edb1ea00ddb41b553ae74b1b29bf5c4a.ppt
- Количество слайдов: 53

Analisi decentrate: un problema od un’ opportunità? Genova 18 maggio 2006 Gianni Messeri Dipartimento Diagnostica di Laboratorio Azienda Ospedaliero-Universitaria Careggi - Firenze

POCT • • Cos’è Perché viene usato Come viene usato Quanto verrà usato

COS’E’

Point of Care Testing Das kind sterbt bald wieder, dessen stirne beim kussen salzig schmeckt The child will die soon, whose forehead tastes salty when kissed

Point of Care Testing 1500 B. C. Hindus in the Ayur Veda recorded that insects and flies were attracted to the urine of some people whose urine tasted sweet. This was associated with certain diseases

Point of Care Testing (POCT) is not new and up until this century was the norm
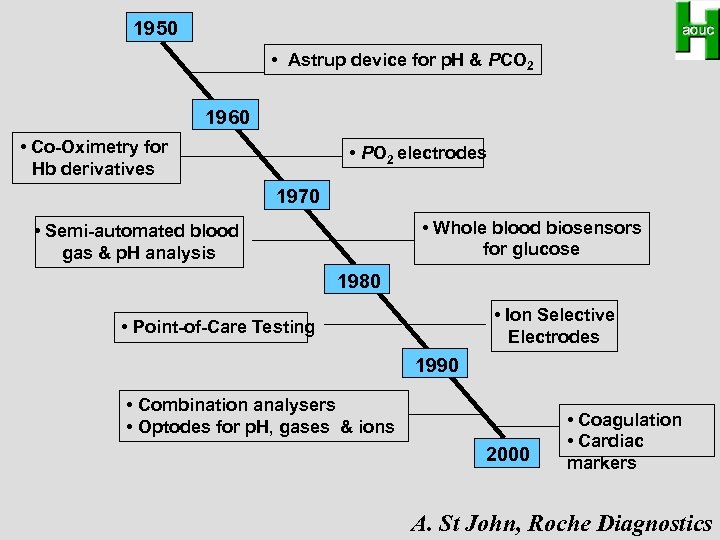
1950 • Astrup device for p. H & PCO 2 1960 • Co-Oximetry for Hb derivatives • PO 2 electrodes 1970 • Whole blood biosensors for glucose • Semi-automated blood gas & p. H analysis 1980 • Ion Selective Electrodes • Point-of-Care Testing 1990 • Combination analysers • Optodes for p. H, gases & ions 2000 • Coagulation • Cardiac markers A. St John, Roche Diagnostics
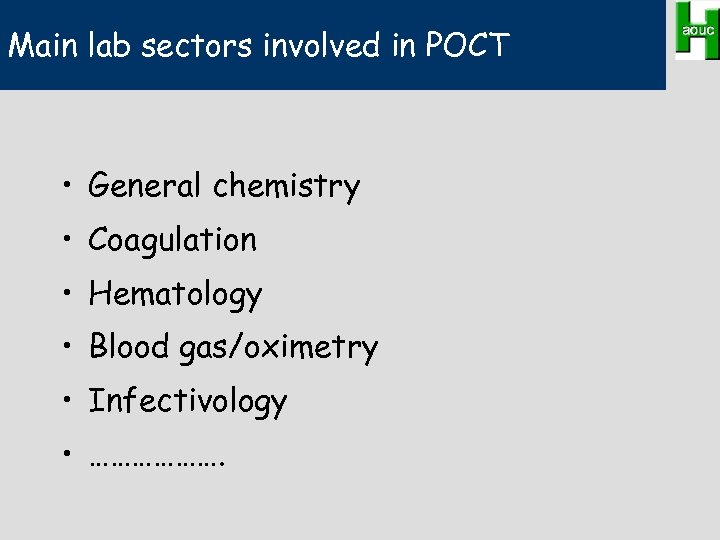
Main lab sectors involved in POCT • General chemistry • Coagulation • Hematology • Blood gas/oximetry • Infectivology • ……………….
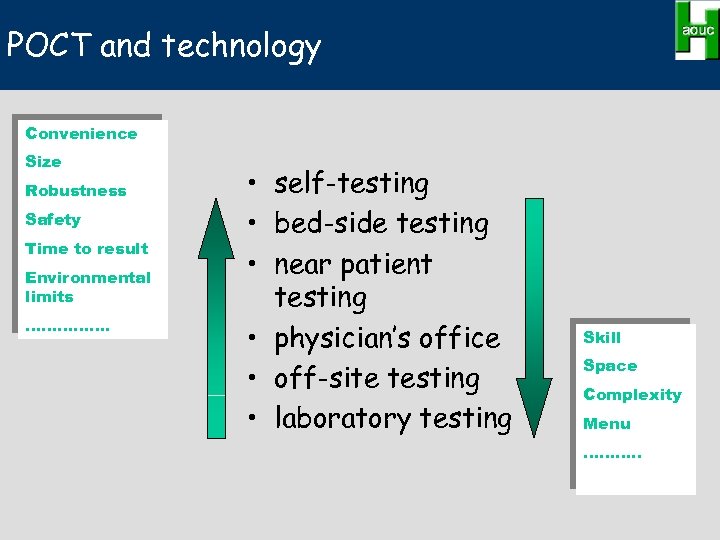
POCT and technology Convenience Size Robustness Safety Time to result Environmental limits ……………. • self-testing • bed-side testing • near patient testing • physician’s office • off-site testing • laboratory testing Skill Space Complexity Menu ………. .

