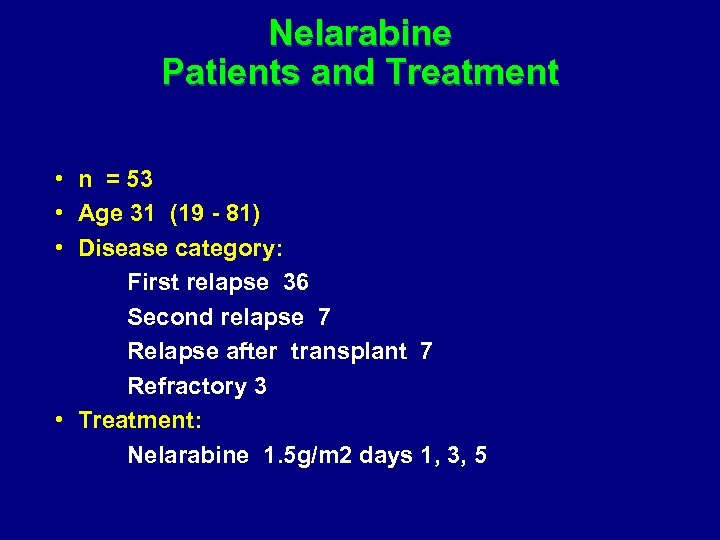
Nelarabine Patients and Treatment • n = 53 • Age 31 (19 - 81) • Disease category: First relapse 36 Second relapse 7 Relapse after transplant 7 Refractory 3 • Treatment: Nelarabine 1. 5 g/m 2 days 1, 3, 5
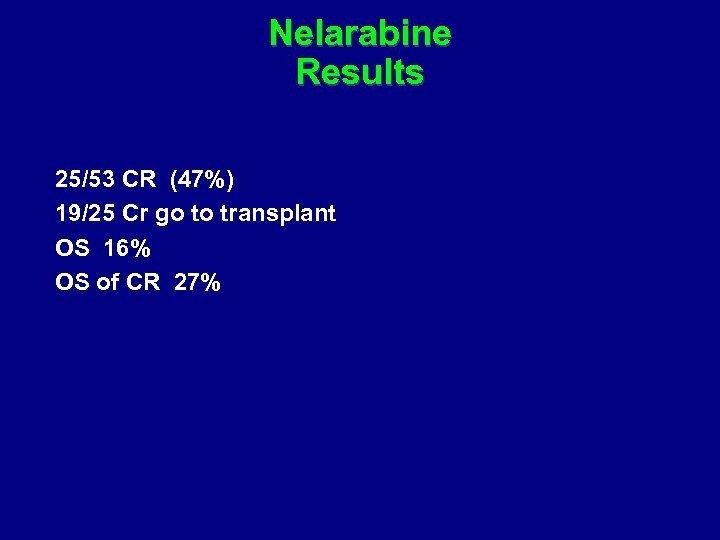
Nelarabine Results 25/53 CR (47%) 19/25 Cr go to transplant OS 16% OS of CR 27%

Nelarabine for T-ALL • Important new agent • Good choice for relapse • Should be tested up front
c86b34d05b6459631be137557427c3ec.ppt