5f4e5ad938883250c42c721201b89a57.ppt
- Количество слайдов: 30
 An Update on Tec. Eco Technology An update on recent advances in Tec and Eco-Cements including the use of high proportions of flyash and SCMS with added reactive magnesia Reactive Magnesia is the most powerful new tool in cement chemistry 16/03/2018 www. tececo. com www. propubs. com 1
An Update on Tec. Eco Technology An update on recent advances in Tec and Eco-Cements including the use of high proportions of flyash and SCMS with added reactive magnesia Reactive Magnesia is the most powerful new tool in cement chemistry 16/03/2018 www. tececo. com www. propubs. com 1
 Tec. Eco Cements • Eco-Cements have relatively high proportions of magnesia which in permeable materials carbonates adding strength and durability. Eco-Cement formulations are generally used for bricks, blocks, pavers, pervious pavements and other permeable cement based products. See http: //www. tececo. com/products. eco-cement. php • Enviro-Cements are made using large quantities of reactive magnesia which reacts to form brucite. Brucite is unique to Tec. Eco Cements and is an ideal mineral for trapping toxic and hazardous wastes due to its layered structure, equilibrium p. H level, durability and low solubility. See http: //www. tececo. com/products. enviro-cement. php • Tec-Cements are cement blends that comprise of a hydraulic cement such as Portland cement mixed with a relatively small proportion of reactive magnesia and optionally pozzolans and/or supplementary cementitious materials which react with Portlandite removing it and making more cement or are activated by Portland cement. They offer a solution to many of the technical problems that plague traditional cement formulations caused by the reactivity of lime (Portlandite) and have significant advantages including faster setting even with a high proportion of non PC additions. See http: //www. tececo. com/products. tec-cement. php
Tec. Eco Cements • Eco-Cements have relatively high proportions of magnesia which in permeable materials carbonates adding strength and durability. Eco-Cement formulations are generally used for bricks, blocks, pavers, pervious pavements and other permeable cement based products. See http: //www. tececo. com/products. eco-cement. php • Enviro-Cements are made using large quantities of reactive magnesia which reacts to form brucite. Brucite is unique to Tec. Eco Cements and is an ideal mineral for trapping toxic and hazardous wastes due to its layered structure, equilibrium p. H level, durability and low solubility. See http: //www. tececo. com/products. enviro-cement. php • Tec-Cements are cement blends that comprise of a hydraulic cement such as Portland cement mixed with a relatively small proportion of reactive magnesia and optionally pozzolans and/or supplementary cementitious materials which react with Portlandite removing it and making more cement or are activated by Portland cement. They offer a solution to many of the technical problems that plague traditional cement formulations caused by the reactivity of lime (Portlandite) and have significant advantages including faster setting even with a high proportion of non PC additions. See http: //www. tececo. com/products. tec-cement. php
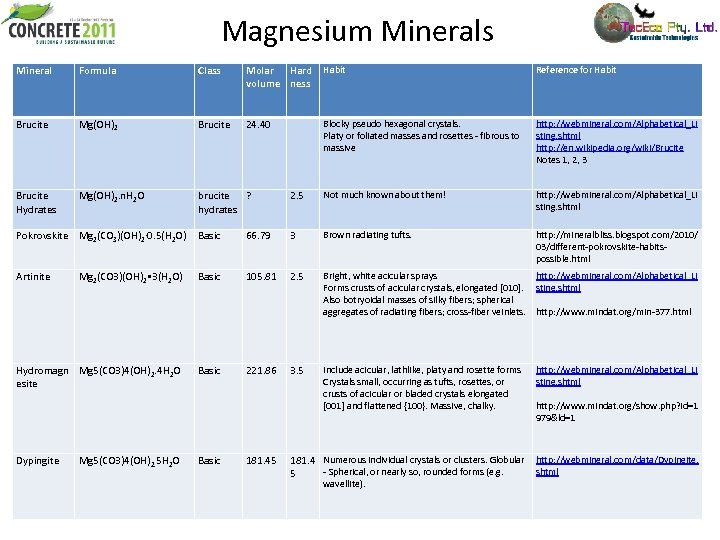 Magnesium Minerals Mineral Formula Class Molar Hard volume ness Habit Reference for Habit Brucite Mg(OH)2 Brucite 24. 40 Blocky pseudo hexagonal crystals. Platy or foliated masses and rosettes - fibrous to massive http: //webmineral. com/Alphabetical_Li sting. shtml http: //en. wikipedia. org/wiki/Brucite Notes 1, 2, 3 Brucite Hydrates Mg(OH)2. n. H 2 O brucite ? hydrates 2. 5 Not much known about them! http: //webmineral. com/Alphabetical_Li sting. shtml Pokrovskite Mg 2(CO 3)(OH)2· 0. 5(H 2 O) Basic 66. 79 3 Brown radiating tufts. http: //mineralbliss. blogspot. com/2010/ 03/different-pokrovskite-habitspossible. html Artinite Mg 2(CO 3)(OH)2 • 3(H 2 O) Basic 105. 81 2. 5 Bright, white acicular sprays Forms crusts of acicular crystals, elongated [010]. Also botryoidal masses of silky fibers; spherical aggregates of radiating fibers; cross-fiber veinlets. http: //webmineral. com/Alphabetical_Li sting. shtml http: //www. mindat. org/min-377. html Hydromagn Mg 5(CO 3)4(OH)2. 4 H 2 O esite Basic 221. 86 3. 5 Include acicular, lathlike, platy and rosette forms Crystals small, occurring as tufts, rosettes, or crusts of acicular or bladed crystals elongated [001] and flattened {100}. Massive, chalky. http: //webmineral. com/Alphabetical_Li sting. shtml http: //www. mindat. org/show. php? id=1 979&ld=1 Dypingite Basic 181. 45 181. 4 Numerous individual crystals or clusters. Globular http: //webmineral. com/data/Dypingite. - Spherical, or nearly so, rounded forms (e. g. shtml 5 Mg 5(CO 3)4(OH)2. 5 H 2 O wavellite).
Magnesium Minerals Mineral Formula Class Molar Hard volume ness Habit Reference for Habit Brucite Mg(OH)2 Brucite 24. 40 Blocky pseudo hexagonal crystals. Platy or foliated masses and rosettes - fibrous to massive http: //webmineral. com/Alphabetical_Li sting. shtml http: //en. wikipedia. org/wiki/Brucite Notes 1, 2, 3 Brucite Hydrates Mg(OH)2. n. H 2 O brucite ? hydrates 2. 5 Not much known about them! http: //webmineral. com/Alphabetical_Li sting. shtml Pokrovskite Mg 2(CO 3)(OH)2· 0. 5(H 2 O) Basic 66. 79 3 Brown radiating tufts. http: //mineralbliss. blogspot. com/2010/ 03/different-pokrovskite-habitspossible. html Artinite Mg 2(CO 3)(OH)2 • 3(H 2 O) Basic 105. 81 2. 5 Bright, white acicular sprays Forms crusts of acicular crystals, elongated [010]. Also botryoidal masses of silky fibers; spherical aggregates of radiating fibers; cross-fiber veinlets. http: //webmineral. com/Alphabetical_Li sting. shtml http: //www. mindat. org/min-377. html Hydromagn Mg 5(CO 3)4(OH)2. 4 H 2 O esite Basic 221. 86 3. 5 Include acicular, lathlike, platy and rosette forms Crystals small, occurring as tufts, rosettes, or crusts of acicular or bladed crystals elongated [001] and flattened {100}. Massive, chalky. http: //webmineral. com/Alphabetical_Li sting. shtml http: //www. mindat. org/show. php? id=1 979&ld=1 Dypingite Basic 181. 45 181. 4 Numerous individual crystals or clusters. Globular http: //webmineral. com/data/Dypingite. - Spherical, or nearly so, rounded forms (e. g. shtml 5 Mg 5(CO 3)4(OH)2. 5 H 2 O wavellite).