POCT Devices l Disposable Analytical Devices l Hand-Held Sensor Systems l Bench-top Technology

Drug of abuse testing Visual reading

POCT Devices l Disposable Analytical Devices l Hand-Held Systems l Bench-top Technology

Hand-Held Systems

POCT Devices l Disposable Analytical Devices l Hand-Held Sensor Systems l Bench-top Technology

Blood gas analyser: - Electrode base technology

Blood gas analyser: - Optode base technology

Perché viene usato

Motivazioni all’uso del POCT • • • Lay-out della struttura Efficienza del Laboratorio Efficienza del Sistema di trasporto Complessità delle patologie trattate Leadership del Laboratorio …………. .
Area Careggi

Come viene usato

La più importante caratteristica di un risultato di laboratorio, indipendentemente da dove questo è prodotto è… …accuracy and precision because ultimately they have the greatest impact on patient outcome! Callum Fraser, 2001
W hile point-of-care testing (POCT) has significantly improved the timely delivery of diagnostic information for clinical decision making, the wide range of settings and operators involved in POCT add a layer of complexity to an institution’s effort to ensure consistently high-quality results. ” Gerald J. Kost, MD, Ph. D. “Using operator lockout to improve the performance of point-of-care blood glucose monitoring. ” 2000.


Sorgenti ed amplificatori degli errori nell’uso del POCT • Sorgenti – Scarsa competenza degli operatori – Mancato rispetto delle procedure – Uso di materiali/strumenti fuori controllo • Amplificatori – Regolamentazione confusa – Rapida disponibilità del risultato – Immediate implicazioni terapeutiche FA Meier et al, Arch Pathol Lab Med. 129, 2005

Classificazione degli errori nel POCT • Fase pre-analitica – – richiesta del test identificazione campione/paziente raccolta del campione valutazione del campione FA Meier et al, Arch Pathol Lab Med. 129, 2005

Classificazione degli errori nel POCT • Fase analitica – – calibrazione interferenza da parte del campione generazione del risultato validazione del risultato FA Meier et al, Arch Pathol Lab Med. 129, 2005

Classificazione degli errori nel POCT • Fase post-analitica – – – formato della risposta mancata evidenziazione della criticità mancata comunicazione del risultato mancanza di registrazione incoeerenza fra dato generato e registrato FA Meier et al, Arch Pathol Lab Med. 129, 2005

