df3a32ffd34acb79aea9bff58f83e63b.ppt
- Количество слайдов: 43
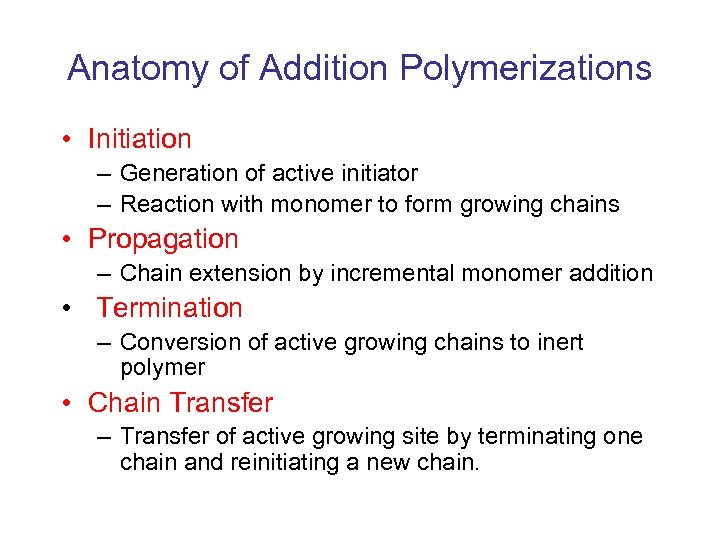 Anatomy of Addition Polymerizations • Initiation – Generation of active initiator – Reaction with monomer to form growing chains • Propagation – Chain extension by incremental monomer addition • Termination – Conversion of active growing chains to inert polymer • Chain Transfer – Transfer of active growing site by terminating one chain and reinitiating a new chain.
Anatomy of Addition Polymerizations • Initiation – Generation of active initiator – Reaction with monomer to form growing chains • Propagation – Chain extension by incremental monomer addition • Termination – Conversion of active growing chains to inert polymer • Chain Transfer – Transfer of active growing site by terminating one chain and reinitiating a new chain.
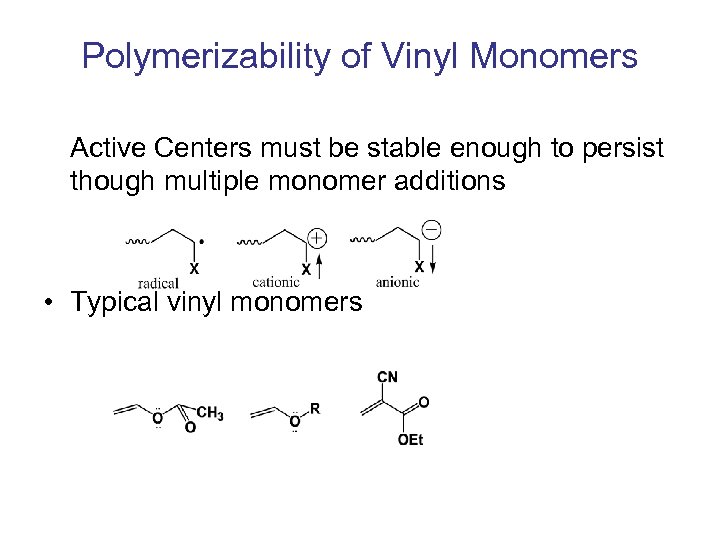 Polymerizability of Vinyl Monomers Active Centers must be stable enough to persist though multiple monomer additions • Typical vinyl monomers
Polymerizability of Vinyl Monomers Active Centers must be stable enough to persist though multiple monomer additions • Typical vinyl monomers
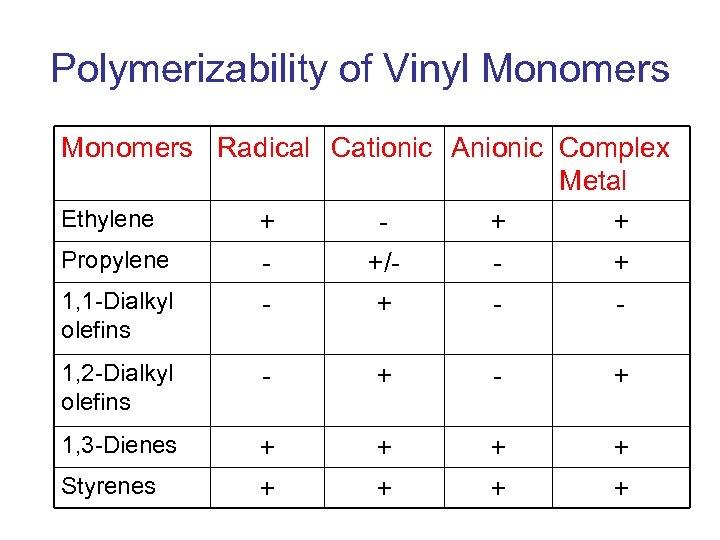 Polymerizability of Vinyl Monomers Radical Cationic Anionic Complex Metal Ethylene + + + Propylene +/+ 1, 1 -Dialkyl + olefins 1, 2 -Dialkyl olefins - + 1, 3 -Dienes + + + + Styrenes
Polymerizability of Vinyl Monomers Radical Cationic Anionic Complex Metal Ethylene + + + Propylene +/+ 1, 1 -Dialkyl + olefins 1, 2 -Dialkyl olefins - + 1, 3 -Dienes + + + + Styrenes
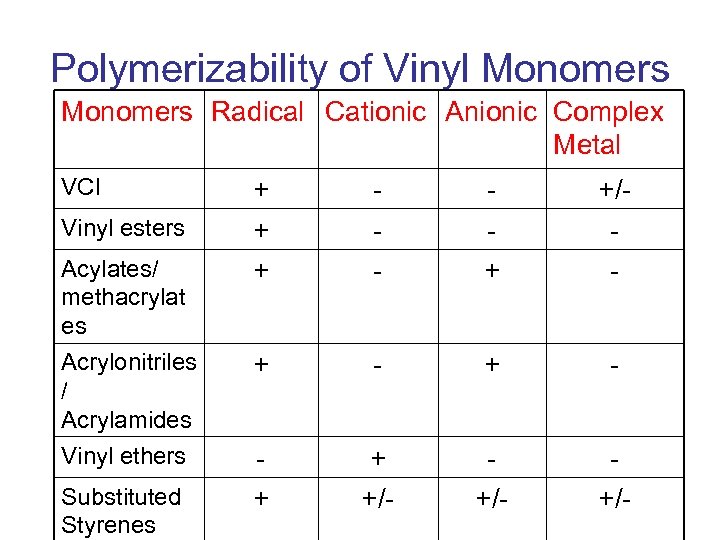 Polymerizability of Vinyl Monomers Radical Cationic Anionic Complex Metal VCl Vinyl esters Acylates/ methacrylat es Acrylonitriles / Acrylamides Vinyl ethers Substituted Styrenes + + + - + +/- + - + + +/- +/-
Polymerizability of Vinyl Monomers Radical Cationic Anionic Complex Metal VCl Vinyl esters Acylates/ methacrylat es Acrylonitriles / Acrylamides Vinyl ethers Substituted Styrenes + + + - + +/- + - + + +/- +/-
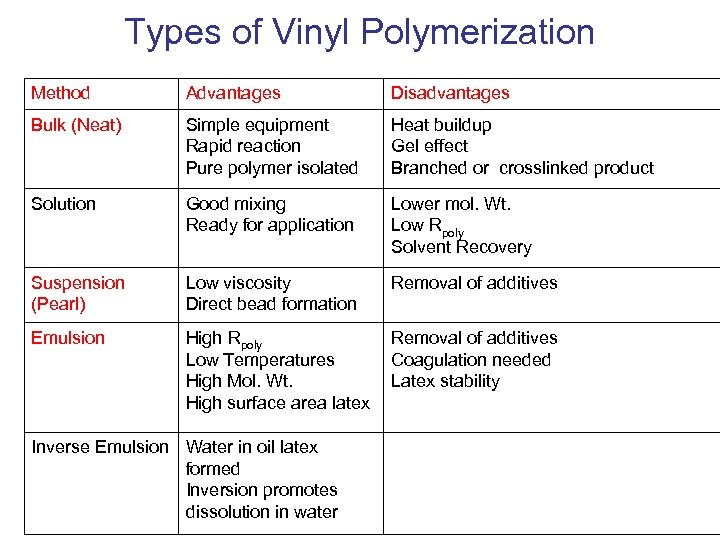 Types of Vinyl Polymerization Method Advantages Disadvantages Bulk (Neat) Simple equipment Rapid reaction Pure polymer isolated Heat buildup Gel effect Branched or crosslinked product Solution Good mixing Ready for application Lower mol. Wt. Low Rpoly Solvent Recovery Suspension (Pearl) Low viscosity Direct bead formation Removal of additives Emulsion High Rpoly Low Temperatures High Mol. Wt. High surface area latex Removal of additives Coagulation needed Latex stability Inverse Emulsion Water in oil latex formed Inversion promotes dissolution in water
Types of Vinyl Polymerization Method Advantages Disadvantages Bulk (Neat) Simple equipment Rapid reaction Pure polymer isolated Heat buildup Gel effect Branched or crosslinked product Solution Good mixing Ready for application Lower mol. Wt. Low Rpoly Solvent Recovery Suspension (Pearl) Low viscosity Direct bead formation Removal of additives Emulsion High Rpoly Low Temperatures High Mol. Wt. High surface area latex Removal of additives Coagulation needed Latex stability Inverse Emulsion Water in oil latex formed Inversion promotes dissolution in water
