5b5c4d5a0662add20b147037e27d77a0.ppt
- Количество слайдов: 35

An Update… CDC Surveillance Project on Bleeding Disorders Diane Aschman Administrative PI Marilyn Manco-Johnson Scientific PI 1

Goals of the Surveillance • Provide descriptive knowledge about the • demographics, diagnoses and health service utilization of populations with bleeding disorders and venous thromboembolism receiving care at HTCs Monitor health indicators among populations with bleeding disorders – – Assess trends over time Measure rates of, and risk factors for, complications Identify high risk populations for prevention Identify issues that require research 2
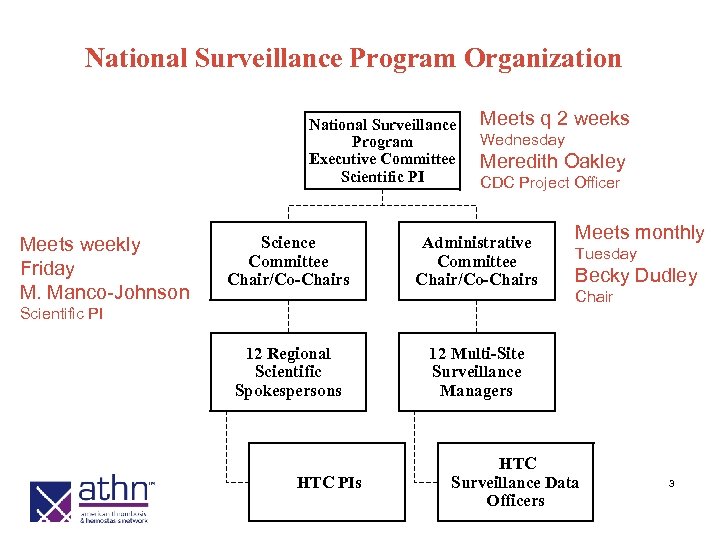
National Surveillance Program Organization National Surveillance Program Executive Committee Scientific PI Meets weekly Friday M. Manco-Johnson Meets q 2 weeks Wednesday Meredith Oakley CDC Project Officer Science Committee Chair/Co-Chairs Administrative Committee Chair/Co-Chairs 12 Regional Scientific Spokespersons Meets monthly 12 Multi-Site Surveillance Managers Scientific PI HTC PIs Tuesday Becky Dudley Chair HTC Surveillance Data Officers 3
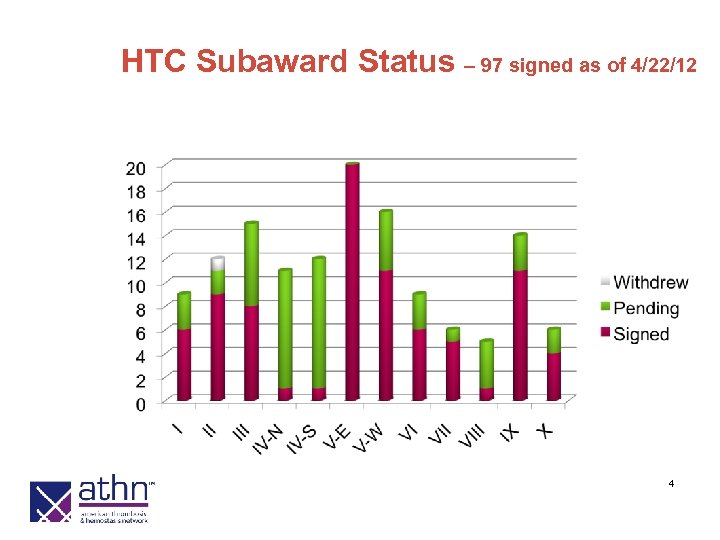
HTC Subaward Status – 97 signed as of 4/22/12 4

Thank You for Signing On! Region VI • Gulf States • Louisiana • Arkansas • Fort Worth • South Texas • Texas Children’s Region VII • Children’s Mercy • Iowa • John Bouhasin • U of Missouri • U of Nebraska 5