Efficacy of feedback from quarterly laboratory comparison in maintaining quality • 61 nurses continued with the practice of regular quarterly split-sample comparisons with feedback. • 63 nurses did no comparisons over the 12 month study • Primary outcome of accuracy was determined through 5 -7 additional samples at 0, 6, 12 months Jones HE. Diabetes Care, 1996
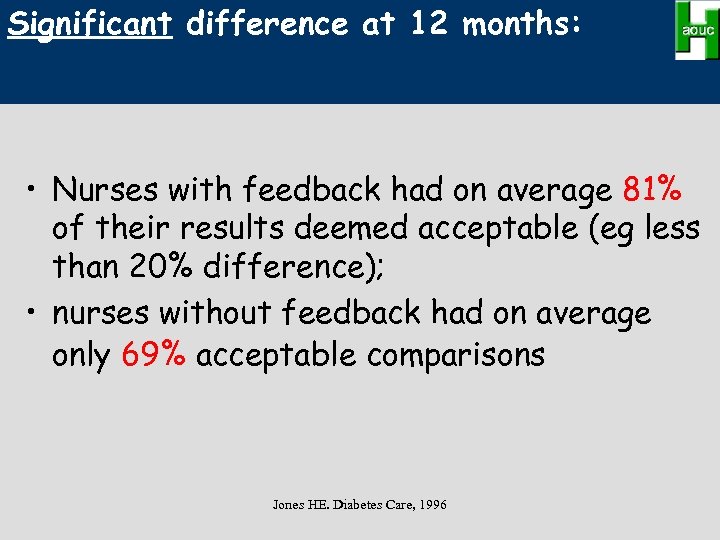
Significant difference at 12 months: • Nurses with feedback had on average 81% of their results deemed acceptable (eg less than 20% difference); • nurses without feedback had on average only 69% acceptable comparisons Jones HE. Diabetes Care, 1996
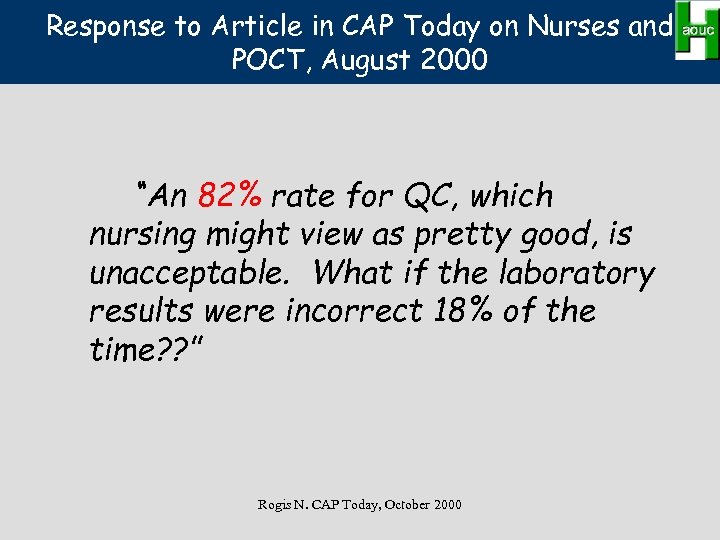
Response to Article in CAP Today on Nurses and POCT, August 2000 “An 82% rate for QC, which nursing might view as pretty good, is unacceptable. What if the laboratory results were incorrect 18% of the time? ? ” Rogis N. CAP Today, October 2000

Six Sigma • Measures errors per million • Focused on “Process Improvements” • Improve quality and reduce errors