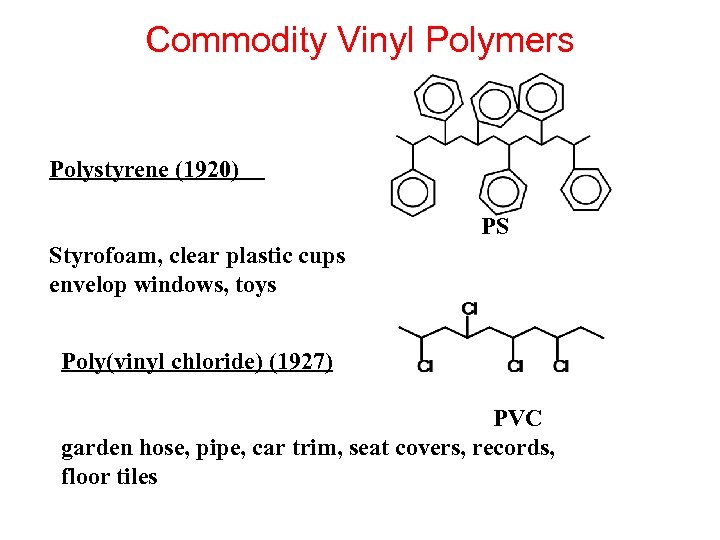 Commodity Vinyl Polymers Polystyrene (1920) PS Styrofoam, clear plastic cups envelop windows, toys Poly(vinyl chloride) (1927) PVC garden hose, pipe, car trim, seat covers, records, floor tiles
Commodity Vinyl Polymers Polystyrene (1920) PS Styrofoam, clear plastic cups envelop windows, toys Poly(vinyl chloride) (1927) PVC garden hose, pipe, car trim, seat covers, records, floor tiles
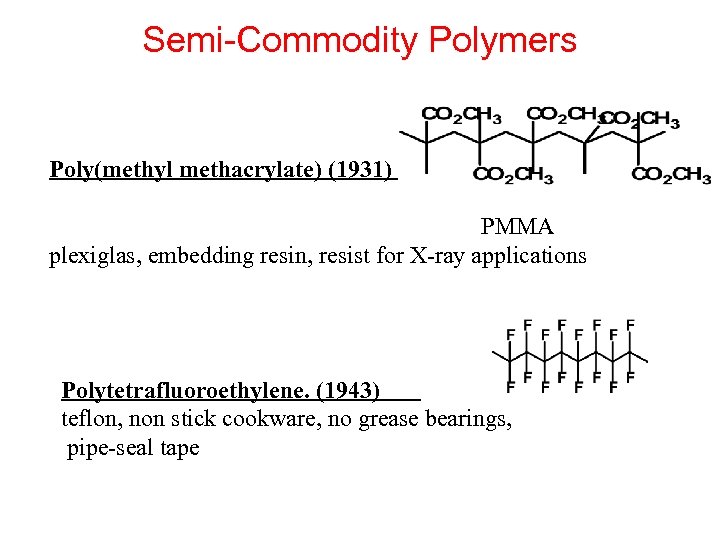 Semi-Commodity Polymers Poly(methyl methacrylate) (1931) PMMA plexiglas, embedding resin, resist for X-ray applications Polytetrafluoroethylene. (1943) teflon, non stick cookware, no grease bearings, pipe-seal tape
Semi-Commodity Polymers Poly(methyl methacrylate) (1931) PMMA plexiglas, embedding resin, resist for X-ray applications Polytetrafluoroethylene. (1943) teflon, non stick cookware, no grease bearings, pipe-seal tape
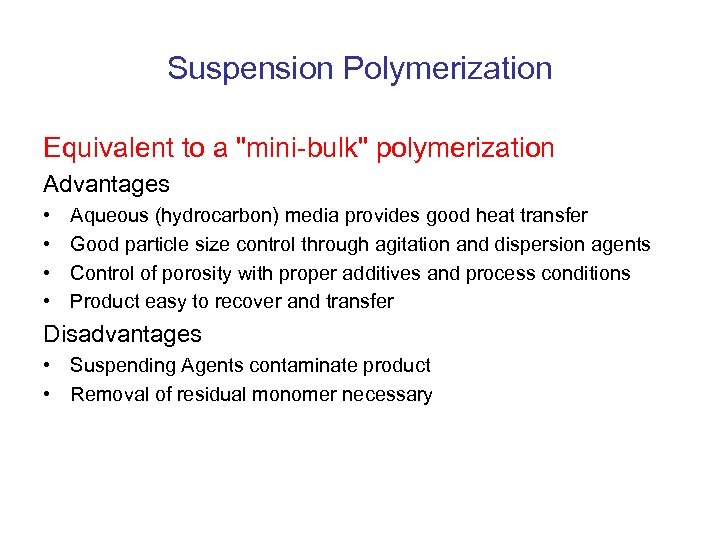 Suspension Polymerization Equivalent to a "mini-bulk" polymerization Advantages • • Aqueous (hydrocarbon) media provides good heat transfer Good particle size control through agitation and dispersion agents Control of porosity with proper additives and process conditions Product easy to recover and transfer Disadvantages • Suspending Agents contaminate product • Removal of residual monomer necessary
Suspension Polymerization Equivalent to a "mini-bulk" polymerization Advantages • • Aqueous (hydrocarbon) media provides good heat transfer Good particle size control through agitation and dispersion agents Control of porosity with proper additives and process conditions Product easy to recover and transfer Disadvantages • Suspending Agents contaminate product • Removal of residual monomer necessary
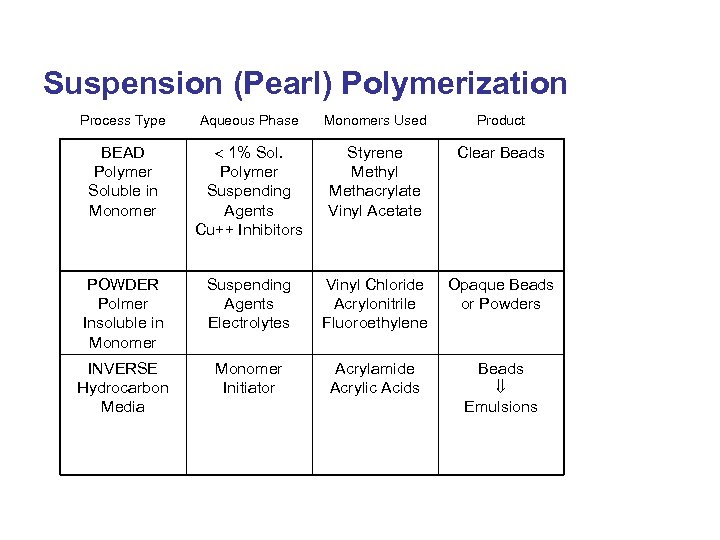 Suspension (Pearl) Polymerization Process Type Aqueous Phase Monomers Used Product BEAD Polymer Soluble in Monomer 1% Sol. Polymer Suspending Agents Cu++ Inhibitors Styrene Methyl Methacrylate Vinyl Acetate Clear Beads POWDER Polmer Insoluble in Monomer Suspending Agents Electrolytes Vinyl Chloride Acrylonitrile Fluoroethylene Opaque Beads or Powders INVERSE Hydrocarbon Media Monomer Initiator Acrylamide Acrylic Acids Beads Emulsions
Suspension (Pearl) Polymerization Process Type Aqueous Phase Monomers Used Product BEAD Polymer Soluble in Monomer 1% Sol. Polymer Suspending Agents Cu++ Inhibitors Styrene Methyl Methacrylate Vinyl Acetate Clear Beads POWDER Polmer Insoluble in Monomer Suspending Agents Electrolytes Vinyl Chloride Acrylonitrile Fluoroethylene Opaque Beads or Powders INVERSE Hydrocarbon Media Monomer Initiator Acrylamide Acrylic Acids Beads Emulsions
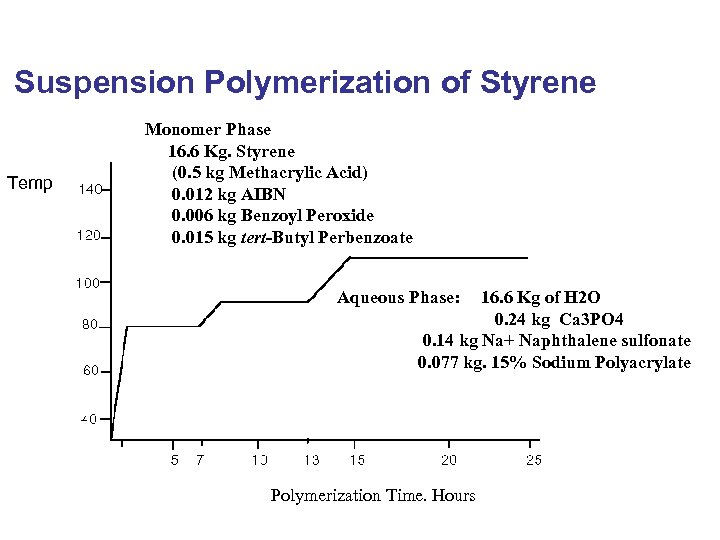 Suspension Polymerization of Styrene Temp Monomer Phase 16. 6 Kg. Styrene (0. 5 kg Methacrylic Acid) 0. 012 kg AIBN 0. 006 kg Benzoyl Peroxide 0. 015 kg tert-Butyl Perbenzoate Aqueous Phase: 16. 6 Kg of H 2 O 0. 24 kg Ca 3 PO 4 0. 14 kg Na+ Naphthalene sulfonate 0. 077 kg. 15% Sodium Polyacrylate Polymerization Time. Hours
