31ed71c405ff54bb47883cf5cea08f2d.ppt
- Количество слайдов: 29

Analysis Table Translation Tool -From English to Chinese Jessie Zhao, intern Mina Chen, intern Shengying Cheng, Project Manager of the Internship Program Yanyun Shen, Site Head of APAC Statistical Programming & Analysis GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations 1

Topics I. High level introduction about the project II. Table translation process and tool features III. Recommended working process GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations

High level introduction about the project GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations
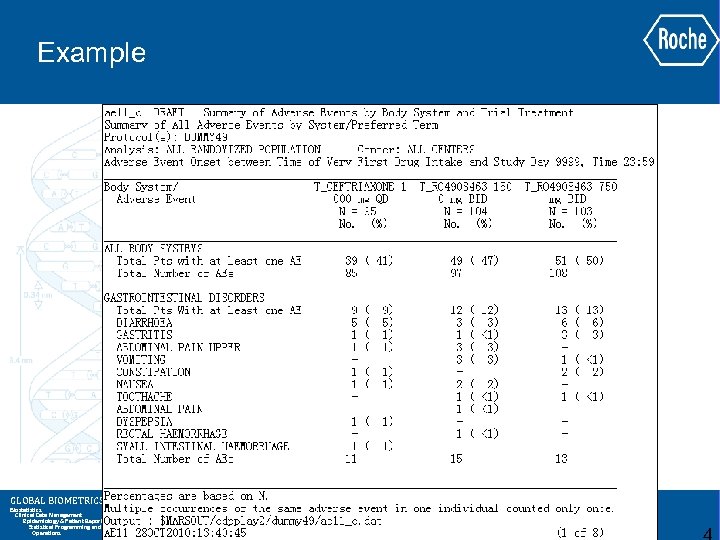
Example GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations
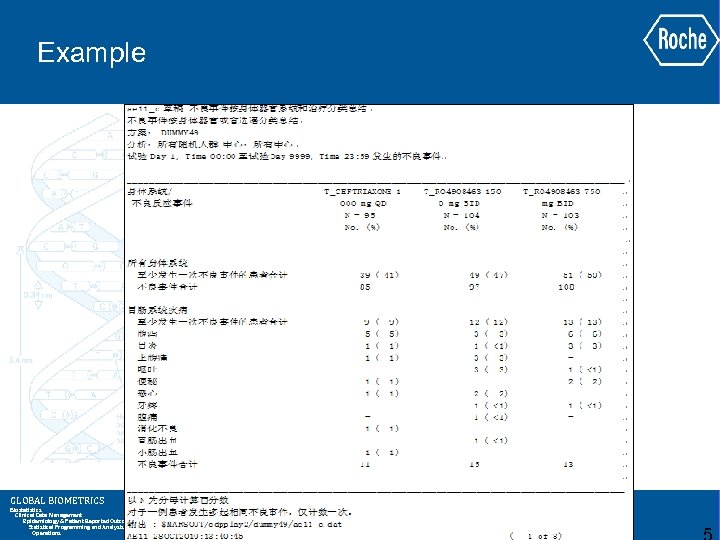
Example GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations
Why is it necessary to develop this tool? Ø According to s. FDA (State Food and Drug Administration) requirement, all CSR including in-text tables should be in Chinese Ø Previously the translation was performed manually by vendor companies which require statisticians and clinical science’s review which is both costly and extra time consuming Ø There was risk to edit the statistical result incidentally Ø Due to system restrictions from the company, Chinese analysis tables can not be generated directly from SAS software GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations

What’s the benefit of this tool? Ø Produce high quality tables in Chinese ü To translate AE terms, we utilize the Med. DRA and its Chinese translation published by MSSO (Maintenance and Support Services Organization) ü Quality check is not necessary to be repeated once we have a dictionary to translate some other frequent used terms Ø Accelerate the submission ü Reduce the time for CSR translation ü Our colleagues will save time for checking the quality and discussion back and forth ü Our colleagues don’t need reformat the translated tables manually because we can generate the Chinese tables with the same format as those tables in English GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations