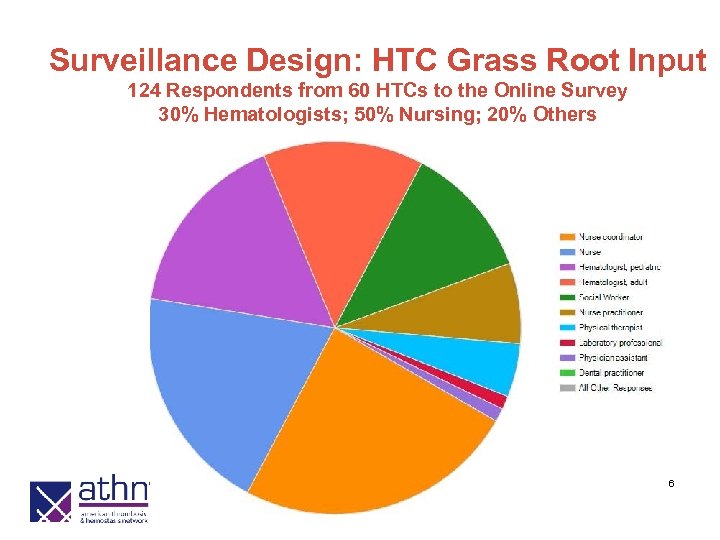
Surveillance Design: HTC Grass Root Input 124 Respondents from 60 HTCs to the Online Survey 30% Hematologists; 50% Nursing; 20% Others 6
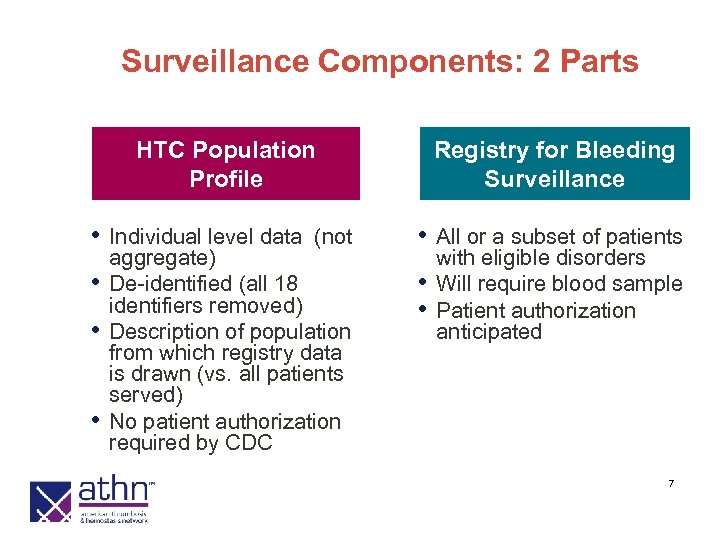
Surveillance Components: 2 Parts HTC Population Profile • Individual level data • • • (not aggregate) De-identified (all 18 identifiers removed) Description of population from which registry data is drawn (vs. all patients served) No patient authorization required by CDC Registry for Bleeding Surveillance • All or a subset of patients • • with eligible disorders Will require blood sample Patient authorization anticipated 7
Surveillance Components: 2 Parts HTC Population Profile Registry for Bleeding Surveillance 8
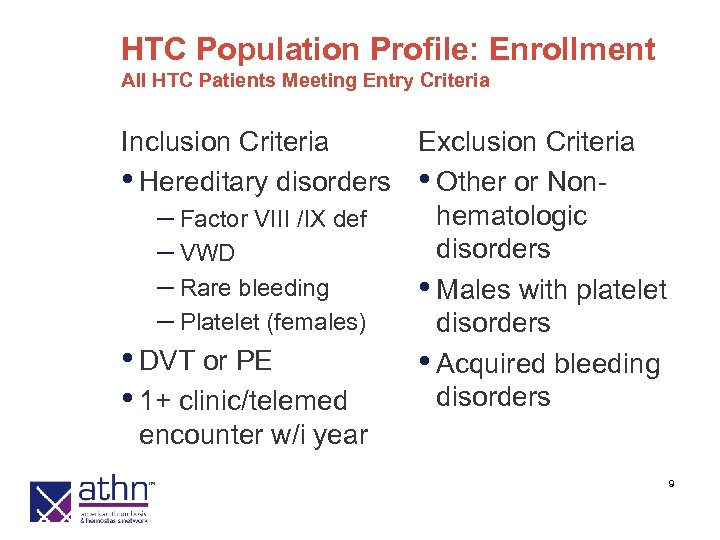
HTC Population Profile: Enrollment All HTC Patients Meeting Entry Criteria Inclusion Criteria Exclusion Criteria • Hereditary disorders • Other or Nonhematologic – Factor VIII /IX def disorders – VWD – Rare bleeding • Males with platelet – Platelet (females) disorders • DVT or PE • Acquired bleeding disorders • 1+ clinic/telemed encounter w/i year 9
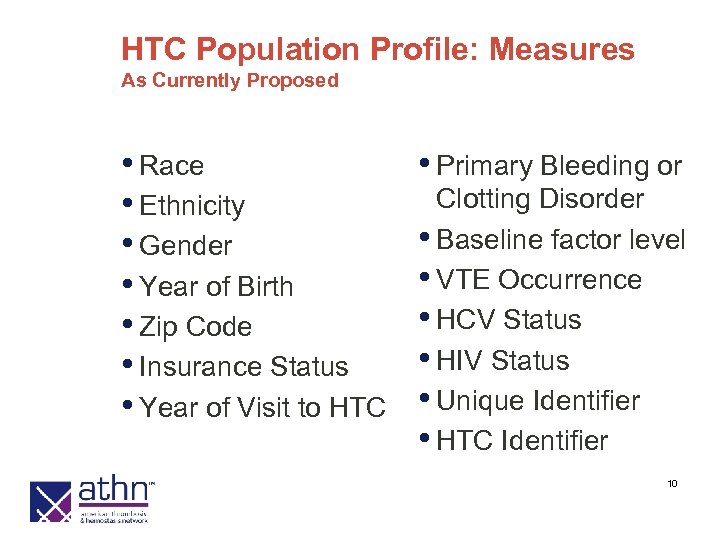