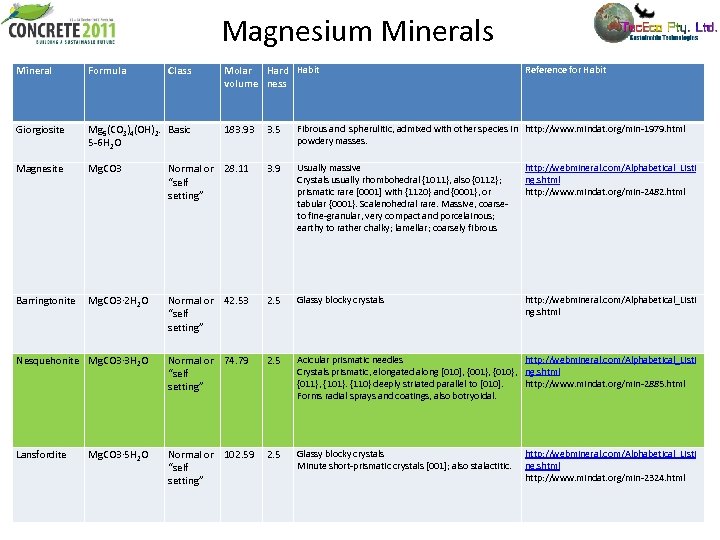 Magnesium Minerals Mineral Formula Class Giorgiosite Mg 5(CO 3)4(OH)2. Basic 5 -6 H 2 O Magnesite Mg. CO 3 Barringtonite Molar Hard Habit volume ness 3. 5 Fibrous and spherulitic, admixed with other species in http: //www. mindat. org/min-1979. html powdery masses. Normal or 28. 11 “self setting” 3. 9 Usually massive Crystals usually rhombohedral {1011}, also {0112}; prismatic rare [0001] with {1120} and {0001}, or tabular {0001}. Scalenohedral rare. Massive, coarse- to fine-granular, very compact and porcelainous; earthy to rather chalky; lamellar; coarsely fibrous http: //webmineral. com/Alphabetical_Listi ng. shtml http: //www. mindat. org/min-2482. html Mg. CO 3· 2 H 2 O Normal or 42. 53 “self setting” 2. 5 Glassy blocky crystals http: //webmineral. com/Alphabetical_Listi ng. shtml Nesquehonite Mg. CO 3· 3 H 2 O Normal or 74. 79 “self setting” 2. 5 Acicular prismatic needles http: //webmineral. com/Alphabetical_Listi Crystals prismatic, elongated along [010], {001}, {010}, ng. shtml {011}, {101}. {110} deeply striated parallel to [010]. http: //www. mindat. org/min-2885. html Forms radial sprays and coatings, also botryoidal. Lansfordite Normal or 102. 59 “self setting” 2. 5 Glassy blocky crystals Minute short-prismatic crystals [001]; also stalactitic. Mg. CO 3· 5 H 2 O 183. 93 Reference for Habit http: //webmineral. com/Alphabetical_Listi ng. shtml http: //www. mindat. org/min-2324. html
Magnesium Minerals Mineral Formula Class Giorgiosite Mg 5(CO 3)4(OH)2. Basic 5 -6 H 2 O Magnesite Mg. CO 3 Barringtonite Molar Hard Habit volume ness 3. 5 Fibrous and spherulitic, admixed with other species in http: //www. mindat. org/min-1979. html powdery masses. Normal or 28. 11 “self setting” 3. 9 Usually massive Crystals usually rhombohedral {1011}, also {0112}; prismatic rare [0001] with {1120} and {0001}, or tabular {0001}. Scalenohedral rare. Massive, coarse- to fine-granular, very compact and porcelainous; earthy to rather chalky; lamellar; coarsely fibrous http: //webmineral. com/Alphabetical_Listi ng. shtml http: //www. mindat. org/min-2482. html Mg. CO 3· 2 H 2 O Normal or 42. 53 “self setting” 2. 5 Glassy blocky crystals http: //webmineral. com/Alphabetical_Listi ng. shtml Nesquehonite Mg. CO 3· 3 H 2 O Normal or 74. 79 “self setting” 2. 5 Acicular prismatic needles http: //webmineral. com/Alphabetical_Listi Crystals prismatic, elongated along [010], {001}, {010}, ng. shtml {011}, {101}. {110} deeply striated parallel to [010]. http: //www. mindat. org/min-2885. html Forms radial sprays and coatings, also botryoidal. Lansfordite Normal or 102. 59 “self setting” 2. 5 Glassy blocky crystals Minute short-prismatic crystals [001]; also stalactitic. Mg. CO 3· 5 H 2 O 183. 93 Reference for Habit http: //webmineral. com/Alphabetical_Listi ng. shtml http: //www. mindat. org/min-2324. html
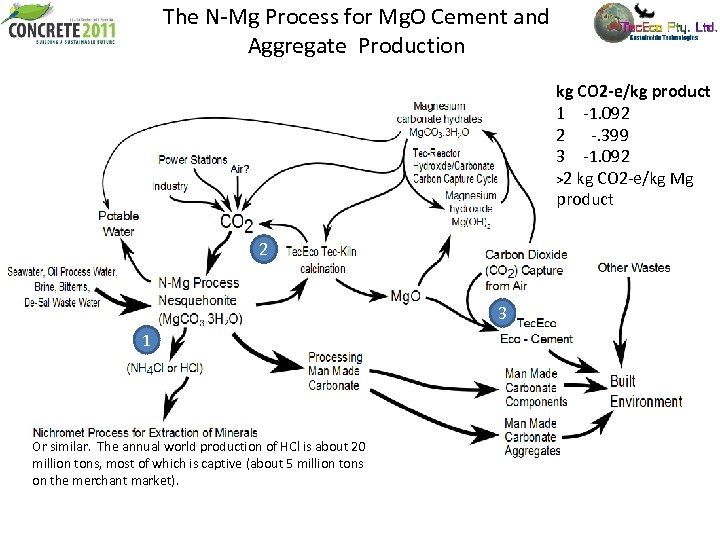 The N-Mg Process for Mg. O Cement and Aggregate Production kg CO 2 -e/kg product 1 -1. 092 2 -. 399 3 -1. 092 >2 kg CO 2 -e/kg Mg product 2 3 1 Or similar. The annual world production of HCl is about 20 million tons, most of which is captive (about 5 million tons on the merchant market).
The N-Mg Process for Mg. O Cement and Aggregate Production kg CO 2 -e/kg product 1 -1. 092 2 -. 399 3 -1. 092 >2 kg CO 2 -e/kg Mg product 2 3 1 Or similar. The annual world production of HCl is about 20 million tons, most of which is captive (about 5 million tons on the merchant market).