What’s the benefit of this tool? (cont. ) Ø Efficiency gains ü This tool can be reused for any studies in any therapeutic areas ü This tool is user friendly. It’s very easy to learn how to use it Ø It’s applicable to translate tables from English to other languages ü It can be used for other language translation by using the same program ü We only need update the dictionaries GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations

Challenges Ø We can’t translate medication terms as the same way as we translate adverse event terms. Ø We are using in-house dictionary which is only in English to do medical coding for medication. Ø The company didn’t purchase WHODD (WHO Drug Dictionary) which has Chinese translation for medication terms Ø There are two ways to solve this issue, either to create our own Chinese translation for all medications used in our studies or purchase any commercial dictionary. It needs to be further investigated. GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations

Introduction of the Tool Design and the Features GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations
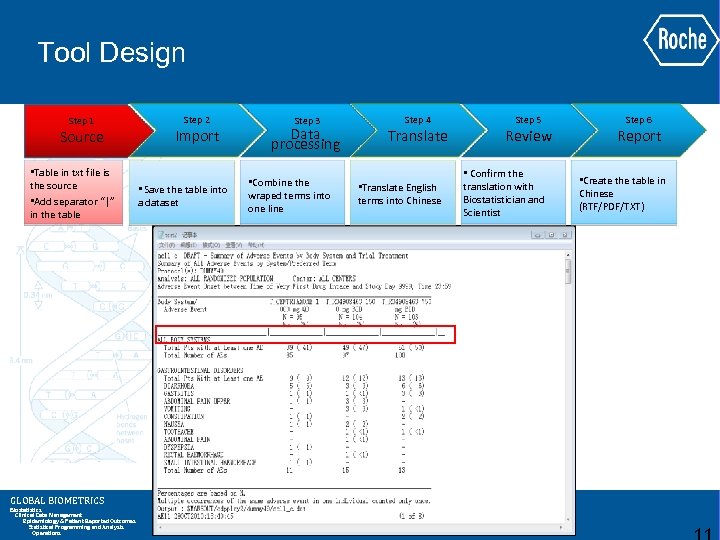
Tool Design Step 1 Source • Table in txt file is the source • Add separator “|” in the table GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations Step 2 Import • Save the table into a dataset Step 3 Data processing • Combine the wraped terms into one line Step 4 Translate • Translate English terms into Chinese Step 5 Review • Confirm the translation with Biostatistician and Scientist Step 6 Report • Create the table in Chinese (RTF/PDF/TXT)
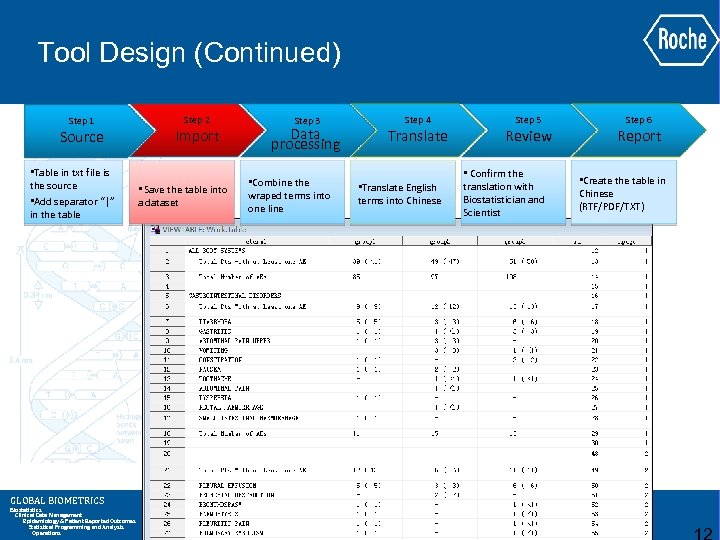
Tool Design (Continued) Step 1 Source • Table in txt file is the source • Add separator “|” in the table GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations Step 2 Import • Save the table into a dataset Step 3 Data processing • Combine the wraped terms into one line Step 4 Translate • Translate English terms into Chinese Step 5 Review • Confirm the translation with Biostatistician and Scientist Step 6 Report • Create the table in Chinese (RTF/PDF/TXT)