HTC Population Profile: Measures As Currently Proposed • Race • Ethnicity • Gender • Year of Birth • Zip Code • Insurance Status • Year of Visit to HTC • Primary Bleeding or Clotting Disorder • Baseline factor level • VTE Occurrence • HCV Status • HIV Status • Unique Identifier • HTC Identifier 10
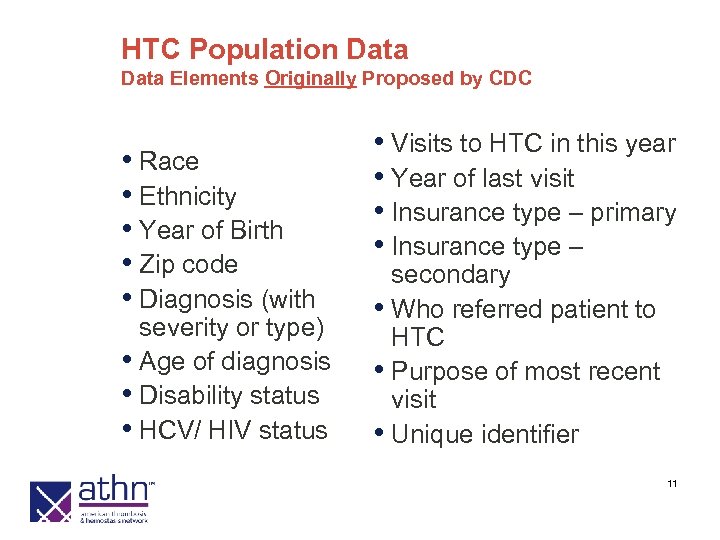
HTC Population Data Elements Originally Proposed by CDC • Race • Ethnicity • Year of Birth • Zip code • Diagnosis (with severity or type) • Age of diagnosis • Disability status • HCV/ HIV status • Visits to HTC in this year • Year of last visit • Insurance type – primary • Insurance type – secondary • Who referred patient to HTC • Purpose of most recent visit • Unique identifier 11
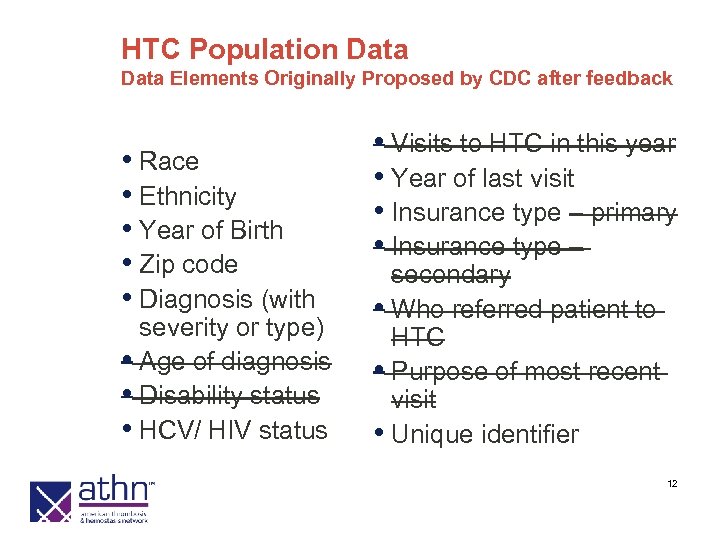
HTC Population Data Elements Originally Proposed by CDC after feedback • Race • Ethnicity • Year of Birth • Zip code • Diagnosis (with severity or type) • Age of diagnosis • Disability status • HCV/ HIV status • Visits to HTC in this year • Year of last visit • Insurance type – primary • Insurance type – secondary • Who referred patient to HTC • Purpose of most recent visit • Unique identifier 12
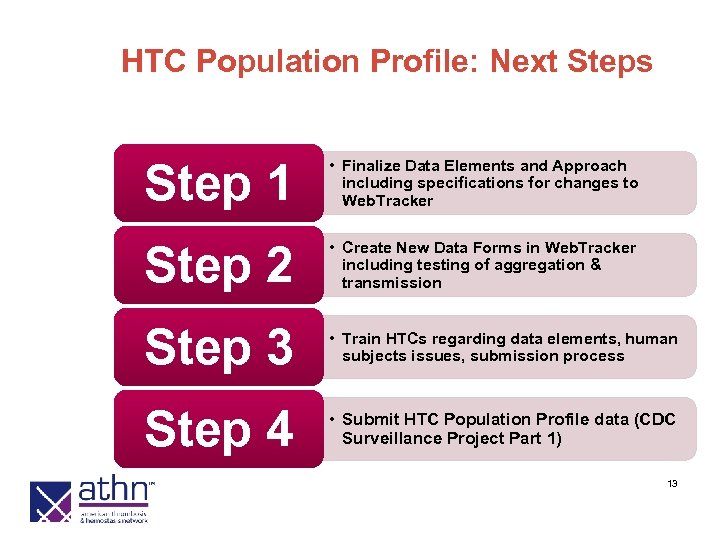
HTC Population Profile: Next Steps Step 1 • Finalize Data Elements and Approach including specifications for changes to Web. Tracker Step 2 • Create New Data Forms in Web. Tracker including testing of aggregation & transmission Step 3 • Train HTCs regarding data elements, human subjects issues, submission process Step 4 • Submit HTC Population Profile data (CDC Surveillance Project Part 1) 13