 The N-Mg Process HCl NH 3 and a small amount of CO 2 H 2 O Tec-Kiln Mg rich water Ammoniacal Mg rich water Mg. CO 3. 3 H 2 O Mg(OH)2 Steam Mg. CO 3. 3 H 2 O Filter NH 4 Cl and a small amount of NH 4 HCO 3 The N-Mg Process - A Modified Solvay Process for Nesquehonite
The N-Mg Process HCl NH 3 and a small amount of CO 2 H 2 O Tec-Kiln Mg rich water Ammoniacal Mg rich water Mg. CO 3. 3 H 2 O Mg(OH)2 Steam Mg. CO 3. 3 H 2 O Filter NH 4 Cl and a small amount of NH 4 HCO 3 The N-Mg Process - A Modified Solvay Process for Nesquehonite
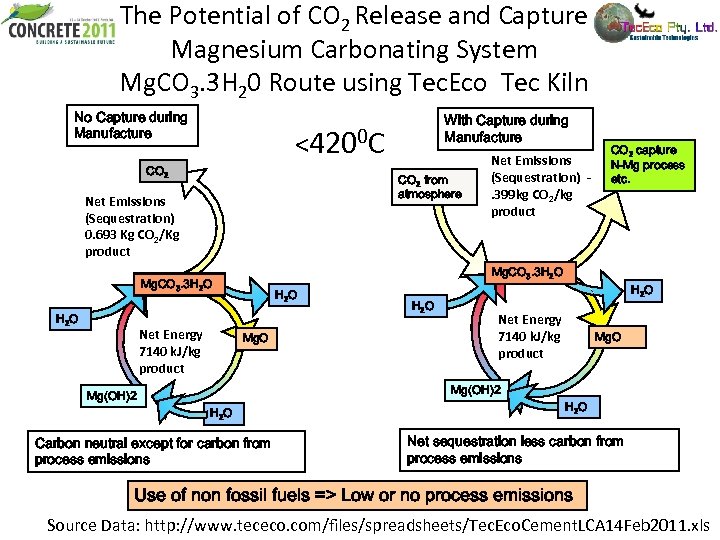 The Potential of CO 2 Release and Capture Magnesium Carbonating System Mg. CO 3. 3 H 20 Route using Tec. Eco Tec Kiln No Capture during Manufacture <4200 C CO 2 from atmosphere Net Emissions (Sequestration) 0. 693 Kg CO 2/Kg product Net Energy 7140 k. J/kg product Net Emissions (Sequestration). 399 kg CO 2/kg product CO 2 capture N-Mg process etc. Mg. CO 3. 3 H 2 O With Capture during Manufacture H 2 O Mg. O H 2 O Net Energy 7140 k. J/kg product Mg. O Mg(OH)2 H 2 O Carbon neutral except for carbon from process emissions H 2 O Net sequestration less carbon from process emissions Use of non fossil fuels => Low or no process emissions Source Data: http: //www. tececo. com/files/spreadsheets/Tec. Eco. Cement. LCA 14 Feb 2011. xls
The Potential of CO 2 Release and Capture Magnesium Carbonating System Mg. CO 3. 3 H 20 Route using Tec. Eco Tec Kiln No Capture during Manufacture <4200 C CO 2 from atmosphere Net Emissions (Sequestration) 0. 693 Kg CO 2/Kg product Net Energy 7140 k. J/kg product Net Emissions (Sequestration). 399 kg CO 2/kg product CO 2 capture N-Mg process etc. Mg. CO 3. 3 H 2 O With Capture during Manufacture H 2 O Mg. O H 2 O Net Energy 7140 k. J/kg product Mg. O Mg(OH)2 H 2 O Carbon neutral except for carbon from process emissions H 2 O Net sequestration less carbon from process emissions Use of non fossil fuels => Low or no process emissions Source Data: http: //www. tececo. com/files/spreadsheets/Tec. Eco. Cement. LCA 14 Feb 2011. xls
 The Tec. Eco Tec-Kiln An obvious future requirement will be to make cements without releases so Tec. Eco are developing a top secret kiln for low temperature calcination of alkali metal carbonates and the pyro processing and simultaneous grinding of other minerals such as clays. The Tec. Eco Tec-Kiln makes no releases and is an essential part of Tec. Eco's plan to sequester massive amounts of CO 2 as man made carbonate in the built environment. The Tec. Eco Tec-Kiln has the following features: • • • Operates in a closed system and therefore does not release CO 2 or other volatiles substances to the atmosphere Can be powered by various potentially cheaper non fossil sources of energy such as intermittent solar or wind energy. Grinds and calcines at the same time thereby running 25% to 30% more efficiently. Produces more precisely definable product. (Secret as disclosure would give away the design) The CO 2 produced can be sold or re-used in for example the N-Mg process. Cement made with the Tec-Kiln will be eligible for carbon offsets. To further develop the Tec-Kiln, Tec. Eco require not only additional funding but also partners able to provide expertise.
The Tec. Eco Tec-Kiln An obvious future requirement will be to make cements without releases so Tec. Eco are developing a top secret kiln for low temperature calcination of alkali metal carbonates and the pyro processing and simultaneous grinding of other minerals such as clays. The Tec. Eco Tec-Kiln makes no releases and is an essential part of Tec. Eco's plan to sequester massive amounts of CO 2 as man made carbonate in the built environment. The Tec. Eco Tec-Kiln has the following features: • • • Operates in a closed system and therefore does not release CO 2 or other volatiles substances to the atmosphere Can be powered by various potentially cheaper non fossil sources of energy such as intermittent solar or wind energy. Grinds and calcines at the same time thereby running 25% to 30% more efficiently. Produces more precisely definable product. (Secret as disclosure would give away the design) The CO 2 produced can be sold or re-used in for example the N-Mg process. Cement made with the Tec-Kiln will be eligible for carbon offsets. To further develop the Tec-Kiln, Tec. Eco require not only additional funding but also partners able to provide expertise.
 Tec. Eco Tec-Kiln, N-Mg route The calcination of nesquehonite has a relatively high enthalpy but there is significant scope for reducing energy using waste heat Initial weight loss below 1000 C consists almost entirely of water (1. 3 molecules per molecule of nesquehonite). Between 100 and 1500 C volatilization of further water is associated with a small loss of carbon dioxide (~3 -5 %). From 1500 C to 2500 C, the residual water content varies between 0 -6 and 0 -2 molecules per molecule of Mg. C 03. Above 3000 C, loss of carbon dioxide becomes appreciable and is virtually complete by 4200 C, leaving Mg. O with a small residual water content. Dell, R. M. and S. W. Weller (1959). "The Thermal Decomposition of Nesquehonite Mg. CO 3 3 H 20 And Magnesium Ammonium Carbonate Mg. CO 3 (NH 4)2 CO 3 4 H 2 O. " Trans Faraday Soc 55(10): 2203 - 2220. Energy could be saved using a two stage calcination process using waste energy for the first stage.
Tec. Eco Tec-Kiln, N-Mg route The calcination of nesquehonite has a relatively high enthalpy but there is significant scope for reducing energy using waste heat Initial weight loss below 1000 C consists almost entirely of water (1. 3 molecules per molecule of nesquehonite). Between 100 and 1500 C volatilization of further water is associated with a small loss of carbon dioxide (~3 -5 %). From 1500 C to 2500 C, the residual water content varies between 0 -6 and 0 -2 molecules per molecule of Mg. C 03. Above 3000 C, loss of carbon dioxide becomes appreciable and is virtually complete by 4200 C, leaving Mg. O with a small residual water content. Dell, R. M. and S. W. Weller (1959). "The Thermal Decomposition of Nesquehonite Mg. CO 3 3 H 20 And Magnesium Ammonium Carbonate Mg. CO 3 (NH 4)2 CO 3 4 H 2 O. " Trans Faraday Soc 55(10): 2203 - 2220. Energy could be saved using a two stage calcination process using waste energy for the first stage.