Suspension Polymerization of Styrene Temp Monomer Phase 16. 6 Kg. Styrene (0. 5 kg Methacrylic Acid) 0. 012 kg AIBN 0. 006 kg Benzoyl Peroxide 0. 015 kg tert-Butyl Perbenzoate Aqueous Phase: 16. 6 Kg of H 2 O 0. 24 kg Ca 3 PO 4 0. 14 kg Na+ Naphthalene sulfonate 0. 077 kg. 15% Sodium Polyacrylate Polymerization Time. Hours
![EMULSION POLYMERIZATION • Advantages: • High rate of polymerization ~ kp[M] Npart/2 • High EMULSION POLYMERIZATION • Advantages: • High rate of polymerization ~ kp[M] Npart/2 • High](https://present5.com/presentation/df3a32ffd34acb79aea9bff58f83e63b/image-11.jpg) EMULSION POLYMERIZATION • Advantages: • High rate of polymerization ~ kp[M] Npart/2 • High molecular weights, ( ) of particles/ R. sec-1 = N kp [M] / Ri • • • Few side reactions High Conversion achieved Efficient heat transfer Low viscosity medium Polymer never in solution Low tendancy to agglomerate Emulsified polymer may be stabilized and used directly Disadvantages: Polymer surface contaminated by surface active agents Coagulation introduces salts; Poor electrical properties
EMULSION POLYMERIZATION • Advantages: • High rate of polymerization ~ kp[M] Npart/2 • High molecular weights, ( ) of particles/ R. sec-1 = N kp [M] / Ri • • • Few side reactions High Conversion achieved Efficient heat transfer Low viscosity medium Polymer never in solution Low tendancy to agglomerate Emulsified polymer may be stabilized and used directly Disadvantages: Polymer surface contaminated by surface active agents Coagulation introduces salts; Poor electrical properties
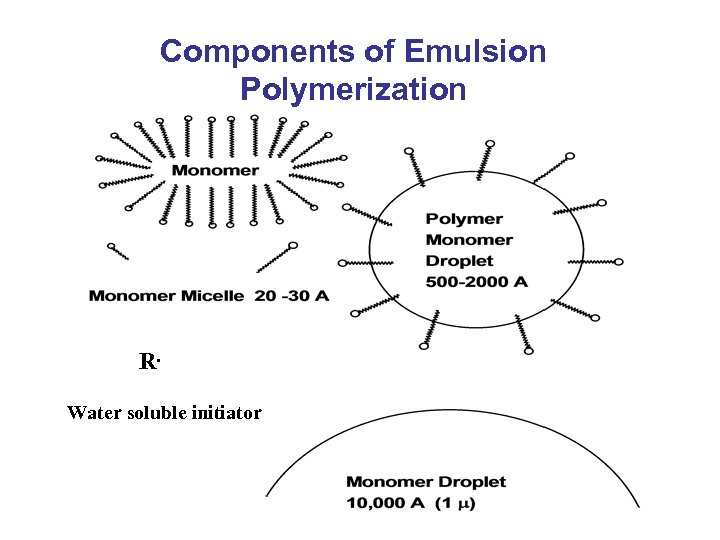 Components of Emulsion Polymerization R. Water soluble initiator
Components of Emulsion Polymerization R. Water soluble initiator
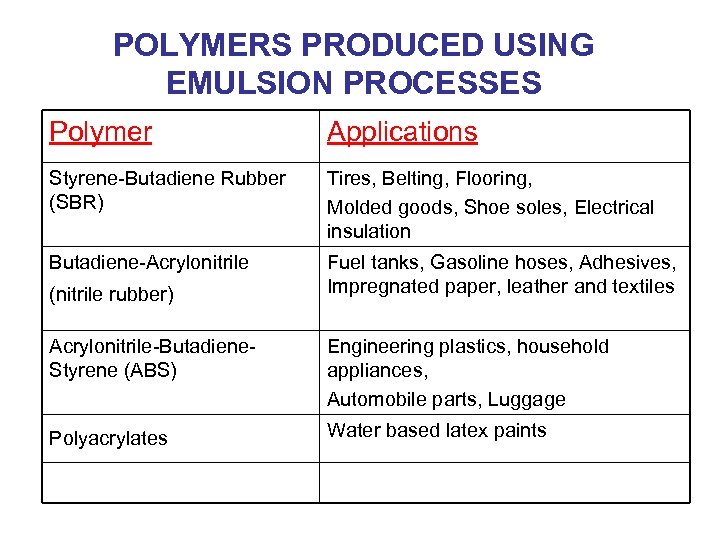 POLYMERS PRODUCED USING EMULSION PROCESSES Polymer Applications Styrene-Butadiene Rubber (SBR) Tires, Belting, Flooring, Molded goods, Shoe soles, Electrical insulation Butadiene-Acrylonitrile Fuel tanks, Gasoline hoses, Adhesives, Impregnated paper, leather and textiles (nitrile rubber) Acrylonitrile-Butadiene. Styrene (ABS) Engineering plastics, household appliances, Automobile parts, Luggage Polyacrylates Water based latex paints
POLYMERS PRODUCED USING EMULSION PROCESSES Polymer Applications Styrene-Butadiene Rubber (SBR) Tires, Belting, Flooring, Molded goods, Shoe soles, Electrical insulation Butadiene-Acrylonitrile Fuel tanks, Gasoline hoses, Adhesives, Impregnated paper, leather and textiles (nitrile rubber) Acrylonitrile-Butadiene. Styrene (ABS) Engineering plastics, household appliances, Automobile parts, Luggage Polyacrylates Water based latex paints
 Ziegler-Natta (Metal-Coordinated) Polymerization • • Stereochemical Control Polydisperse products Statistical Compositions and Sequences Limited set of useful monomers, i. e. olefins • SINGLE SITE CATALYSTS
Ziegler-Natta (Metal-Coordinated) Polymerization • • Stereochemical Control Polydisperse products Statistical Compositions and Sequences Limited set of useful monomers, i. e. olefins • SINGLE SITE CATALYSTS