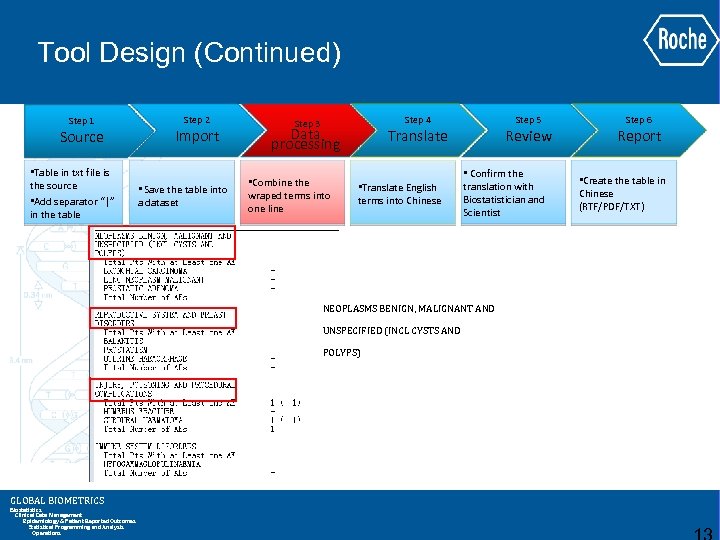
Tool Design (Continued) Step 1 Source • Table in txt file is the source • Add separator “|” in the table Step 2 Import • Save the table into a dataset Step 4 Step 3 Data processing • Combine the wraped terms into one line Step 5 Translate • Translate English terms into Chinese Review • Confirm the translation with Biostatistician and Scientist NEOPLASMS BENIGN, MALIGNANT AND UNSPECIFIED (INCL CYSTS AND POLYPS) GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations Step 6 Report • Create the table in Chinese (RTF/PDF/TXT)
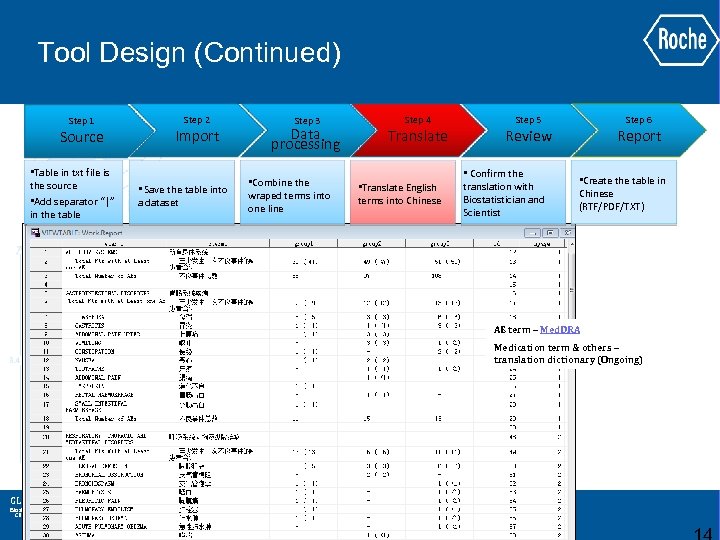
Tool Design (Continued) Step 1 Source • Table in txt file is the source • Add separator “|” in the table Step 2 Import • Save the table into a dataset Step 3 Data processing • Combine the wraped terms into one line Step 4 Translate • Translate English terms into Chinese Step 6 Step 5 Report Review • Confirm the translation with Biostatistician and Scientist • Create the table in Chinese (RTF/PDF/TXT) AE term – Med. DRA Medication term & others – translation dictionary (Ongoing) GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations
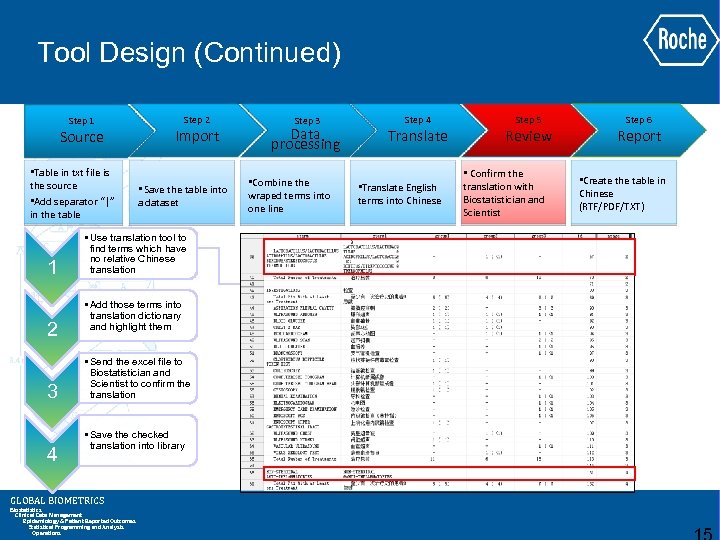
Tool Design (Continued) Step 1 Source • Table in txt file is the source • Add separator “|” in the table 1 2 3 4 Step 2 Import • Save the table into a dataset • Use translation tool to find terms which have no relative Chinese translation • Add those terms into translation dictionary and highlight them • Send the excel file to Biostatistician and Scientist to confirm the translation • Save the checked translation into library GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations Step 3 Data processing • Combine the wraped terms into one line Step 4 Translate • Translate English terms into Chinese Step 5 Review • Confirm the translation with Biostatistician and Scientist Step 6 Report • Create the table in Chinese (RTF/PDF/TXT)