 Tec. Eco-Cements are blends of one or more hydraulic cements and relatively high proportions of reactive magnesia with or without pozzolans and supplementary cementitious additions. They will only carbonate in gas permeable substrates forming strong fibrous minerals. Water vapour and CO 2 must be available for carbonation to ensue. Eco-Cements can be used in a wide range of products from foamed concretes to bricks, blocks and pavers, mortars renders, grouts and pervious concretes such as our own permeacocrete. Somewhere in the vicinity of the Pareto proportion (80%) of conventional concretes could be replaced by Eco-Cement. Left: Recent Eco-Cement blocks made, transported and erected in a week. Laying and Eco-Cement floor. Eco-Cement mortar & Eco-cement mud bricks. Right: Eco-Cement permeacocretes and foamed concretes
Tec. Eco-Cements are blends of one or more hydraulic cements and relatively high proportions of reactive magnesia with or without pozzolans and supplementary cementitious additions. They will only carbonate in gas permeable substrates forming strong fibrous minerals. Water vapour and CO 2 must be available for carbonation to ensue. Eco-Cements can be used in a wide range of products from foamed concretes to bricks, blocks and pavers, mortars renders, grouts and pervious concretes such as our own permeacocrete. Somewhere in the vicinity of the Pareto proportion (80%) of conventional concretes could be replaced by Eco-Cement. Left: Recent Eco-Cement blocks made, transported and erected in a week. Laying and Eco-Cement floor. Eco-Cement mortar & Eco-cement mud bricks. Right: Eco-Cement permeacocretes and foamed concretes
 Forced Carbonation ~ Optimisation Forced Carbonation (Cambridge) Kinetic Optimisation (Tec. Eco) Steps Multistep process Less steps = lower costs Rate Variable Varying on weather conditions (wet dry best and gas permeability) % Carbonation in 6 months 70% (reported, could be more if permeable) 100% Ease of general implementation Require point sources CO 2 Can be implemented very quickly Can use large quantities of fine wastes like wastes fly ash that are not necessarily pozzolanic Fine wastes tend to reduce gas permeability Safety Are carbonation rooms safe? No issues Key requirements Special carbonation rooms Optimal kinetics including gas permeability Physical rate considerations Other issues Doubling the concentration of CO 2 doubles Doubling the pore size quadruples the rate of carbonation. Able to be sealed with paint etc as pre Some sealing paints will slow down carbonation carbonated According to ECN "The CO 2 concentration in power station flue gas ranges from about 4% (by volume)for natural gas fired combined cycle plants to about 14% for pulverised coal fired boilers. " At 10% the rate increase over atmospheric could be expected to be 10/. 038 = 263 times provided other kinetic barriers such as the delivery of water do not set in. Ref : http: //www. ecn. nl/en/h 2 sf/products-services/co 2 -capture/r-d-activities/post-combustion-co 2 -capture/ accessed 24 Mar 08. Forced carbonation of silicate phases as promoted by some is nonsense
Forced Carbonation ~ Optimisation Forced Carbonation (Cambridge) Kinetic Optimisation (Tec. Eco) Steps Multistep process Less steps = lower costs Rate Variable Varying on weather conditions (wet dry best and gas permeability) % Carbonation in 6 months 70% (reported, could be more if permeable) 100% Ease of general implementation Require point sources CO 2 Can be implemented very quickly Can use large quantities of fine wastes like wastes fly ash that are not necessarily pozzolanic Fine wastes tend to reduce gas permeability Safety Are carbonation rooms safe? No issues Key requirements Special carbonation rooms Optimal kinetics including gas permeability Physical rate considerations Other issues Doubling the concentration of CO 2 doubles Doubling the pore size quadruples the rate of carbonation. Able to be sealed with paint etc as pre Some sealing paints will slow down carbonation carbonated According to ECN "The CO 2 concentration in power station flue gas ranges from about 4% (by volume)for natural gas fired combined cycle plants to about 14% for pulverised coal fired boilers. " At 10% the rate increase over atmospheric could be expected to be 10/. 038 = 263 times provided other kinetic barriers such as the delivery of water do not set in. Ref : http: //www. ecn. nl/en/h 2 sf/products-services/co 2 -capture/r-d-activities/post-combustion-co 2 -capture/ accessed 24 Mar 08. Forced carbonation of silicate phases as promoted by some is nonsense
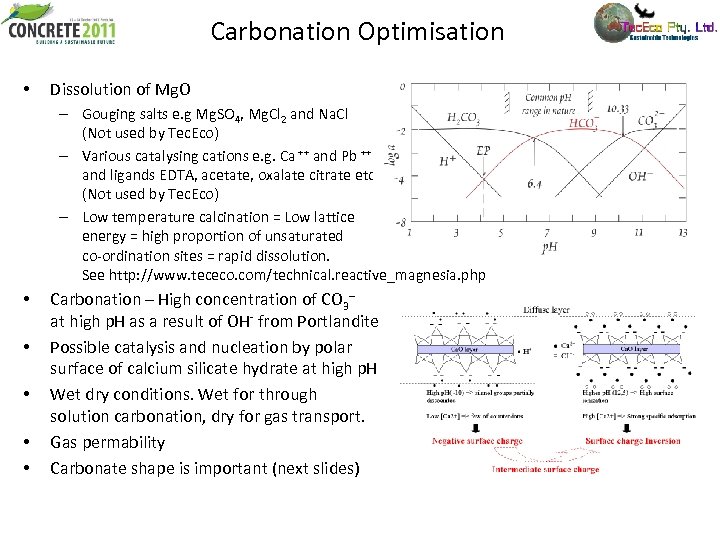 Carbonation Optimisation • Dissolution of Mg. O – Gouging salts e. g Mg. SO 4, Mg. Cl 2 and Na. Cl (Not used by Tec. Eco) – Various catalysing cations e. g. Ca ++ and Pb ++ and ligands EDTA, acetate, oxalate citrate etc. (Not used by Tec. Eco) – Low temperature calcination = Low lattice energy = high proportion of unsaturated co-ordination sites = rapid dissolution. See http: //www. tececo. com/technical. reactive_magnesia. php • • • Carbonation – High concentration of CO 3 -- at high p. H as a result of OH- from Portlandite Possible catalysis and nucleation by polar surface of calcium silicate hydrate at high p. H Wet dry conditions. Wet for through solution carbonation, dry for gas transport. Gas permability Carbonate shape is important (next slides)
Carbonation Optimisation • Dissolution of Mg. O – Gouging salts e. g Mg. SO 4, Mg. Cl 2 and Na. Cl (Not used by Tec. Eco) – Various catalysing cations e. g. Ca ++ and Pb ++ and ligands EDTA, acetate, oxalate citrate etc. (Not used by Tec. Eco) – Low temperature calcination = Low lattice energy = high proportion of unsaturated co-ordination sites = rapid dissolution. See http: //www. tececo. com/technical. reactive_magnesia. php • • • Carbonation – High concentration of CO 3 -- at high p. H as a result of OH- from Portlandite Possible catalysis and nucleation by polar surface of calcium silicate hydrate at high p. H Wet dry conditions. Wet for through solution carbonation, dry for gas transport. Gas permability Carbonate shape is important (next slides)
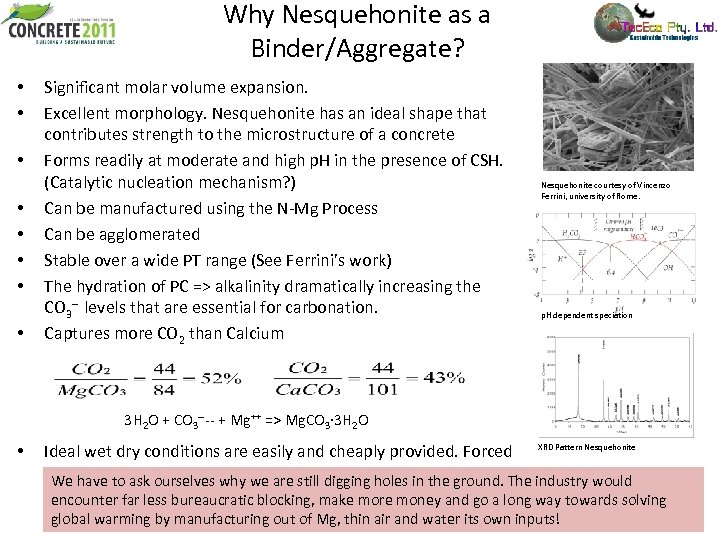 Why Nesquehonite as a Binder/Aggregate? • • Significant molar volume expansion. Excellent morphology. Nesquehonite has an ideal shape that contributes strength to the microstructure of a concrete Forms readily at moderate and high p. H in the presence of CSH. (Catalytic nucleation mechanism? ) Can be manufactured using the N-Mg Process Can be agglomerated Stable over a wide PT range (See Ferrini’s work) The hydration of PC => alkalinity dramatically increasing the CO 3 -- levels that are essential for carbonation. Captures more CO 2 than Calcium Nesquehonite courtesy of Vincenzo Ferrini, university of Rome. p. H dependent speciation 3 H 2 O + CO 3 ---- + Mg++ => Mg. CO 3· 3 H 2 O • Ideal wet dry conditions are easily and cheaply provided. Forced XRD Pattern Nesquehonite carbonation is not required (Cambridge uni and others) We have to ask ourselves why we are still digging holes in the ground. The industry would encounter far less bureaucratic blocking, make more money and go a long way towards solving global warming by manufacturing out of Mg, thin air and water its own inputs!