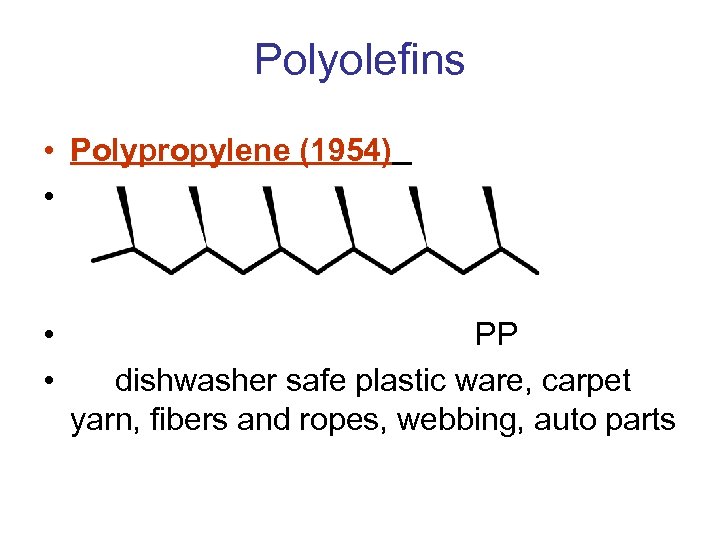 Polyolefins • Polypropylene (1954) • • • PP dishwasher safe plastic ware, carpet yarn, fibers and ropes, webbing, auto parts
Polyolefins • Polypropylene (1954) • • • PP dishwasher safe plastic ware, carpet yarn, fibers and ropes, webbing, auto parts
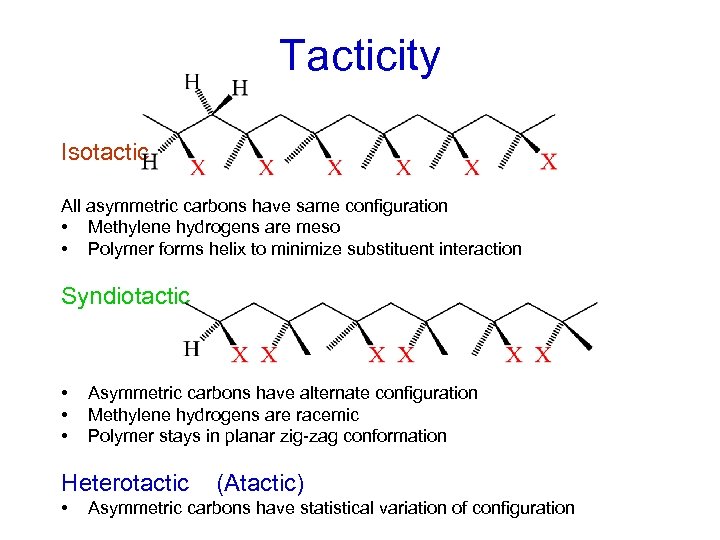 Tacticity Isotactic All asymmetric carbons have same configuration • Methylene hydrogens are meso • Polymer forms helix to minimize substituent interaction Syndiotactic • • • Asymmetric carbons have alternate configuration Methylene hydrogens are racemic Polymer stays in planar zig-zag conformation Heterotactic • (Atactic) Asymmetric carbons have statistical variation of configuration
Tacticity Isotactic All asymmetric carbons have same configuration • Methylene hydrogens are meso • Polymer forms helix to minimize substituent interaction Syndiotactic • • • Asymmetric carbons have alternate configuration Methylene hydrogens are racemic Polymer stays in planar zig-zag conformation Heterotactic • (Atactic) Asymmetric carbons have statistical variation of configuration
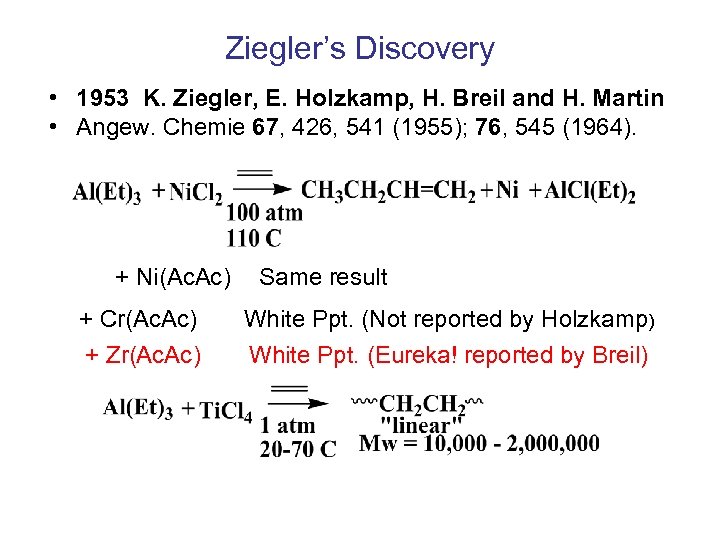 Ziegler’s Discovery • 1953 K. Ziegler, E. Holzkamp, H. Breil and H. Martin • Angew. Chemie 67, 426, 541 (1955); 76, 545 (1964). + Ni(Ac. Ac) + Cr(Ac. Ac) + Zr(Ac. Ac) Same result White Ppt. (Not reported by Holzkamp) White Ppt. (Eureka! reported by Breil)
Ziegler’s Discovery • 1953 K. Ziegler, E. Holzkamp, H. Breil and H. Martin • Angew. Chemie 67, 426, 541 (1955); 76, 545 (1964). + Ni(Ac. Ac) + Cr(Ac. Ac) + Zr(Ac. Ac) Same result White Ppt. (Not reported by Holzkamp) White Ppt. (Eureka! reported by Breil)
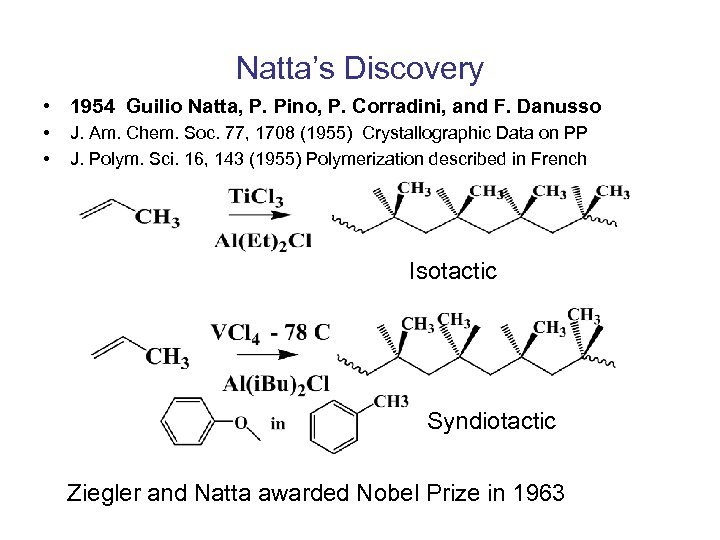 Natta’s Discovery • 1954 Guilio Natta, P. Pino, P. Corradini, and F. Danusso • • J. Am. Chem. Soc. 77, 1708 (1955) Crystallographic Data on PP J. Polym. Sci. 16, 143 (1955) Polymerization described in French Isotactic Syndiotactic Ziegler and Natta awarded Nobel Prize in 1963
Natta’s Discovery • 1954 Guilio Natta, P. Pino, P. Corradini, and F. Danusso • • J. Am. Chem. Soc. 77, 1708 (1955) Crystallographic Data on PP J. Polym. Sci. 16, 143 (1955) Polymerization described in French Isotactic Syndiotactic Ziegler and Natta awarded Nobel Prize in 1963
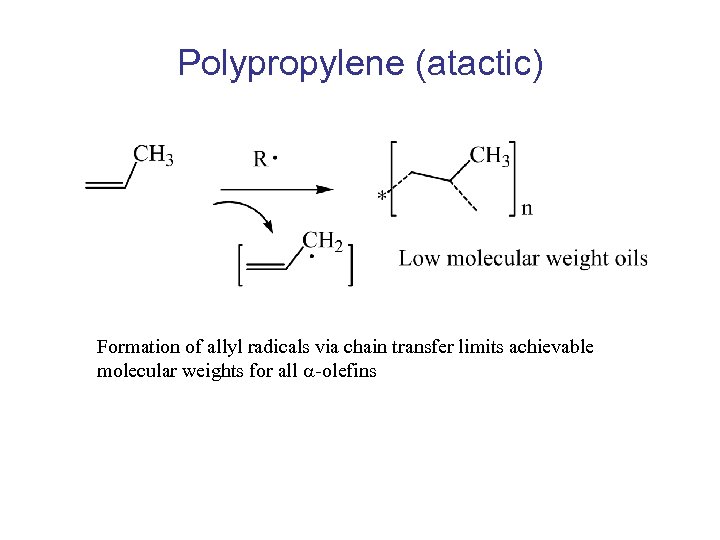 Polypropylene (atactic) Formation of allyl radicals via chain transfer limits achievable molecular weights for all -olefins
Polypropylene (atactic) Formation of allyl radicals via chain transfer limits achievable molecular weights for all -olefins
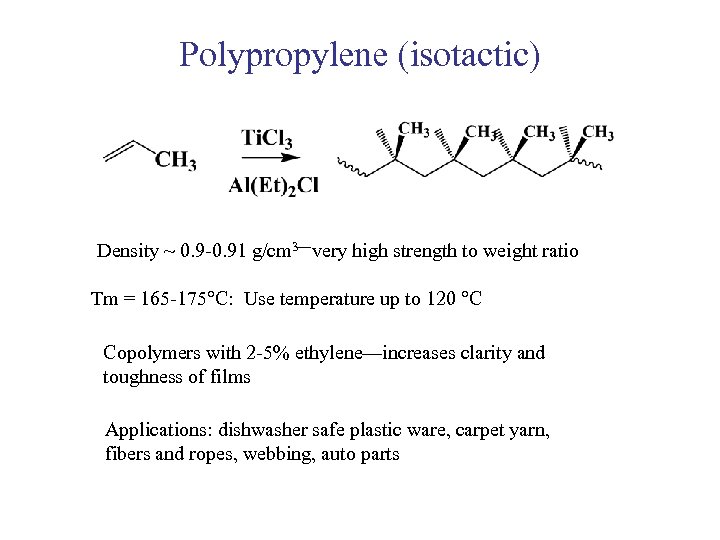 Polypropylene (isotactic) Density ~ 0. 9 -0. 91 g/cm 3—very high strength to weight ratio Tm = 165 -175 C: Use temperature up to 120 C Copolymers with 2 -5% ethylene—increases clarity and toughness of films Applications: dishwasher safe plastic ware, carpet yarn, fibers and ropes, webbing, auto parts
Polypropylene (isotactic) Density ~ 0. 9 -0. 91 g/cm 3—very high strength to weight ratio Tm = 165 -175 C: Use temperature up to 120 C Copolymers with 2 -5% ethylene—increases clarity and toughness of films Applications: dishwasher safe plastic ware, carpet yarn, fibers and ropes, webbing, auto parts