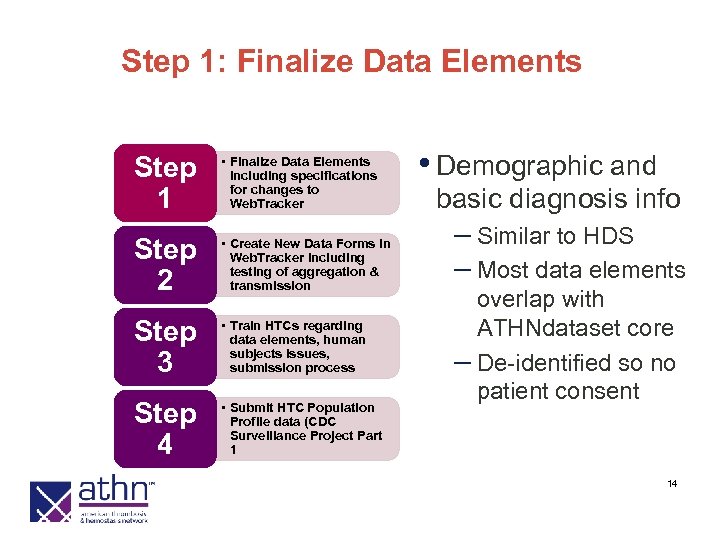
Step 1: Finalize Data Elements Step 1 • Finalize Data Elements including specifications for changes to Web. Tracker Step 2 • Create New Data Forms in Web. Tracker including testing of aggregation & transmission Step 3 • Train HTCs regarding data elements, human subjects issues, submission process Step 4 • Submit HTC Population Profile data (CDC Surveillance Project Part 1 • Demographic and basic diagnosis info – Similar to HDS – Most data elements overlap with ATHNdataset core – De-identified so no patient consent 14
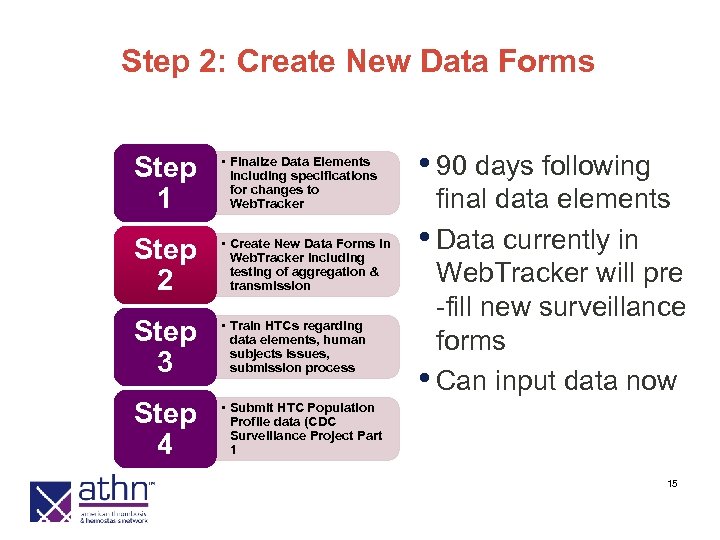
Step 2: Create New Data Forms Step 1 • Finalize Data Elements including specifications for changes to Web. Tracker Step 2 • Create New Data Forms in Web. Tracker including testing of aggregation & transmission Step 3 • Train HTCs regarding data elements, human subjects issues, submission process Step 4 • 90 days following • Submit HTC Population Profile data (CDC Surveillance Project Part 1 final data elements • Data currently in Web. Tracker will pre -fill new surveillance forms • Can input data now 15
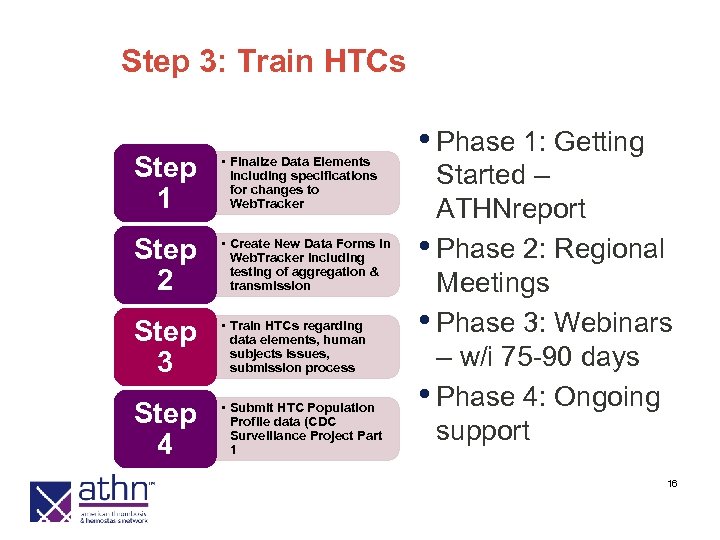