Why Nesquehonite as a Binder/Aggregate? • • Significant molar volume expansion. Excellent morphology. Nesquehonite has an ideal shape that contributes strength to the microstructure of a concrete Forms readily at moderate and high p. H in the presence of CSH. (Catalytic nucleation mechanism? ) Can be manufactured using the N-Mg Process Can be agglomerated Stable over a wide PT range (See Ferrini’s work) The hydration of PC => alkalinity dramatically increasing the CO 3 -- levels that are essential for carbonation. Captures more CO 2 than Calcium Nesquehonite courtesy of Vincenzo Ferrini, university of Rome. p. H dependent speciation 3 H 2 O + CO 3 ---- + Mg++ => Mg. CO 3· 3 H 2 O • Ideal wet dry conditions are easily and cheaply provided. Forced XRD Pattern Nesquehonite carbonation is not required (Cambridge uni and others) We have to ask ourselves why we are still digging holes in the ground. The industry would encounter far less bureaucratic blocking, make more money and go a long way towards solving global warming by manufacturing out of Mg, thin air and water its own inputs!
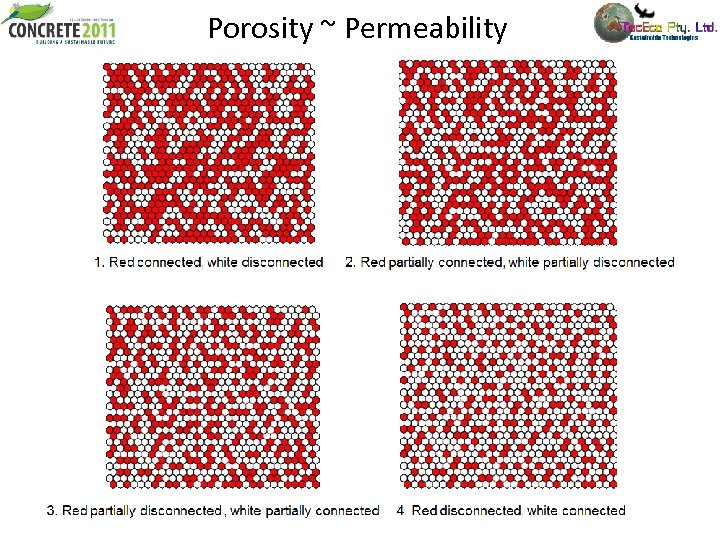 Porosity ~ Permeability
Porosity ~ Permeability
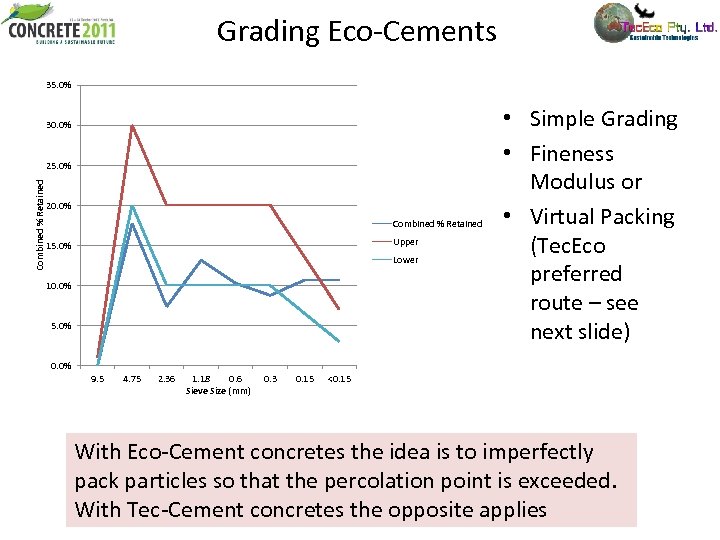 Grading Eco-Cements 35. 0% 30. 0% Combined % Retained 25. 0% 20. 0% Combined % Retained Upper 15. 0% Lower 10. 0% 5. 0% • Simple Grading • Fineness Modulus or • Virtual Packing (Tec. Eco preferred route – see next slide) 0. 0% 9. 5 4. 75 2. 36 1. 18 0. 6 Sieve Size (mm) 0. 3 0. 15 <0. 15 With Eco-Cement concretes the idea is to imperfectly pack particles so that the percolation point is exceeded. With Tec-Cement concretes the opposite applies
Grading Eco-Cements 35. 0% 30. 0% Combined % Retained 25. 0% 20. 0% Combined % Retained Upper 15. 0% Lower 10. 0% 5. 0% • Simple Grading • Fineness Modulus or • Virtual Packing (Tec. Eco preferred route – see next slide) 0. 0% 9. 5 4. 75 2. 36 1. 18 0. 6 Sieve Size (mm) 0. 3 0. 15 <0. 15 With Eco-Cement concretes the idea is to imperfectly pack particles so that the percolation point is exceeded. With Tec-Cement concretes the opposite applies
 Economics of Magnesium Carbonate Binder Based Masonry Products What this embedded spreadsheet demonstrates is that Magnesium Carbonate Block formulations are uneconomic unless the price of reactive Mg. O approaches that of PC or there is a high price for carbon or alternatively less Mg. O can be used! Because of molar volume growth less can be used but we must still address supply chain issues. This embedded spreadsheet looks only at the binder price and assumes all other factors remain the same
Economics of Magnesium Carbonate Binder Based Masonry Products What this embedded spreadsheet demonstrates is that Magnesium Carbonate Block formulations are uneconomic unless the price of reactive Mg. O approaches that of PC or there is a high price for carbon or alternatively less Mg. O can be used! Because of molar volume growth less can be used but we must still address supply chain issues. This embedded spreadsheet looks only at the binder price and assumes all other factors remain the same
 Permeacocretes • Permeacocretes are an example of a product where the other advantages of using reactive Mg. O overcome its high cost and lack of a suitable market for carbon trading. • The use of Mg. O gives an ideal rheology which makes it possible to make permeacocrete pervious pavements using conventional road laying equipment therefore substantially reducing labour costs. • There are many other advantages of pervious pavements see http: //www. tececo. com/files/conf erence%20 presentations/Tec. Eco. P resentation. SGA 25 Mar 2010. ppt
Permeacocretes • Permeacocretes are an example of a product where the other advantages of using reactive Mg. O overcome its high cost and lack of a suitable market for carbon trading. • The use of Mg. O gives an ideal rheology which makes it possible to make permeacocrete pervious pavements using conventional road laying equipment therefore substantially reducing labour costs. • There are many other advantages of pervious pavements see http: //www. tececo. com/files/conf erence%20 presentations/Tec. Eco. P resentation. SGA 25 Mar 2010. ppt
 Tec-Cements • Tec-Cements (5 -20% Mg. O, 80 -95% OPC) – contain more Portland cement than reactive magnesia. Reactive magnesia hydrates in the same rate order as Portland cement forming Brucite which uses up excess water reducing the voids: paste ratio, increasing density and possibly raising the short term p. H. – Reactions with pozzolans are more affective. After much of the Portlandite has been consumed Brucite tends to control the long term p. H which is lower and due to it’s low solubility, mobility and reactivity results in greater durability. – Other benefits include improvements in density, strength and rheology, reduced permeability and shrinkage and the use of a wider range of aggregates many of which are potentially wastes without reaction problems.