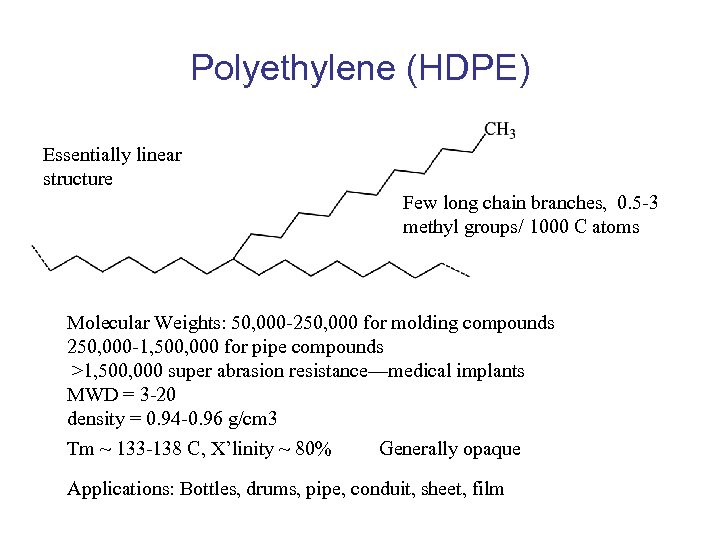 Polyethylene (HDPE) Essentially linear structure Few long chain branches, 0. 5 -3 methyl groups/ 1000 C atoms Molecular Weights: 50, 000 -250, 000 for molding compounds 250, 000 -1, 500, 000 for pipe compounds >1, 500, 000 super abrasion resistance—medical implants MWD = 3 -20 density = 0. 94 -0. 96 g/cm 3 Tm ~ 133 -138 C, X’linity ~ 80% Generally opaque Applications: Bottles, drums, pipe, conduit, sheet, film
Polyethylene (HDPE) Essentially linear structure Few long chain branches, 0. 5 -3 methyl groups/ 1000 C atoms Molecular Weights: 50, 000 -250, 000 for molding compounds 250, 000 -1, 500, 000 for pipe compounds >1, 500, 000 super abrasion resistance—medical implants MWD = 3 -20 density = 0. 94 -0. 96 g/cm 3 Tm ~ 133 -138 C, X’linity ~ 80% Generally opaque Applications: Bottles, drums, pipe, conduit, sheet, film
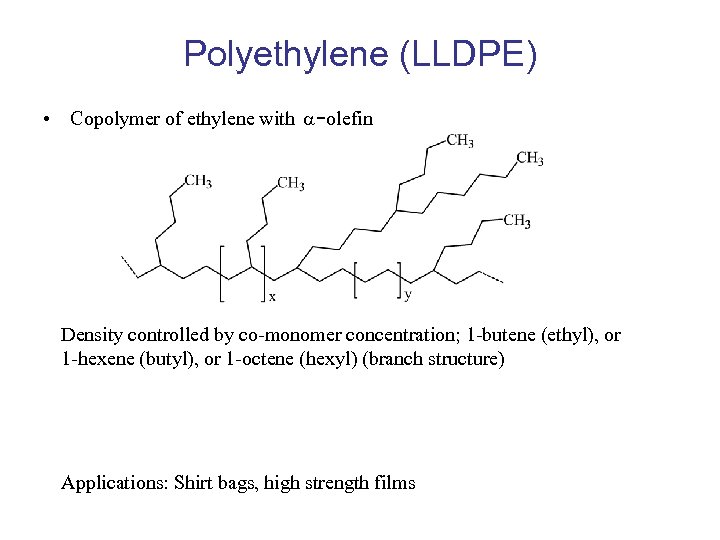 Polyethylene (LLDPE) • Copolymer of ethylene with -olefin Density controlled by co-monomer concentration; 1 -butene (ethyl), or 1 -hexene (butyl), or 1 -octene (hexyl) (branch structure) Applications: Shirt bags, high strength films
Polyethylene (LLDPE) • Copolymer of ethylene with -olefin Density controlled by co-monomer concentration; 1 -butene (ethyl), or 1 -hexene (butyl), or 1 -octene (hexyl) (branch structure) Applications: Shirt bags, high strength films
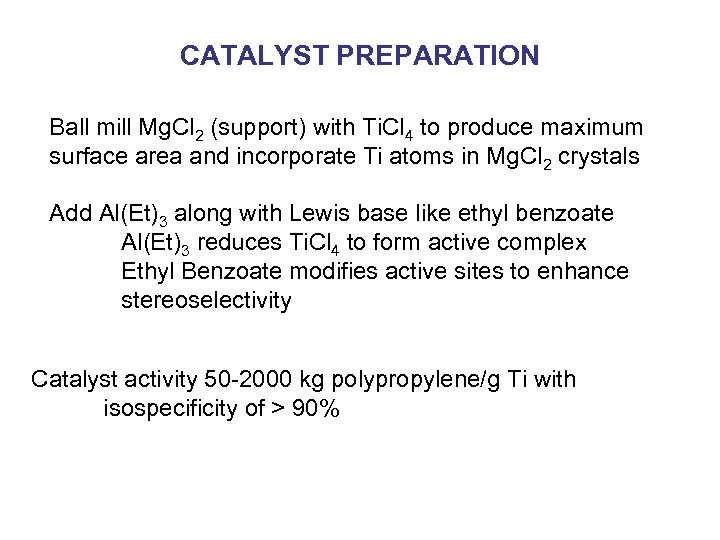 CATALYST PREPARATION Ball mill Mg. Cl 2 (support) with Ti. Cl 4 to produce maximum surface area and incorporate Ti atoms in Mg. Cl 2 crystals Add Al(Et)3 along with Lewis base like ethyl benzoate Al(Et)3 reduces Ti. Cl 4 to form active complex Ethyl Benzoate modifies active sites to enhance stereoselectivity Catalyst activity 50 -2000 kg polypropylene/g Ti with isospecificity of > 90%
CATALYST PREPARATION Ball mill Mg. Cl 2 (support) with Ti. Cl 4 to produce maximum surface area and incorporate Ti atoms in Mg. Cl 2 crystals Add Al(Et)3 along with Lewis base like ethyl benzoate Al(Et)3 reduces Ti. Cl 4 to form active complex Ethyl Benzoate modifies active sites to enhance stereoselectivity Catalyst activity 50 -2000 kg polypropylene/g Ti with isospecificity of > 90%
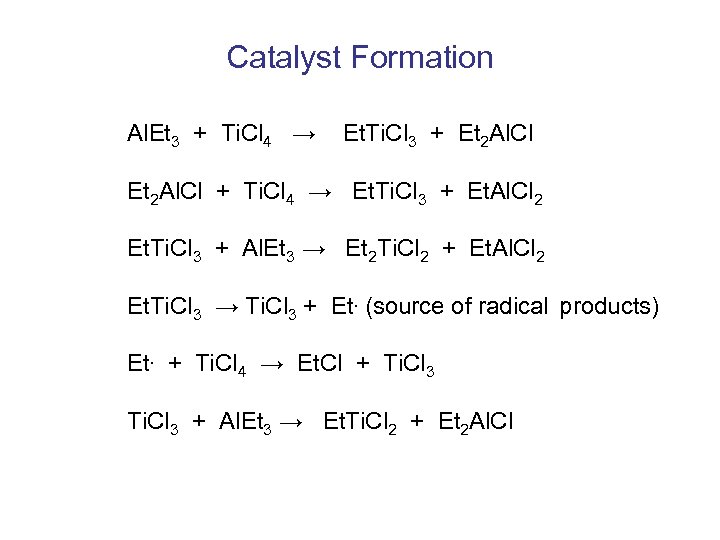 Catalyst Formation Al. Et 3 + Ti. Cl 4 → Et. Ti. Cl 3 + Et 2 Al. Cl + Ti. Cl 4 → Et. Ti. Cl 3 + Et. Al. Cl 2 Et. Ti. Cl 3 + Al. Et 3 → Et 2 Ti. Cl 2 + Et. Al. Cl 2 Et. Ti. Cl 3 → Ti. Cl 3 + Et. (source of radical products) Et. + Ti. Cl 4 → Et. Cl + Ti. Cl 3 + Al. Et 3 → Et. Ti. Cl 2 + Et 2 Al. Cl
Catalyst Formation Al. Et 3 + Ti. Cl 4 → Et. Ti. Cl 3 + Et 2 Al. Cl + Ti. Cl 4 → Et. Ti. Cl 3 + Et. Al. Cl 2 Et. Ti. Cl 3 + Al. Et 3 → Et 2 Ti. Cl 2 + Et. Al. Cl 2 Et. Ti. Cl 3 → Ti. Cl 3 + Et. (source of radical products) Et. + Ti. Cl 4 → Et. Cl + Ti. Cl 3 + Al. Et 3 → Et. Ti. Cl 2 + Et 2 Al. Cl
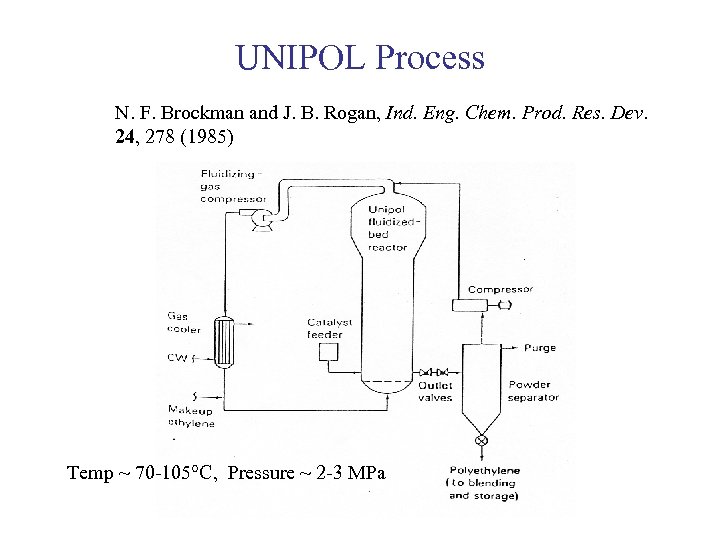 UNIPOL Process N. F. Brockman and J. B. Rogan, Ind. Eng. Chem. Prod. Res. Dev. 24, 278 (1985) Temp ~ 70 -105°C, Pressure ~ 2 -3 MPa