Step 3: Train HTCs Step 1 • Finalize Data Elements including specifications for changes to Web. Tracker Step 2 • Create New Data Forms in Web. Tracker including testing of aggregation & transmission Step 3 • Train HTCs regarding data elements, human subjects issues, submission process Step 4 • Submit HTC Population Profile data (CDC Surveillance Project Part 1 • Phase 1: Getting Started – ATHNreport • Phase 2: Regional Meetings • Phase 3: Webinars – w/i 75 -90 days • Phase 4: Ongoing support 16
Surveillance Components: 2 Parts HTC Population Profile Registry for Bleeding Surveillance 17
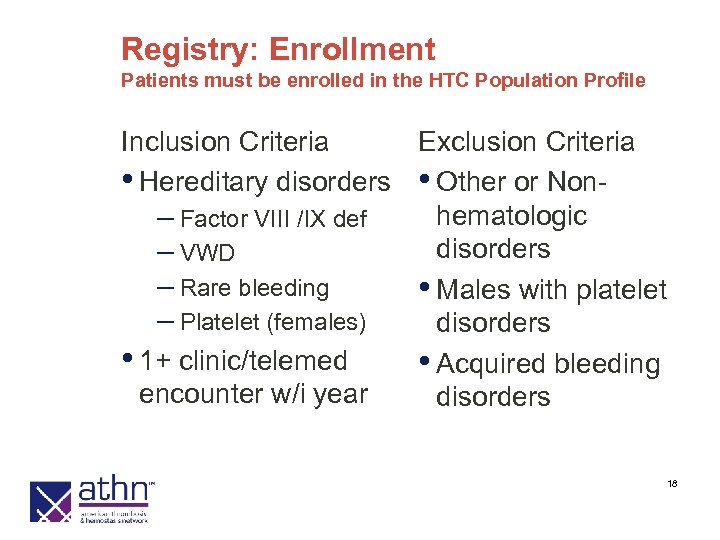
Registry: Enrollment Patients must be enrolled in the HTC Population Profile Inclusion Criteria Exclusion Criteria • Hereditary disorders • Other or Nonhematologic – Factor VIII /IX def disorders – VWD – Rare bleeding • Males with platelet – Platelet (females) disorders • 1+ clinic/telemed • Acquired bleeding encounter w/i year disorders 18
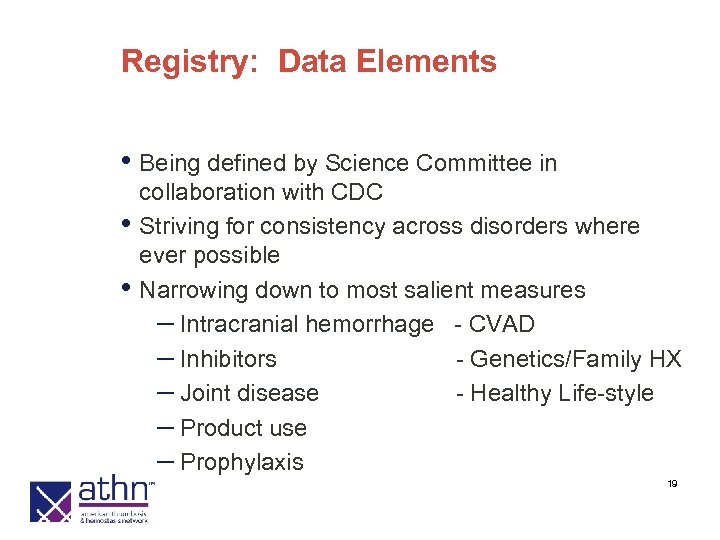
Registry: Data Elements • Being defined by Science Committee in • • collaboration with CDC Striving for consistency across disorders where ever possible Narrowing down to most salient measures – Intracranial hemorrhage - CVAD – Inhibitors - Genetics/Family HX – Joint disease - Healthy Life-style – Product use – Prophylaxis 19