Tec-Cements • Tec-Cements (5 -20% Mg. O, 80 -95% OPC) – contain more Portland cement than reactive magnesia. Reactive magnesia hydrates in the same rate order as Portland cement forming Brucite which uses up excess water reducing the voids: paste ratio, increasing density and possibly raising the short term p. H. – Reactions with pozzolans are more affective. After much of the Portlandite has been consumed Brucite tends to control the long term p. H which is lower and due to it’s low solubility, mobility and reactivity results in greater durability. – Other benefits include improvements in density, strength and rheology, reduced permeability and shrinkage and the use of a wider range of aggregates many of which are potentially wastes without reaction problems.
 PC 50% Modified Ternary Mix with N-Mg Route Mg Carbonate Aggregate • Tec. Eco recently announced a way forward to greater sustainability for the Portland cement industry. • Up to 30% or more strength at all stages with high & very high replacement ternary mixes. (GBFS +- fly ash replacing PC. ) • Finishers can go home early using >50% replacement mixes removing the remaining barrier to their implementation • Brilliant rheology, low shrinkage and little or no cracking. • Excellent durability. • A solution to autogenous shrinkage? • Can tolerate carbon in fly ash and clays to some extent. • Mg++ combines with chloride or sulphate immobilising these cations
PC 50% Modified Ternary Mix with N-Mg Route Mg Carbonate Aggregate • Tec. Eco recently announced a way forward to greater sustainability for the Portland cement industry. • Up to 30% or more strength at all stages with high & very high replacement ternary mixes. (GBFS +- fly ash replacing PC. ) • Finishers can go home early using >50% replacement mixes removing the remaining barrier to their implementation • Brilliant rheology, low shrinkage and little or no cracking. • Excellent durability. • A solution to autogenous shrinkage? • Can tolerate carbon in fly ash and clays to some extent. • Mg++ combines with chloride or sulphate immobilising these cations
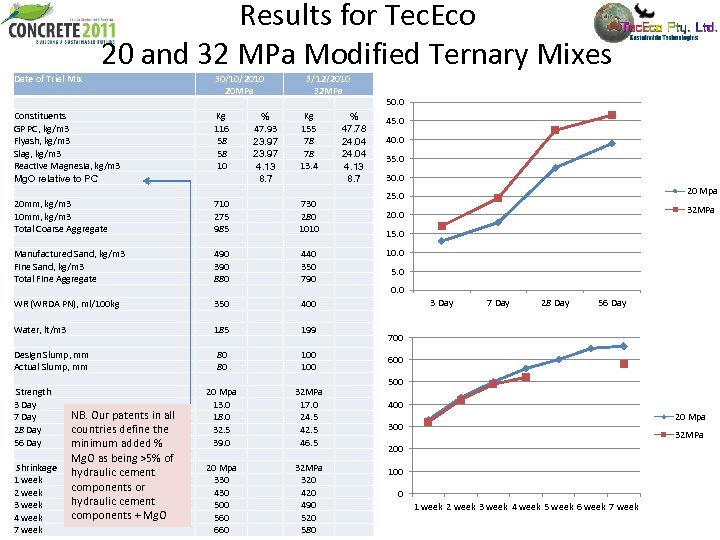 Results for Tec. Eco 20 and 32 MPa Modified Ternary Mixes Date of Trial Mix Constituents GP PC, kg/m 3 Flyash, kg/m 3 Slag, kg/m 3 Reactive Magnesia, kg/m 3 Mg. O relative to PC 20 mm, kg/m 3 10 mm, kg/m 3 Total Coarse Aggregate Manufactured Sand, kg/m 3 Fine Sand, kg/m 3 Total Fine Aggregate WR (WRDA PN), ml/100 kg Water, lt/m 3 Design Slump, mm Actual Slump, mm Strength 3 Day NB. Our patents in all 7 Day 28 Day countries define the 56 Day minimum added % Mg. O as being >5% of Shrinkage hydraulic cement 1 week components or 2 week hydraulic cement 3 week components + Mg. O 4 week 7 week 30/10/2010 20 MPa Kg % 116 47. 93 58 23. 97 10 4. 13 8. 7 710 275 985 490 390 880 350 185 80 80 20 Mpa 13. 0 18. 0 32. 5 39. 0 20 Mpa 330 430 500 560 660 3/12/2010 32 MPa Kg % 155 47. 78 78 24. 04 13. 4 4. 13 8. 7 730 280 1010 440 350 790 400 199 100 32 MPa 17. 0 24. 5 42. 5 46. 5 32 MPa 320 490 520 580 50. 0 45. 0 40. 0 35. 0 30. 0 20 Mpa 25. 0 32 MPa 20. 0 15. 0 10. 0 5. 0 0. 0 3 Day 7 Day 28 Day 56 Day 700 600 500 400 20 Mpa 300 32 MPa 200 100 0 1 week 2 week 3 week 4 week 5 week 6 week 7 week
Results for Tec. Eco 20 and 32 MPa Modified Ternary Mixes Date of Trial Mix Constituents GP PC, kg/m 3 Flyash, kg/m 3 Slag, kg/m 3 Reactive Magnesia, kg/m 3 Mg. O relative to PC 20 mm, kg/m 3 10 mm, kg/m 3 Total Coarse Aggregate Manufactured Sand, kg/m 3 Fine Sand, kg/m 3 Total Fine Aggregate WR (WRDA PN), ml/100 kg Water, lt/m 3 Design Slump, mm Actual Slump, mm Strength 3 Day NB. Our patents in all 7 Day 28 Day countries define the 56 Day minimum added % Mg. O as being >5% of Shrinkage hydraulic cement 1 week components or 2 week hydraulic cement 3 week components + Mg. O 4 week 7 week 30/10/2010 20 MPa Kg % 116 47. 93 58 23. 97 10 4. 13 8. 7 710 275 985 490 390 880 350 185 80 80 20 Mpa 13. 0 18. 0 32. 5 39. 0 20 Mpa 330 430 500 560 660 3/12/2010 32 MPa Kg % 155 47. 78 78 24. 04 13. 4 4. 13 8. 7 730 280 1010 440 350 790 400 199 100 32 MPa 17. 0 24. 5 42. 5 46. 5 32 MPa 320 490 520 580 50. 0 45. 0 40. 0 35. 0 30. 0 20 Mpa 25. 0 32 MPa 20. 0 15. 0 10. 0 5. 0 0. 0 3 Day 7 Day 28 Day 56 Day 700 600 500 400 20 Mpa 300 32 MPa 200 100 0 1 week 2 week 3 week 4 week 5 week 6 week 7 week
 A Tec-Cement Modified Ternary Mix
A Tec-Cement Modified Ternary Mix
 Tec-Cement Mixes Ordinary Mixes Tec. Eco Tec-Cement Mixes Notes Reactive Mg. O as defined None Usually 8 to 10% / PC added 1 Pozzolan (Pos) Should be used Recommended. Supplementary cementitious materials (SCM’s) Should be used Recommended. Limit on additions pozzolans + SCM’s Limited by standards that are increasingly exceeded > 50% recommended especially if a ternary blend Rheology Usually sticky, especially with fly ash. Hard to finish. Slippery and creamy. Easy to finish. Setting time Slow. Especially with flyash only. Much faster. Blends with a high proportion Pos. and SCM’s set like ordinary PC concrete. Shrinkage and cracking Significant Much less Additives Usually used Not necessary Durability Without additions of Pos and SCM’s Excellent especially with questionable. additions of Pos and SCM’s 28 day Strength (prev 20 MPA mix) < . 20 Mpa/Kg PC/m 3 We recommend using both Pos and SCM’s together 2 >. 27 Mpa/Kg PC/m 3 $ Cost Binder/Mpa at 28 days > ($2. 30 -$2. 50) < ($1. 50 -$1. 90) 3 (prev 20 & 32 MPa mixes) Notes 1. See http: //www. tececo. com/technical. reactive_magnesia. php. % is relative to PC and in addition to amount already in PC 2. To keep our patents simple we included supplementary cementitious materials as pozzolans in our specification 3. See economics pages following