Is 99. 9% Good Enough? • • • 1 hour of unsafe drinking water every month. 2 unsafe plane landings per day at O'Hare Airport in Chicago. 12 newborns will be given to the wrong parents daily. 291 pacemaker operations will be performed incorrectly each year. 500 incorrect operations each week. 315 entries in Webster's Dictionary will be misspelled. 18, 322 pieces of mail will be mishandled/hour. 20, 000 incorrect prescriptions every year. 22, 000 checks deducted from the wrong bank account each hour. …………………. What are your POCT compliance rates?
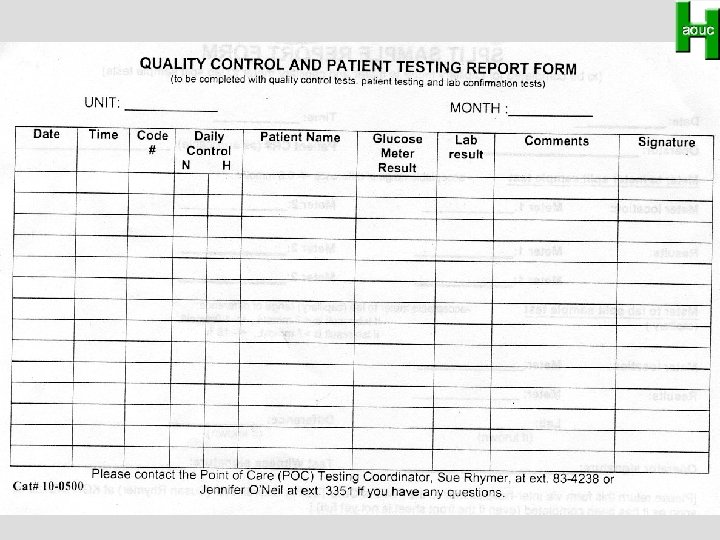
Potential Outcome Indicators • • • # and type of problems reported QA statistics Stat tests / routine tests Repeat frequency Duplicate testing Cost Satisfaction Management issues Personnel issues Collier C. Clin Biochem 1998

POCT & ISO/DIS 22870 Point-of-care testing (POCT) Requirements for quality and competence
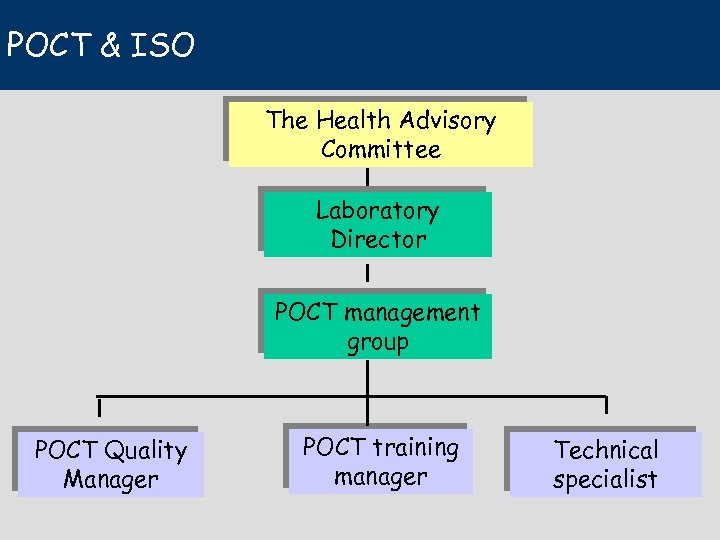
POCT & ISO The Health Advisory Committee Laboratory Director POCT management group POCT Quality Manager POCT training manager Technical specialist

The Health Advisory Group A health professional grouping shall be responsible to the governing body, for defining the scope of POCT to be made available. This shall take into consideration: – – the clinical need for POCT financial implications, technical feasibility the ability of the organization to fulfil the need.

The Laboratory Director • The Laboratory Director or another suitably qualified person, shall be responsible for: • procuring, evaluating, and selecting all POCT devices, reagents, and systems, including quality control material • establishing documented quality policy and protocols for the performance of all POCT and associated quality control and quality assurance.