We Need & Want Your Involvement • Weigh in on Registry Data Elements – Contact regional leadership – Review at www. htcnetwork. org • Start amassing HTC Population Profile data • Keep up to date – ATHNreport – Trainings 20

Announcing… ATHNdataset Diane Aschman A Growing Resource for Bleeding Disorders Community 21

Announcing ATHNdataset: A Growing Resource • Created by American Thrombosis and Hemostasis • • Network (ATHN) in collaboration with its 133 affiliated hemophilia treatment centers Brings together standardized demographic and clinical data into one national dataset Is a “Limited Data Set” as defined by Privacy Rule – Stripped of 16 specified direct identifiers – Used or disclosed for public health, research or health care operations – Disclosure covered by data use agreements 22
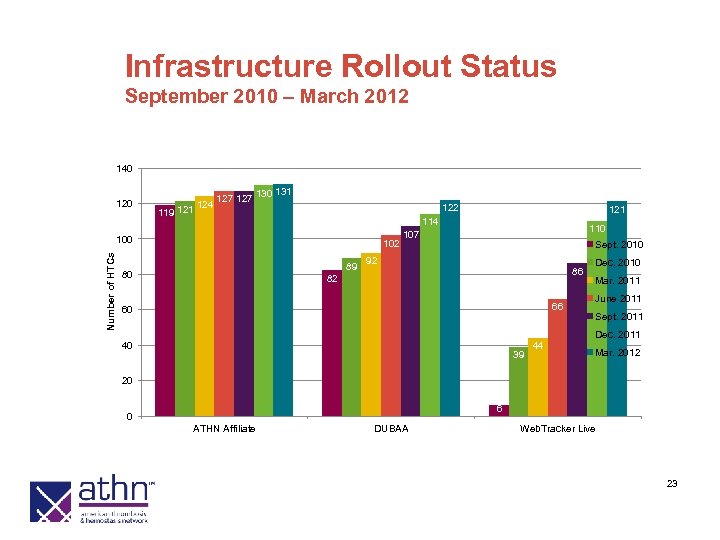
Infrastructure Rollout Status September 2010 – March 2012 140 120 119 121 124 127 130 131 122 Number of HTCs 100 102 89 80 121 114 110 107 Sept. 2010 92 86 82 66 60 Dec. 2010 Mar. 2011 June 2011 Sept. 2011 Dec. 2011 40 39 44 Mar. 2012 20 6 0 ATHN Affiliate DUBAA Web. Tracker Live 23
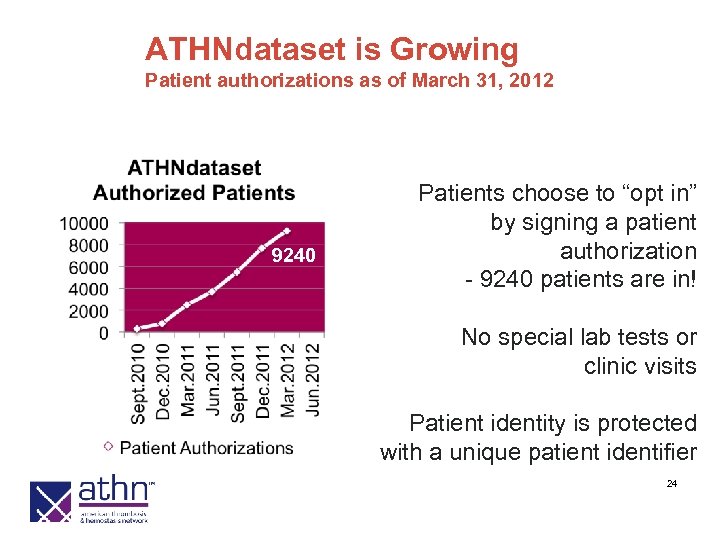
ATHNdataset is Growing Patient authorizations as of March 31, 2012 9240 Patients choose to “opt in” by signing a patient authorization - 9240 patients are in! No special lab tests or clinic visits Patient identity is protected with a unique patient identifier 24