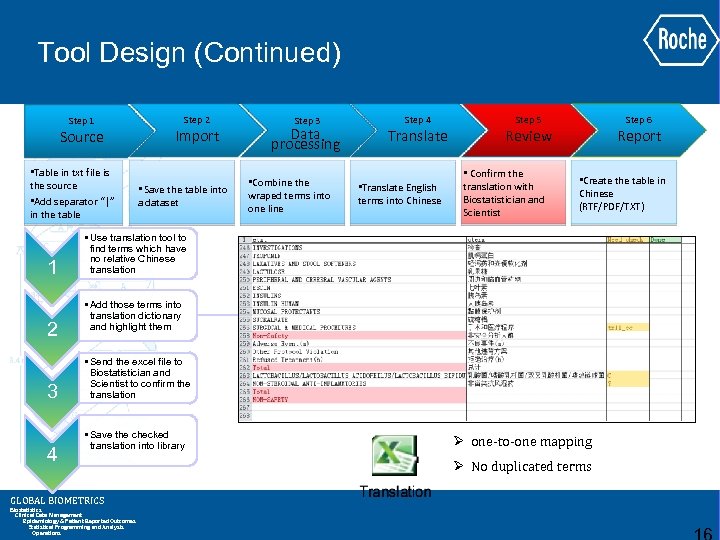
Tool Design (Continued) Step 1 Source • Table in txt file is the source • Add separator “|” in the table 1 2 3 4 Step 2 Import • Save the table into a dataset Step 3 Data processing • Combine the wraped terms into one line Step 4 Translate • Translate English terms into Chinese Step 6 Step 5 Report Review • Confirm the translation with Biostatistician and Scientist • Create the table in Chinese (RTF/PDF/TXT) • Use translation tool to find terms which have no relative Chinese translation • Add those terms into translation dictionary and highlight them • Send the excel file to Biostatistician and Scientist to confirm the translation • Save the checked translation into library GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations Ø one-to-one mapping Ø No duplicated terms
Tool Design (Continued) Step 1 Source • Table in txt file is the source • Add separator “|” in the table 1 2 3 4 Step 2 Import • Save the table into a dataset • Use translation tool to find terms which have no relative Chinese translation • Add those terms into translation dictionary and highlight them • Send the excel file to Biostatistician and Scientist to confirm the translation • Save the checked translation into library GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations Step 3 Data processing • Combine the wraped terms into one line Step 4 Translate • Translate English terms into Chinese Step 5 Review • Confirm the translation with Biostatistician and Scientist Step 6 Report • Create the table in Chinese (RTF/PDF/TXT)
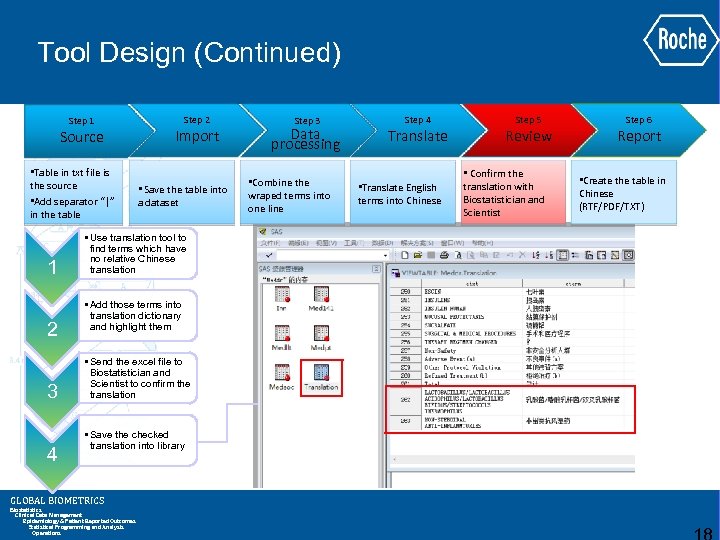