Tec-Cement Mixes Ordinary Mixes Tec. Eco Tec-Cement Mixes Notes Reactive Mg. O as defined None Usually 8 to 10% / PC added 1 Pozzolan (Pos) Should be used Recommended. Supplementary cementitious materials (SCM’s) Should be used Recommended. Limit on additions pozzolans + SCM’s Limited by standards that are increasingly exceeded > 50% recommended especially if a ternary blend Rheology Usually sticky, especially with fly ash. Hard to finish. Slippery and creamy. Easy to finish. Setting time Slow. Especially with flyash only. Much faster. Blends with a high proportion Pos. and SCM’s set like ordinary PC concrete. Shrinkage and cracking Significant Much less Additives Usually used Not necessary Durability Without additions of Pos and SCM’s Excellent especially with questionable. additions of Pos and SCM’s 28 day Strength (prev 20 MPA mix) < . 20 Mpa/Kg PC/m 3 We recommend using both Pos and SCM’s together 2 >. 27 Mpa/Kg PC/m 3 $ Cost Binder/Mpa at 28 days > ($2. 30 -$2. 50) < ($1. 50 -$1. 90) 3 (prev 20 & 32 MPa mixes) Notes 1. See http: //www. tececo. com/technical. reactive_magnesia. php. % is relative to PC and in addition to amount already in PC 2. To keep our patents simple we included supplementary cementitious materials as pozzolans in our specification 3. See economics pages following
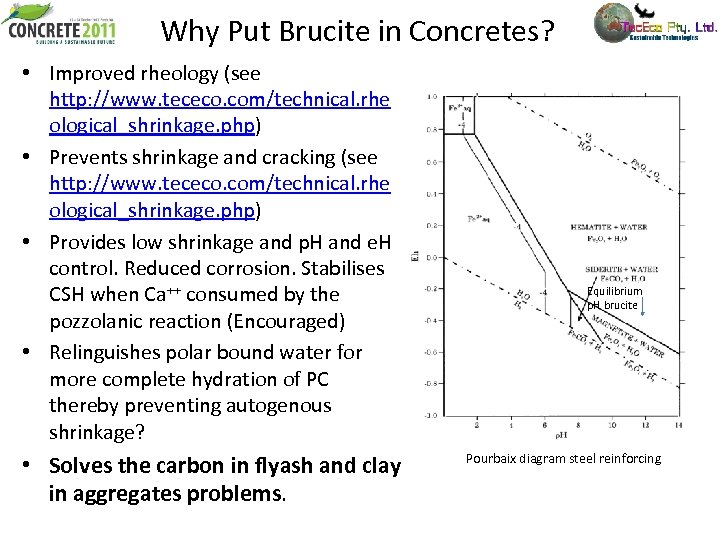 Why Put Brucite in Concretes? • Improved rheology (see http: //www. tececo. com/technical. rhe ological_shrinkage. php) • Prevents shrinkage and cracking (see http: //www. tececo. com/technical. rhe ological_shrinkage. php) • Provides low shrinkage and p. H and e. H control. Reduced corrosion. Stabilises CSH when Ca++ consumed by the pozzolanic reaction (Encouraged) • Relinguishes polar bound water for more complete hydration of PC thereby preventing autogenous shrinkage? • Solves the carbon in flyash and clay in aggregates problems. Equilibrium p. H brucite Pourbaix diagram steel reinforcing
Why Put Brucite in Concretes? • Improved rheology (see http: //www. tececo. com/technical. rhe ological_shrinkage. php) • Prevents shrinkage and cracking (see http: //www. tececo. com/technical. rhe ological_shrinkage. php) • Provides low shrinkage and p. H and e. H control. Reduced corrosion. Stabilises CSH when Ca++ consumed by the pozzolanic reaction (Encouraged) • Relinguishes polar bound water for more complete hydration of PC thereby preventing autogenous shrinkage? • Solves the carbon in flyash and clay in aggregates problems. Equilibrium p. H brucite Pourbaix diagram steel reinforcing
 Wet Stage Properties of Tec. Cement Concretes • Water has cohesivity due to a network of extensive threedimensional hydrogen bonding and this property is strengthened both by Brucite surfaces and the strongly kosmotropic magnesium ion in solution. • The strong polar bonding – Affects all wet stage properties • • • improving rheology markedly reducing early age shrinkage contributing to high early strength Reducing bleed water thereby retaining alkali Making the mixes highly thixotropic – Significantly brings forward the onset of first set with high replacement mixes. • increases the “wet sand effect” effect. • Mg. O goes negative – Helps deliver high early strength Ca++ = 114 picometres Mg++ = 86 picometres
Wet Stage Properties of Tec. Cement Concretes • Water has cohesivity due to a network of extensive threedimensional hydrogen bonding and this property is strengthened both by Brucite surfaces and the strongly kosmotropic magnesium ion in solution. • The strong polar bonding – Affects all wet stage properties • • • improving rheology markedly reducing early age shrinkage contributing to high early strength Reducing bleed water thereby retaining alkali Making the mixes highly thixotropic – Significantly brings forward the onset of first set with high replacement mixes. • increases the “wet sand effect” effect. • Mg. O goes negative – Helps deliver high early strength Ca++ = 114 picometres Mg++ = 86 picometres
 Mg. O has a Bar Magnet Effect The Change in the Surface Charge of Metal Oxides with p. H. Source: Small, R. J. et al. , 2005. Using a buffered rinse solution to minimize metal contamination after wafer cleaning. Micro. Magazine. com. Available at: http: //www. micromagazine. com/archive/98/01/small. html.
Mg. O has a Bar Magnet Effect The Change in the Surface Charge of Metal Oxides with p. H. Source: Small, R. J. et al. , 2005. Using a buffered rinse solution to minimize metal contamination after wafer cleaning. Micro. Magazine. com. Available at: http: //www. micromagazine. com/archive/98/01/small. html.
 Dry Stage Properties of Tec-Cement Concretes • Significantly increased tensile strength • Increased compressive strength (especially early strength) particularly with high replacement mixes containing significant amounts of GBFS compacting factor • Improves • Higher tensile strain capacity? • Greater creep • Less permeable? • Solves autogenous shrinkage problems • May solve other delayed reaction problems Recommended Reading: Du C. A Review of Magnesium Oxide in Concrete - A serendipitous discovery leads to new concrete for dam construction. Concrete International. 2005; (December 2005): 45 - 50.
Dry Stage Properties of Tec-Cement Concretes • Significantly increased tensile strength • Increased compressive strength (especially early strength) particularly with high replacement mixes containing significant amounts of GBFS compacting factor • Improves • Higher tensile strain capacity? • Greater creep • Less permeable? • Solves autogenous shrinkage problems • May solve other delayed reaction problems Recommended Reading: Du C. A Review of Magnesium Oxide in Concrete - A serendipitous discovery leads to new concrete for dam construction. Concrete International. 2005; (December 2005): 45 - 50.