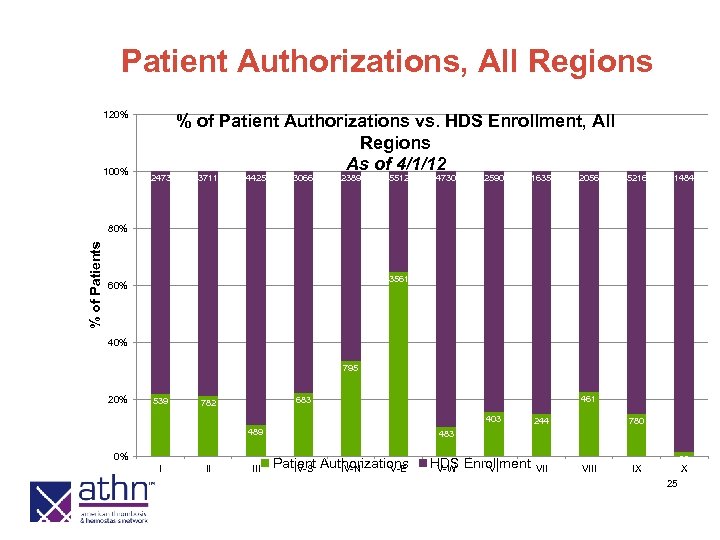
Patient Authorizations, All Regions 120% 100% 2473 % of Patient Authorizations vs. HDS Enrollment, All Regions As of 4/1/12 3711 4425 3066 2389 5512 4730 2590 1635 2056 5216 1484 % of Patients 80% 3561 60% 40% 795 20% 539 461 683 782 403 489 0% I II III 780 244 483 Patient Authorizations IV-S IV-N V-E HDS Enrollment V-W VI VIII 20 IX X 25
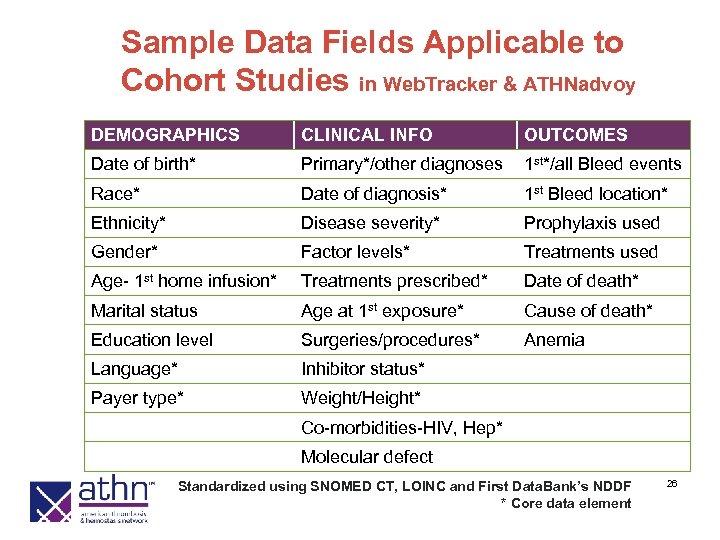
Sample Data Fields Applicable to Cohort Studies in Web. Tracker & ATHNadvoy DEMOGRAPHICS CLINICAL INFO OUTCOMES Date of birth* Primary*/other diagnoses 1 st*/all Bleed events Race* Date of diagnosis* 1 st Bleed location* Ethnicity* Disease severity* Prophylaxis used Gender* Factor levels* Treatments used Age- 1 st home infusion* Treatments prescribed* Date of death* Marital status Age at 1 st exposure* Cause of death* Education level Surgeries/procedures* Anemia Language* Inhibitor status* Payer type* Weight/Height* Co-morbidities-HIV, Hep* Molecular defect Standardized using SNOMED CT, LOINC and First Data. Bank’s NDDF * Core data element 26
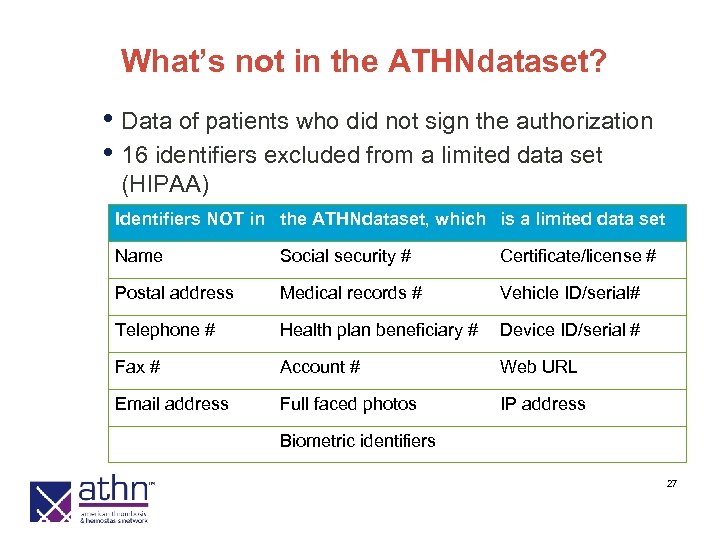
What’s not in the ATHNdataset? • Data of patients who did not sign the authorization • 16 identifiers excluded from a limited data set (HIPAA) Identifiers NOT in the ATHNdataset, which is a limited data set Name