Tool Design (Continued) Step 1 Source • Table in txt file is the source • Add separator “|” in the table 1 2 3 4 Step 2 Import • Save the table into a dataset • Use translation tool to find terms which have no relative Chinese translation • Add those terms into translation dictionary and highlight them • Send the excel file to Biostatistician and Scientist to confirm the translation • Save the checked translation into library GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations Step 3 Data processing • Combine the wraped terms into one line Step 4 Translate • Translate English terms into Chinese Step 5 Review • Confirm the translation with Biostatistician and Scientist Step 6 Report • Create the table in Chinese (RTF/PDF/TXT)
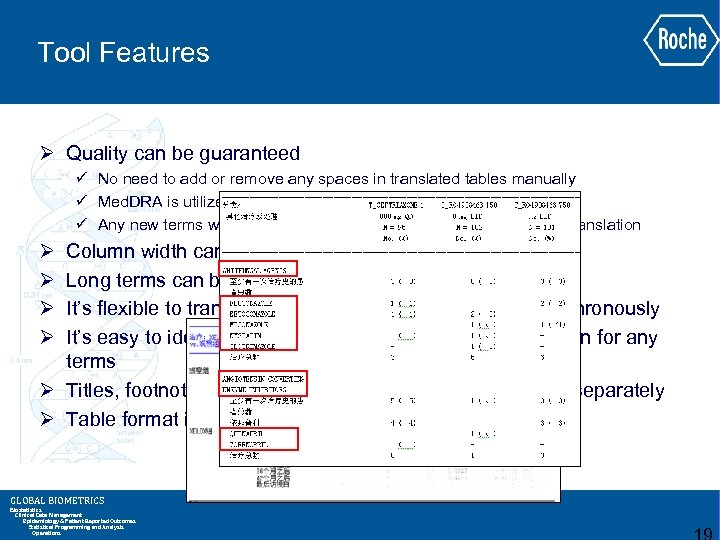
Tool Features Ø Quality can be guaranteed ü No need to add or remove any spaces in translated tables manually ü Med. DRA is utilized to translate adverse events ü Any new terms will be confirmed with Biostat and Scientist prior to translation Ø Ø Column width can be defined automatically Long terms can be wrapped automatically It’s flexible to translate multiple columns in one table synchronously It’s easy to identify if there is no relative Chinese translation for any terms Ø Titles, footnotes and column names should be translated separately Ø Table format is the same as the one in English GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations
Table examples GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations
Table examples GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations
Table examples GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations
Table examples GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations
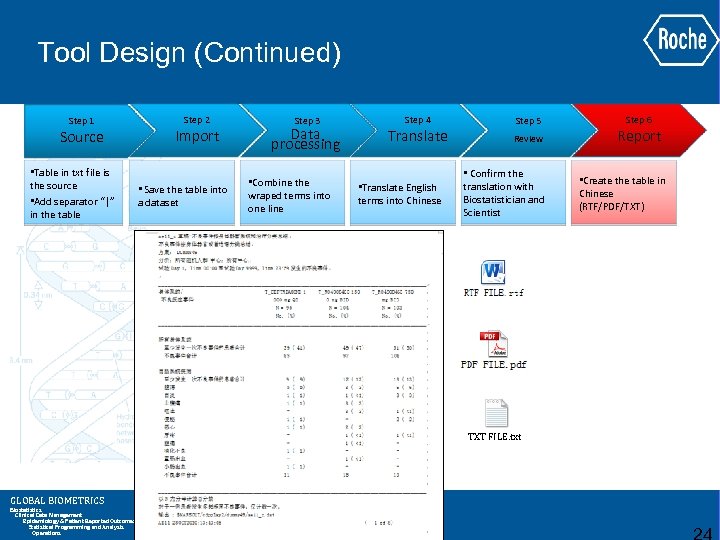