UNIPOL Process N. F. Brockman and J. B. Rogan, Ind. Eng. Chem. Prod. Res. Dev. 24, 278 (1985) Temp ~ 70 -105°C, Pressure ~ 2 -3 MPa
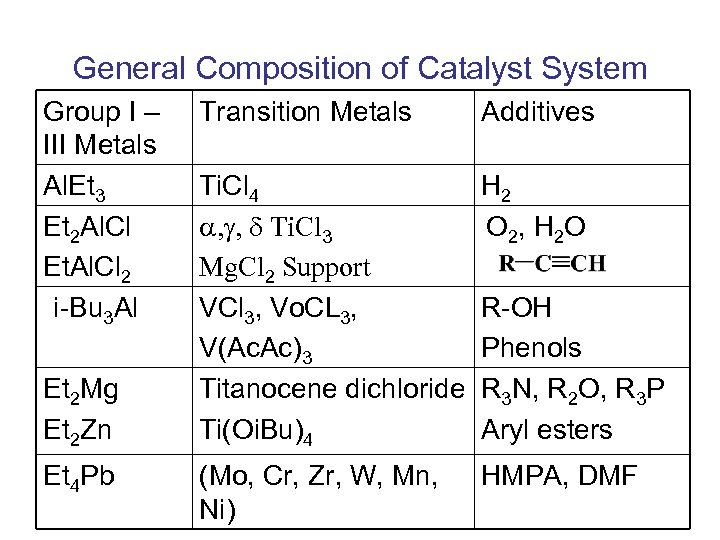 General Composition of Catalyst System Group I – III Metals Al. Et 3 Et 2 Al. Cl Et. Al. Cl 2 i-Bu 3 Al Et 2 Mg Et 2 Zn Et 4 Pb Transition Metals Additives Ti. Cl 4 , g, d Ti. Cl 3 Mg. Cl 2 Support VCl 3, Vo. CL 3, V(Ac. Ac)3 Titanocene dichloride Ti(Oi. Bu)4 H 2 O 2, H 2 O (Mo, Cr, Zr, W, Mn, Ni) HMPA, DMF R-OH Phenols R 3 N, R 2 O, R 3 P Aryl esters
General Composition of Catalyst System Group I – III Metals Al. Et 3 Et 2 Al. Cl Et. Al. Cl 2 i-Bu 3 Al Et 2 Mg Et 2 Zn Et 4 Pb Transition Metals Additives Ti. Cl 4 , g, d Ti. Cl 3 Mg. Cl 2 Support VCl 3, Vo. CL 3, V(Ac. Ac)3 Titanocene dichloride Ti(Oi. Bu)4 H 2 O 2, H 2 O (Mo, Cr, Zr, W, Mn, Ni) HMPA, DMF R-OH Phenols R 3 N, R 2 O, R 3 P Aryl esters
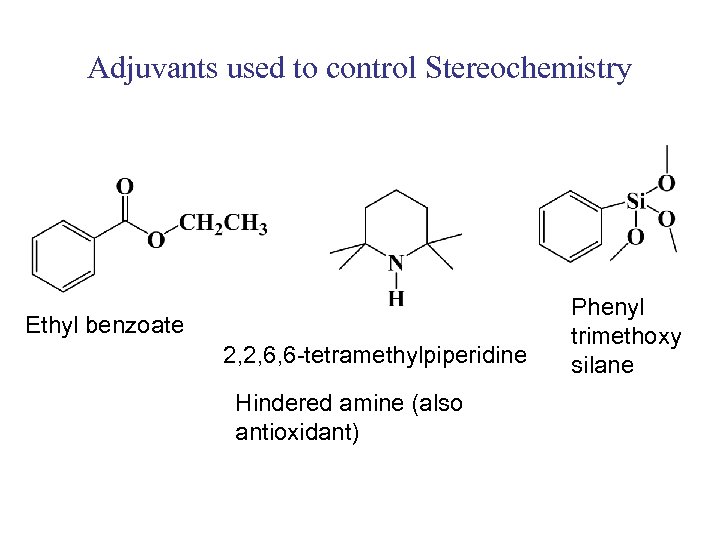 Adjuvants used to control Stereochemistry Ethyl benzoate 2, 2, 6, 6 -tetramethylpiperidine Hindered amine (also antioxidant) Phenyl trimethoxy silane
Adjuvants used to control Stereochemistry Ethyl benzoate 2, 2, 6, 6 -tetramethylpiperidine Hindered amine (also antioxidant) Phenyl trimethoxy silane
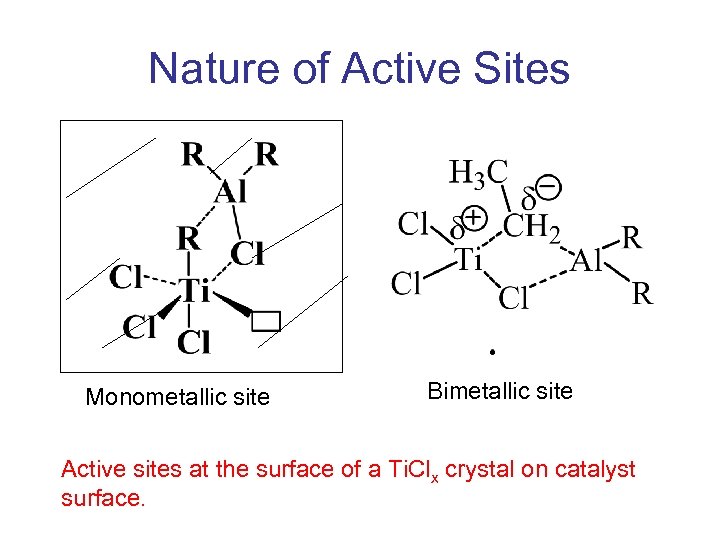 Nature of Active Sites Monometallic site Bimetallic site Active sites at the surface of a Ti. Clx crystal on catalyst surface.
Nature of Active Sites Monometallic site Bimetallic site Active sites at the surface of a Ti. Clx crystal on catalyst surface.
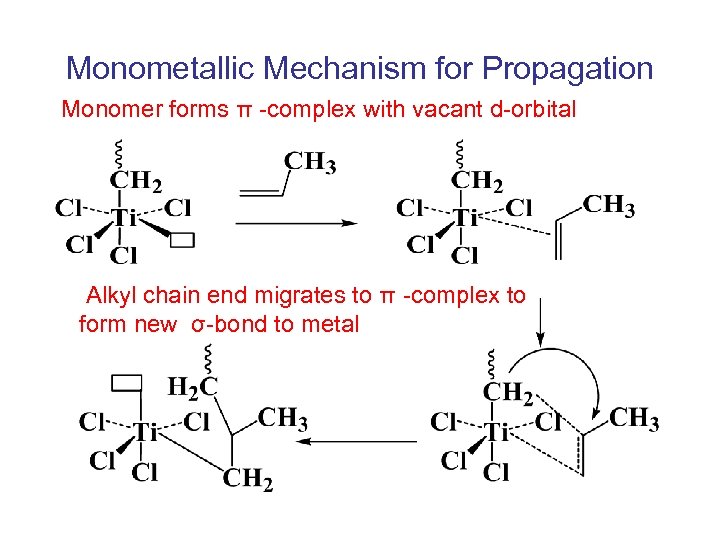 Monometallic Mechanism for Propagation Monomer forms π -complex with vacant d-orbital Alkyl chain end migrates to π -complex to form new σ-bond to metal
Monometallic Mechanism for Propagation Monomer forms π -complex with vacant d-orbital Alkyl chain end migrates to π -complex to form new σ-bond to metal
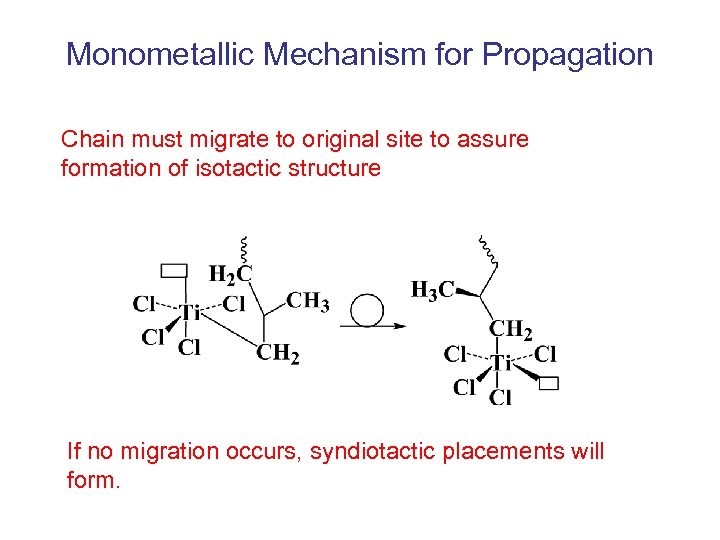 Monometallic Mechanism for Propagation Chain must migrate to original site to assure formation of isotactic structure If no migration occurs, syndiotactic placements will form.
Monometallic Mechanism for Propagation Chain must migrate to original site to assure formation of isotactic structure If no migration occurs, syndiotactic placements will form.
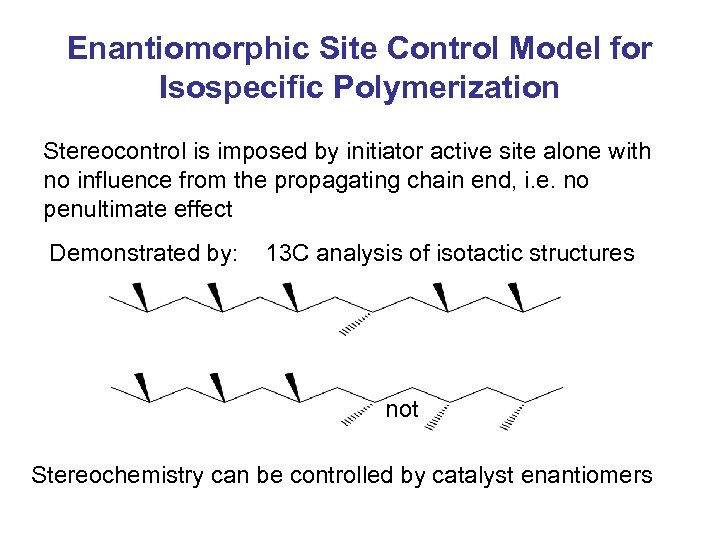 Enantiomorphic Site Control Model for Isospecific Polymerization Stereocontrol is imposed by initiator active site alone with no influence from the propagating chain end, i. e. no penultimate effect Demonstrated by: 13 C analysis of isotactic structures not Stereochemistry can be controlled by catalyst enantiomers
Enantiomorphic Site Control Model for Isospecific Polymerization Stereocontrol is imposed by initiator active site alone with no influence from the propagating chain end, i. e. no penultimate effect Demonstrated by: 13 C analysis of isotactic structures not Stereochemistry can be controlled by catalyst enantiomers
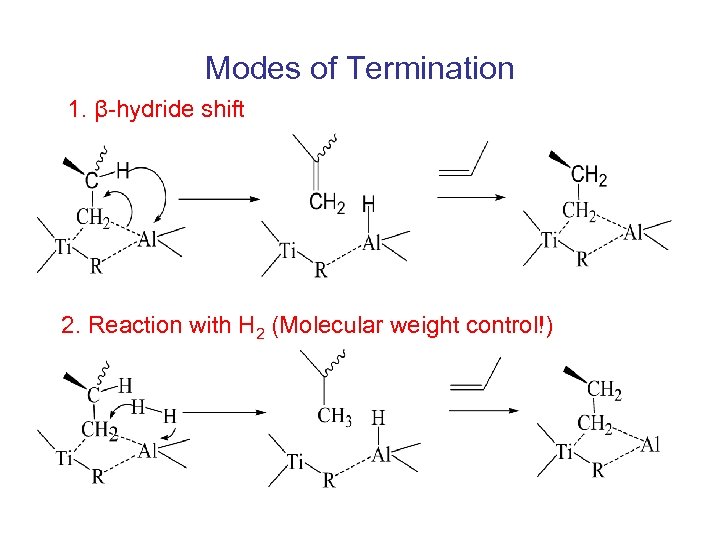 Modes of Termination 1. β-hydride shift 2. Reaction with H 2 (Molecular weight control!)