The Laboratory Director - the equipment The Laboratory Director, or designated suitably qualified person, shall be responsible for the selection criteria and for the procurement of equipment, materials, and reagents. • An inventory shall be maintained of all POCT equipment including serial number and unique identification, manufacturer/supplier, date purchased, and service history, including dates out-of-service. • Reagents, kits, and equipment shall be verified prior to routine use. • There shall be written procedures for the maintenance and operation of POCT equipment • …………………….

The Laboratory Director - the training • The Laboratory Director, or other suitably qualified person, may appoint a person with appropriate training and experience, to manage the training and competency assessment.

The POCT Management group • The Laboratory Director or designate shall appoint a multidisciplinary POCT management group with representation from the laboratory, the administration, and clinical programmes including nursing to advise on the provision of POCT.

The POCT Management Group • shall ensure that responsibilities and authorities are defined and communicated within the organization. • ………………………. • shall consider all proposals to introduce any product, device, or system for POCT

The Quality Manager The management of laboratory services shall: • a) identify the processes needed for the quality management system for POCT throughout the organization; • …. . • appoint a person with appropriate training and experience, as quality manager responsible for POCT quality, which includes review of the requirements related to POCT.

The Training manager • He shall develop, implement, and maintain an appropriate theoretical and practical training programme for all POCT personnel. may assign responsibility for training on a specific POCT instrument/system to an appropriate Technical Specialist or Technologist. • Only personnel who have completed the training and demonstrated competence shall carry out POCT. • Records of training/attestation and of retraining and re -attestation shall be retained. • …………………. .
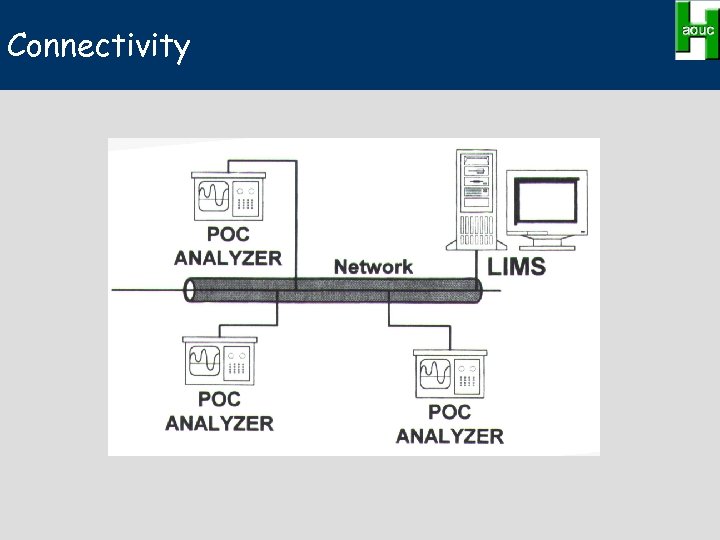
Connectivity
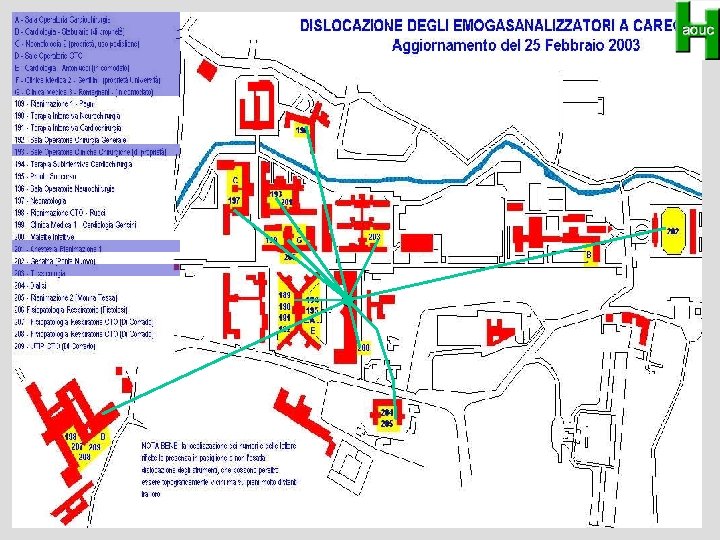
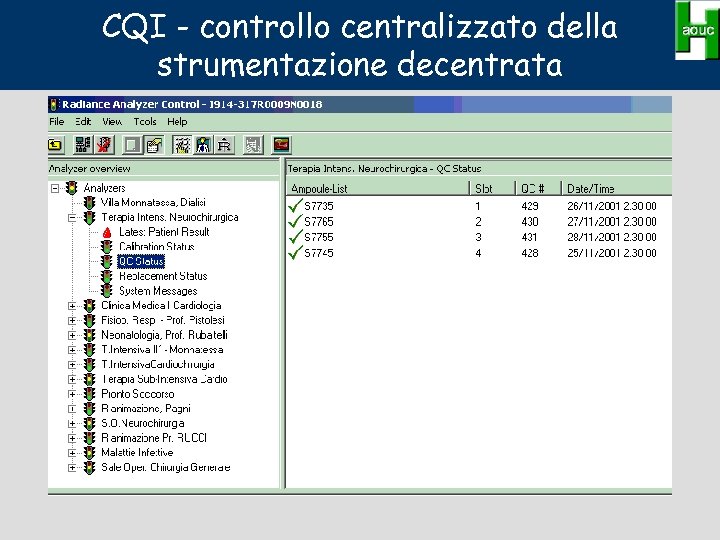
CQI - controllo centralizzato della strumentazione decentrata
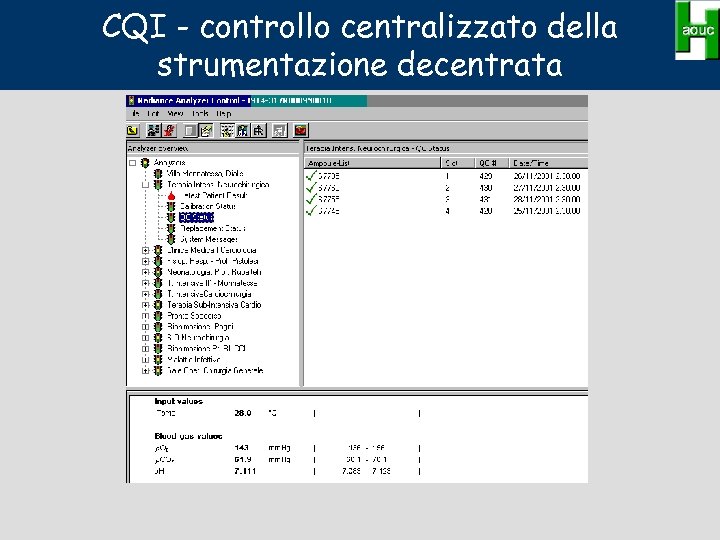
CQI - controllo centralizzato della strumentazione decentrata

Quanto verrà usato?

Nuovi POCT Le nuove microtecnologie inclusi i microarrays per ormoni o screening di polimorfismi del DNAsaranno disponibili in strumenti portatili nei prossimi anni l Entro i prossimi anni strumenti portatili saranno in grado di analizzare un gran numero di analiti, in pochi minuti su di una goccia di sangue al letto del malato l

Le domande I test più comuni verranno eseguiti come POCT? l I test meno urgenti, di uso meno comune o di esecuzione più complessa rimarranno nei Laboratori Centrali? l Questo tipo di impostazione sarà economicamente vantaggiosa? l

Conclusioni • Rivedere il concetto di laboratorio centrale • Approccio di squadra multidisciplinare per la gestione del POCT aziendale • Coordinamento del Laboratorio Centrale per: – – la valutazione dei metodi/strumenti di POCT la formazione degli operatori (medici, infermieri) il QC le valutazioni economiche • Ruolo centrale dell’informatica

Sempre più vicini al paziente!
edb1ea00ddb41b553ae74b1b29bf5c4a.ppt