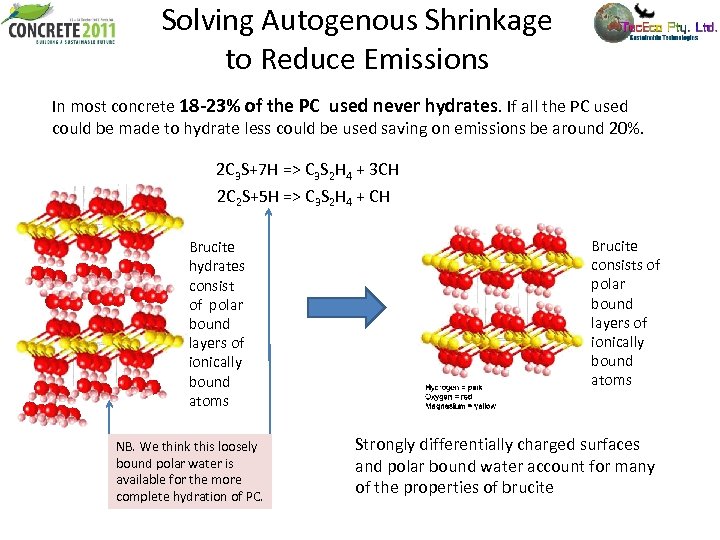 Solving Autogenous Shrinkage to Reduce Emissions In most concrete 18 -23% of the PC used never hydrates. If all the PC used could be made to hydrate less could be used saving on emissions be around 20%. 2 C 3 S+7 H => C 3 S 2 H 4 + 3 CH 2 C 2 S+5 H => C 3 S 2 H 4 + CH Brucite hydrates consist of polar bound layers of ionically bound atoms NB. We think this loosely bound polar water is available for the more complete hydration of PC. Brucite consists of polar bound layers of ionically bound atoms Strongly differentially charged surfaces and polar bound water account for many of the properties of brucite
Solving Autogenous Shrinkage to Reduce Emissions In most concrete 18 -23% of the PC used never hydrates. If all the PC used could be made to hydrate less could be used saving on emissions be around 20%. 2 C 3 S+7 H => C 3 S 2 H 4 + 3 CH 2 C 2 S+5 H => C 3 S 2 H 4 + CH Brucite hydrates consist of polar bound layers of ionically bound atoms NB. We think this loosely bound polar water is available for the more complete hydration of PC. Brucite consists of polar bound layers of ionically bound atoms Strongly differentially charged surfaces and polar bound water account for many of the properties of brucite
 Economics of Tec-Cements Binder Prices Only This embedded spreadsheet looks only at the binder price and assumes all other factors remain the same
Economics of Tec-Cements Binder Prices Only This embedded spreadsheet looks only at the binder price and assumes all other factors remain the same
 The Case for Agglomeration of Carbonates, Fly ash and other Wastes • Sand stone aggregate are in short supply in some areas. • Nesquehonite is an ideal micro aggregate so why not agglomerate it and/or other magnesium carbonates to make man made manufactured aggregate? • Mg. O binders will be suitable for this purpose and Tec. Eco are seeking funding to demonstrate the technology. • Tec. Eco can already agglomerate fly ash and nesquehonite without additional energy. We just can’t tell you how as we have not had the money to pursue a patent.
The Case for Agglomeration of Carbonates, Fly ash and other Wastes • Sand stone aggregate are in short supply in some areas. • Nesquehonite is an ideal micro aggregate so why not agglomerate it and/or other magnesium carbonates to make man made manufactured aggregate? • Mg. O binders will be suitable for this purpose and Tec. Eco are seeking funding to demonstrate the technology. • Tec. Eco can already agglomerate fly ash and nesquehonite without additional energy. We just can’t tell you how as we have not had the money to pursue a patent.
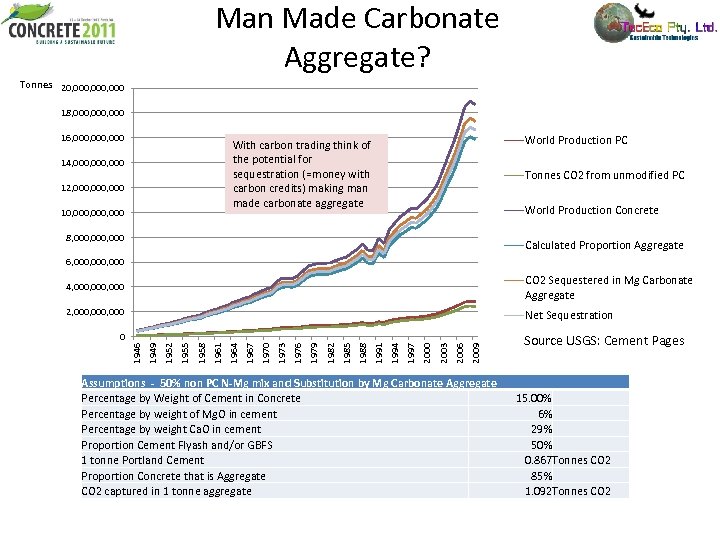 Man Made Carbonate Aggregate? Tonnes 20, 000, 000 18, 000, 000 16, 000, 000 World Production PC With carbon trading think of the potential for sequestration (=money with carbon credits) making man made carbonate aggregate 14, 000, 000 12, 000, 000 10, 000, 000 Tonnes CO 2 from unmodified PC World Production Concrete 8, 000, 000 Calculated Proportion Aggregate 6, 000, 000 4, 000, 000 CO 2 Sequestered in Mg Carbonate Aggregate 2, 000, 000 Net Sequestration 2009 2006 2003 2000 1997 1994 1991 1988 1985 1982 1979 1976 1973 1970 1967 1964 1961 1958 1955 1952 1949 1946 0 Assumptions - 50% non PC N-Mg mix and Substitution by Mg Carbonate Aggregate Percentage by Weight of Cement in Concrete Percentage by weight of Mg. O in cement Percentage by weight Ca. O in cement Proportion Cement Flyash and/or GBFS 1 tonne Portland Cement Proportion Concrete that is Aggregate CO 2 captured in 1 tonne aggregate Source USGS: Cement Pages 15. 00% 6% 29% 50% 0. 867 Tonnes CO 2 85% 1. 092 Tonnes CO 2
Man Made Carbonate Aggregate? Tonnes 20, 000, 000 18, 000, 000 16, 000, 000 World Production PC With carbon trading think of the potential for sequestration (=money with carbon credits) making man made carbonate aggregate 14, 000, 000 12, 000, 000 10, 000, 000 Tonnes CO 2 from unmodified PC World Production Concrete 8, 000, 000 Calculated Proportion Aggregate 6, 000, 000 4, 000, 000 CO 2 Sequestered in Mg Carbonate Aggregate 2, 000, 000 Net Sequestration 2009 2006 2003 2000 1997 1994 1991 1988 1985 1982 1979 1976 1973 1970 1967 1964 1961 1958 1955 1952 1949 1946 0 Assumptions - 50% non PC N-Mg mix and Substitution by Mg Carbonate Aggregate Percentage by Weight of Cement in Concrete Percentage by weight of Mg. O in cement Percentage by weight Ca. O in cement Proportion Cement Flyash and/or GBFS 1 tonne Portland Cement Proportion Concrete that is Aggregate CO 2 captured in 1 tonne aggregate Source USGS: Cement Pages 15. 00% 6% 29% 50% 0. 867 Tonnes CO 2 85% 1. 092 Tonnes CO 2