Modes of Termination 1. β-hydride shift 2. Reaction with H 2 (Molecular weight control!)
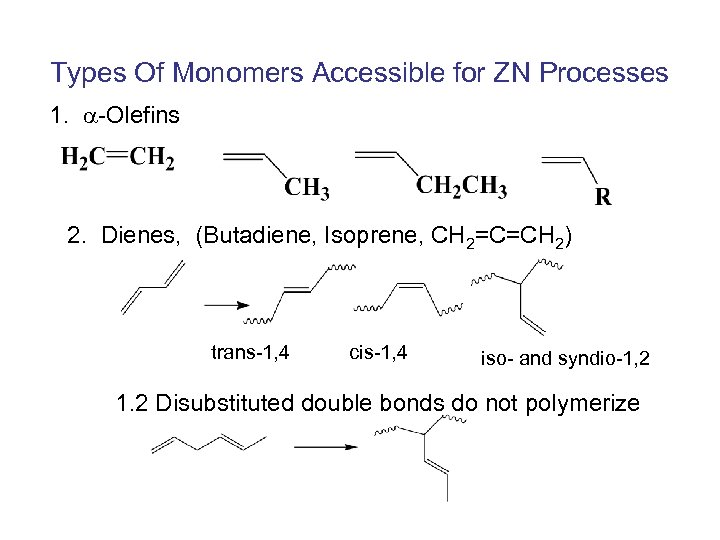 Types Of Monomers Accessible for ZN Processes 1. -Olefins 2. Dienes, (Butadiene, Isoprene, CH 2=C=CH 2) trans-1, 4 cis-1, 4 iso- and syndio-1, 2 1. 2 Disubstituted double bonds do not polymerize
Types Of Monomers Accessible for ZN Processes 1. -Olefins 2. Dienes, (Butadiene, Isoprene, CH 2=C=CH 2) trans-1, 4 cis-1, 4 iso- and syndio-1, 2 1. 2 Disubstituted double bonds do not polymerize
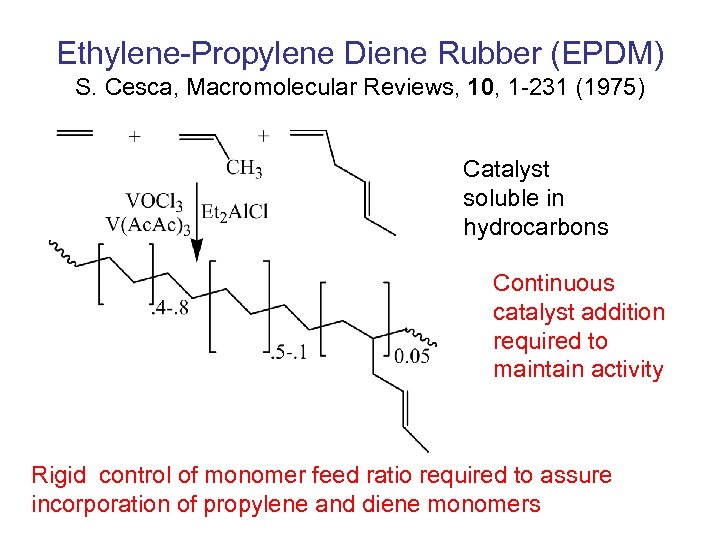 Ethylene-Propylene Diene Rubber (EPDM) S. Cesca, Macromolecular Reviews, 10, 1 -231 (1975) Catalyst soluble in hydrocarbons Continuous catalyst addition required to maintain activity Rigid control of monomer feed ratio required to assure incorporation of propylene and diene monomers
Ethylene-Propylene Diene Rubber (EPDM) S. Cesca, Macromolecular Reviews, 10, 1 -231 (1975) Catalyst soluble in hydrocarbons Continuous catalyst addition required to maintain activity Rigid control of monomer feed ratio required to assure incorporation of propylene and diene monomers
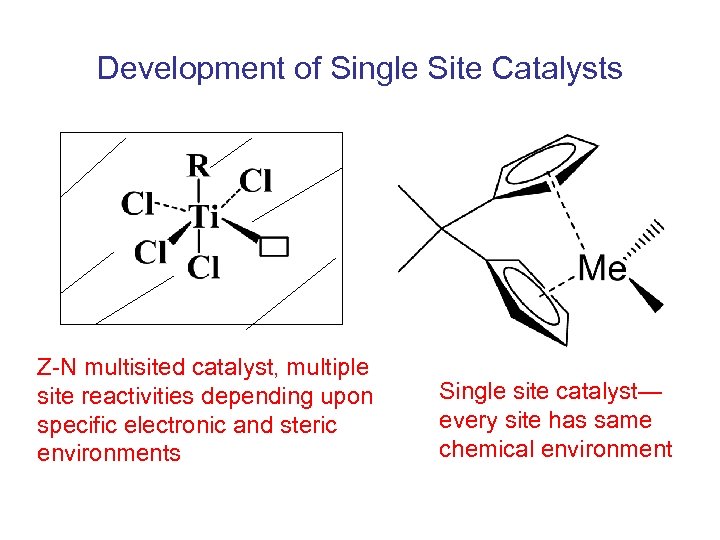 Development of Single Site Catalysts Z-N multisited catalyst, multiple site reactivities depending upon specific electronic and steric environments Single site catalyst— every site has same chemical environment
Development of Single Site Catalysts Z-N multisited catalyst, multiple site reactivities depending upon specific electronic and steric environments Single site catalyst— every site has same chemical environment
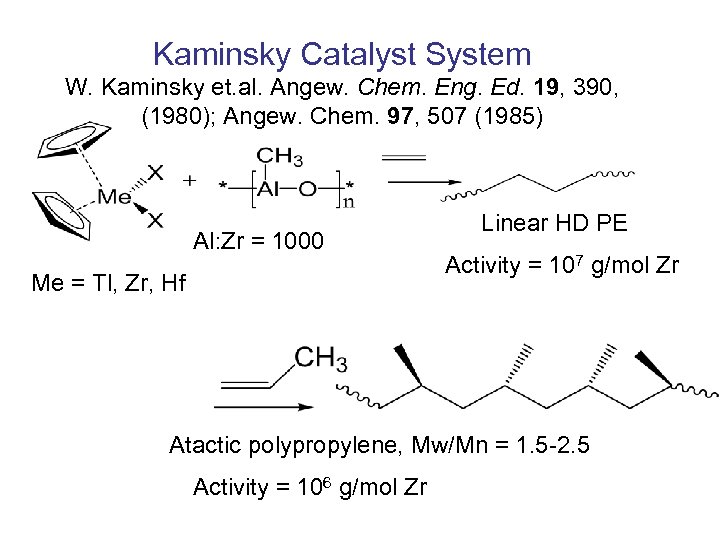 Kaminsky Catalyst System W. Kaminsky et. al. Angew. Chem. Eng. Ed. 19, 390, (1980); Angew. Chem. 97, 507 (1985) Al: Zr = 1000 Me = Tl, Zr, Hf Linear HD PE Activity = 107 g/mol Zr Atactic polypropylene, Mw/Mn = 1. 5 -2. 5 Activity = 106 g/mol Zr
Kaminsky Catalyst System W. Kaminsky et. al. Angew. Chem. Eng. Ed. 19, 390, (1980); Angew. Chem. 97, 507 (1985) Al: Zr = 1000 Me = Tl, Zr, Hf Linear HD PE Activity = 107 g/mol Zr Atactic polypropylene, Mw/Mn = 1. 5 -2. 5 Activity = 106 g/mol Zr
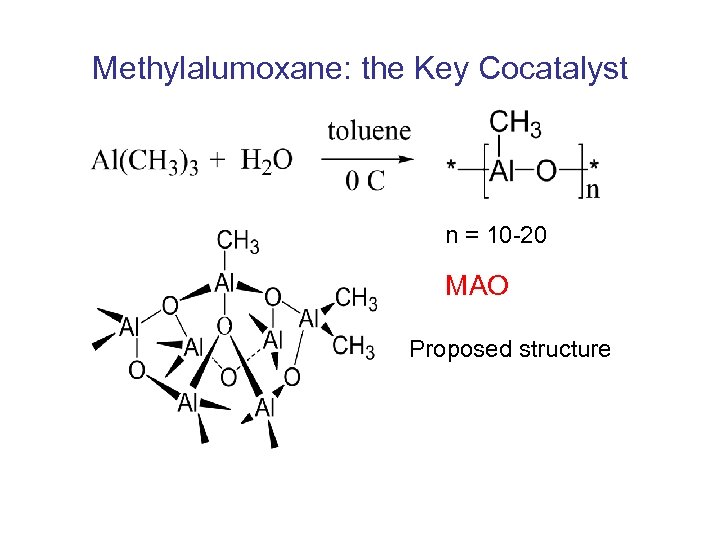 Methylalumoxane: the Key Cocatalyst n = 10 -20 MAO Proposed structure
Methylalumoxane: the Key Cocatalyst n = 10 -20 MAO Proposed structure
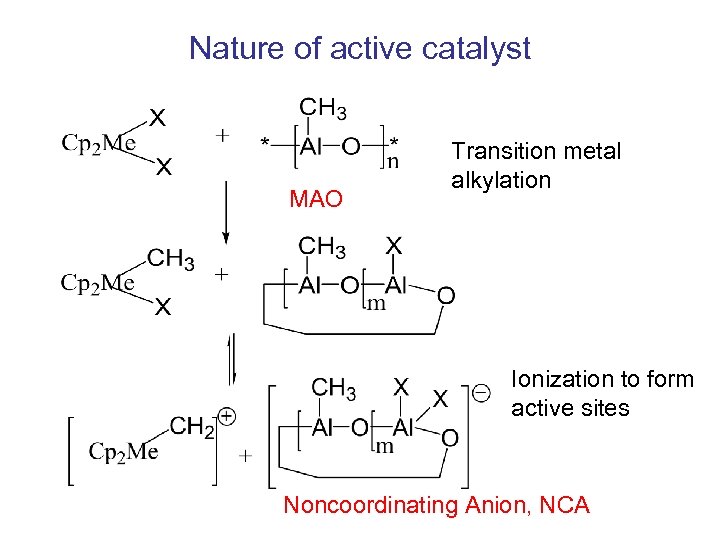 Nature of active catalyst MAO Transition metal alkylation Ionization to form active sites Noncoordinating Anion, NCA