Tool Design (Continued) Step 1 Source • Table in txt file is the source • Add separator “|” in the table Step 2 Import • Save the table into a dataset Step 3 Data processing • Combine the wraped terms into one line Step 4 Translate • Translate English terms into Chinese Step 5 Review • Confirm the translation with Biostatistician and Scientist TXT FILE. txt GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations Step 6 Report • Create the table in Chinese (RTF/PDF/TXT)
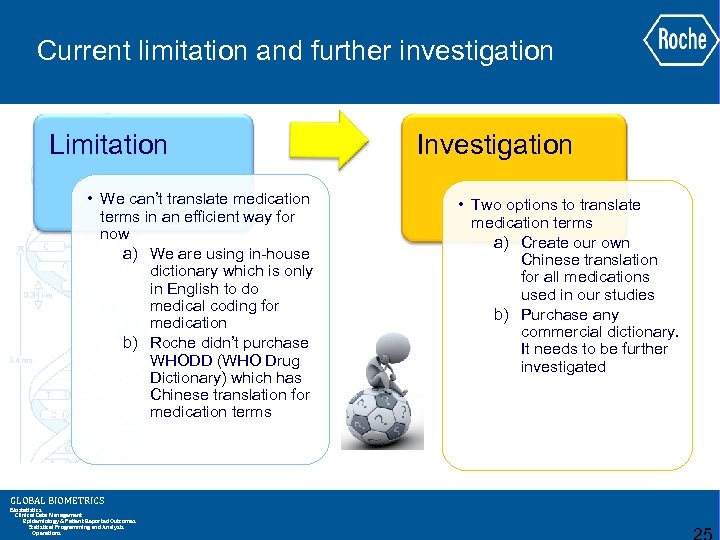
Current limitation and further investigation Limitation • We can’t translate medication terms in an efficient way for now a) We are using in-house dictionary which is only in English to do medical coding for medication b) Roche didn’t purchase WHODD (WHO Drug Dictionary) which has Chinese translation for medication terms GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations Investigation • Two options to translate medication terms a) Create our own Chinese translation for all medications used in our studies b) Purchase any commercial dictionary. It needs to be further investigated

Recommended working process GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations

Recommended working process Ø Suggestion to biostatistician and scientist ü Add identifier for tables which need translation in LOPO to ensure most of tables would be translated prior to CSR generation ü Continue to add identifiers in LOPO prior to CSR finalization and update with SPA GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations

Summary Ø This tool can translate tables from English to Chinese (or other languages) in good quality Ø It has great potential to accelerate the submission Ø Need further investigation to solve current limitations Ø It is a great internship program to provide an opportunity for staff development GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations

Thank you! GLOBAL BIOMETRICS Biostatistics Clinical Data Management Epidemiology & Patient Reported Outcomes Statistical Programming and Analysis Operations
31ed71c405ff54bb47883cf5cea08f2d.ppt