Nature of active catalyst MAO Transition metal alkylation Ionization to form active sites Noncoordinating Anion, NCA
 Homogeneous Z-N Polymerization Advantages: High Catalytic Activity Impressive control of stereochemistry Well defined catalyst precursors Design of Polymer microstructures, including chiral polymers Disadvantages: Requires large excess of Aluminoxane (counter-ion) Higher tendency for chain termination: β-H elimination, etc. Limited control of molecular weight distribution
Homogeneous Z-N Polymerization Advantages: High Catalytic Activity Impressive control of stereochemistry Well defined catalyst precursors Design of Polymer microstructures, including chiral polymers Disadvantages: Requires large excess of Aluminoxane (counter-ion) Higher tendency for chain termination: β-H elimination, etc. Limited control of molecular weight distribution
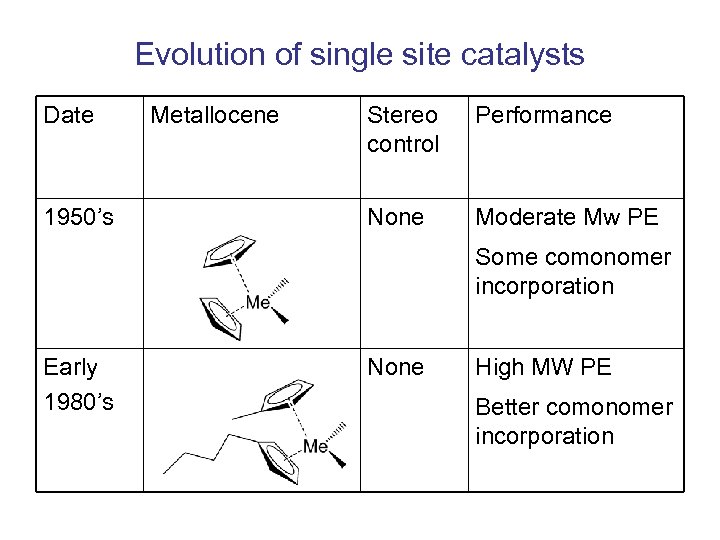 Evolution of single site catalysts Date 1950’s Metallocene Stereo control Performance None Moderate Mw PE Some comonomer incorporation Early 1980’s None High MW PE Better comonomer incorporation
Evolution of single site catalysts Date 1950’s Metallocene Stereo control Performance None Moderate Mw PE Some comonomer incorporation Early 1980’s None High MW PE Better comonomer incorporation
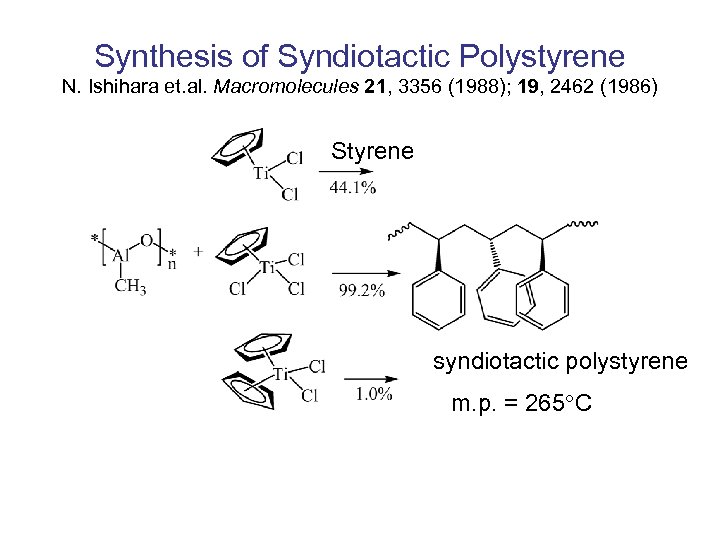 Synthesis of Syndiotactic Polystyrene N. Ishihara et. al. Macromolecules 21, 3356 (1988); 19, 2462 (1986) Styrene syndiotactic polystyrene m. p. = 265 C
Synthesis of Syndiotactic Polystyrene N. Ishihara et. al. Macromolecules 21, 3356 (1988); 19, 2462 (1986) Styrene syndiotactic polystyrene m. p. = 265 C
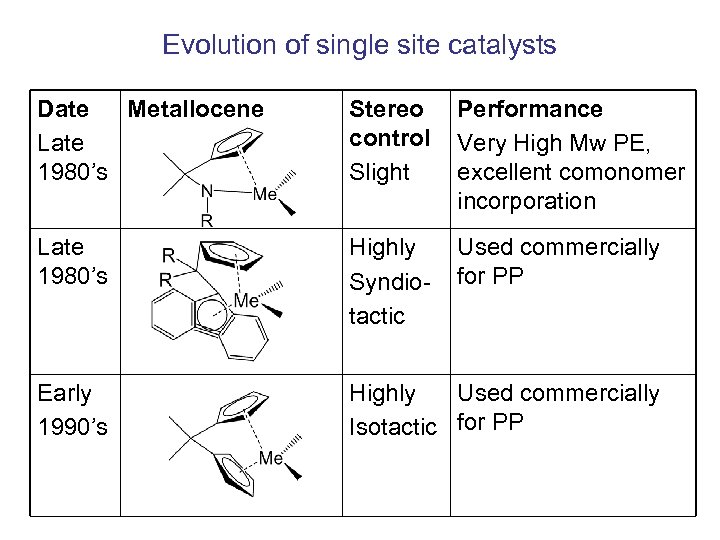 Evolution of single site catalysts Date Metallocene Late 1980’s Stereo control Slight Performance Very High Mw PE, excellent comonomer incorporation Late 1980’s Highly Syndiotactic Used commercially for PP Early 1990’s Highly Used commercially Isotactic for PP
Evolution of single site catalysts Date Metallocene Late 1980’s Stereo control Slight Performance Very High Mw PE, excellent comonomer incorporation Late 1980’s Highly Syndiotactic Used commercially for PP Early 1990’s Highly Used commercially Isotactic for PP
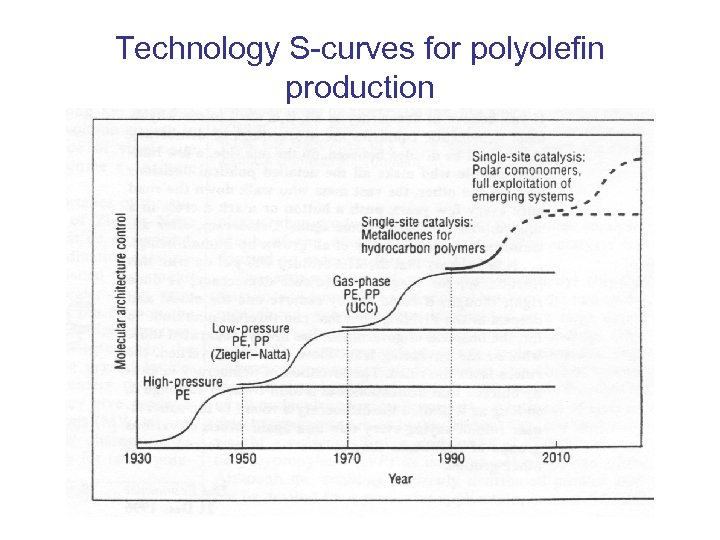 Technology S-curves for polyolefin production
Technology S-curves for polyolefin production