Social security # Certificate/license # Postal address Medical records # Vehicle ID/serial# Telephone # Health plan beneficiary # Device ID/serial # Fax # Account # Web URL Email address Full faced photos IP address Biometric identifiers 27
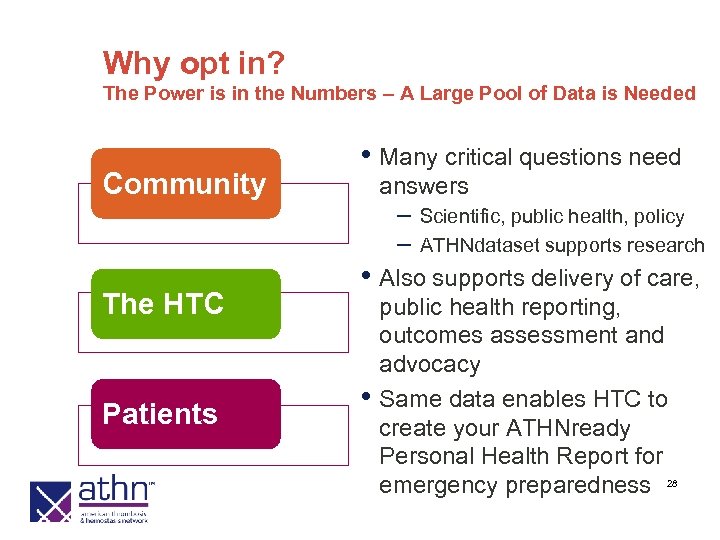
Why opt in? The Power is in the Numbers – A Large Pool of Data is Needed Community • Many critical questions need answers – – The HTC Patients Scientific, public health, policy ATHNdataset supports research • Also supports delivery of care, • public health reporting, outcomes assessment and advocacy Same data enables HTC to create your ATHNready Personal Health Report for emergency preparedness 28
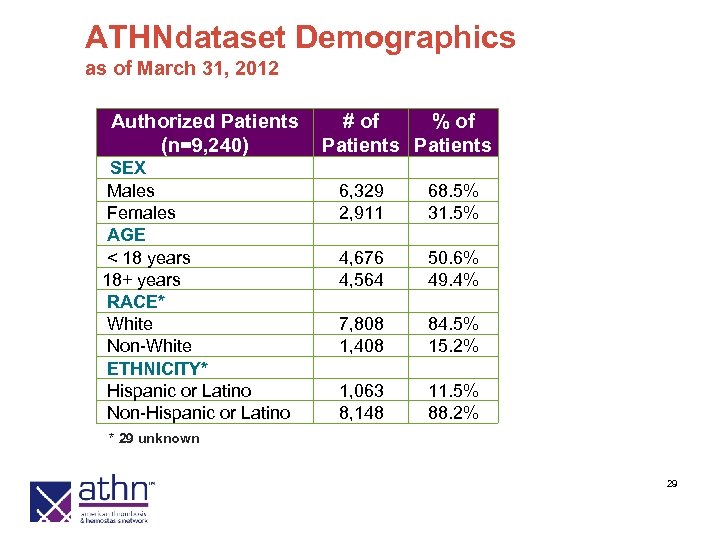
ATHNdataset Demographics as of March 31, 2012 Authorized Patients (n=9, 240) SEX Males Females AGE < 18 years 18+ years RACE* White Non-White ETHNICITY* Hispanic or Latino Non-Hispanic or Latino # of % of Patients 6, 329 2, 911 68. 5% 31. 5% 4, 676 4, 564 50. 6% 49. 4% 7, 808 1, 408 84. 5% 15. 2% 1, 063 8, 148 11. 5% 88. 2% * 29 unknown 29
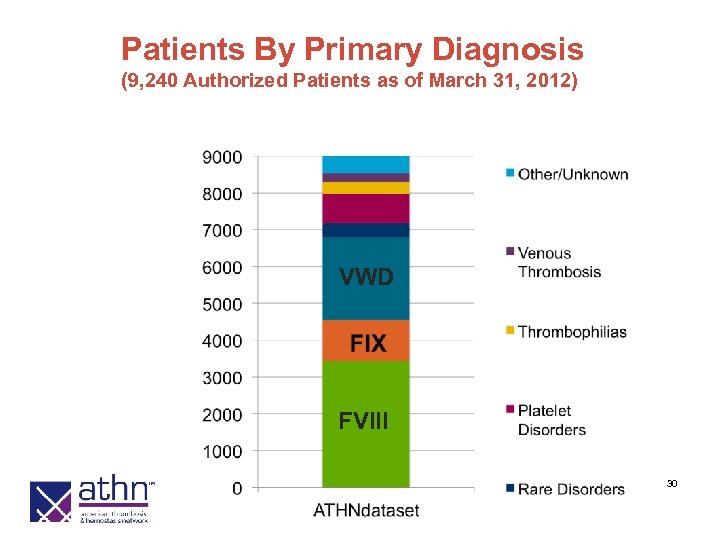
Patients By Primary Diagnosis (9, 240 Authorized Patients as of March 31, 2012) VWD FVIII 30
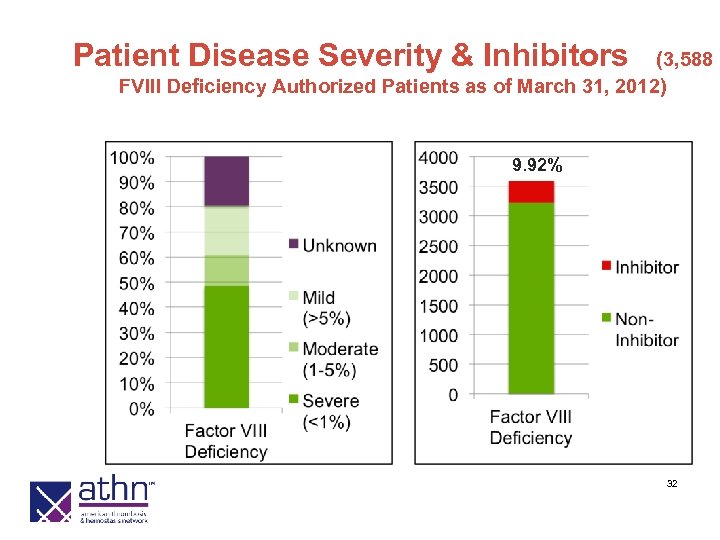
Patient Disease Severity & Inhibitors (3, 588 FVIII Deficiency Authorized Patients as of March 31, 2012) 9. 92% 32
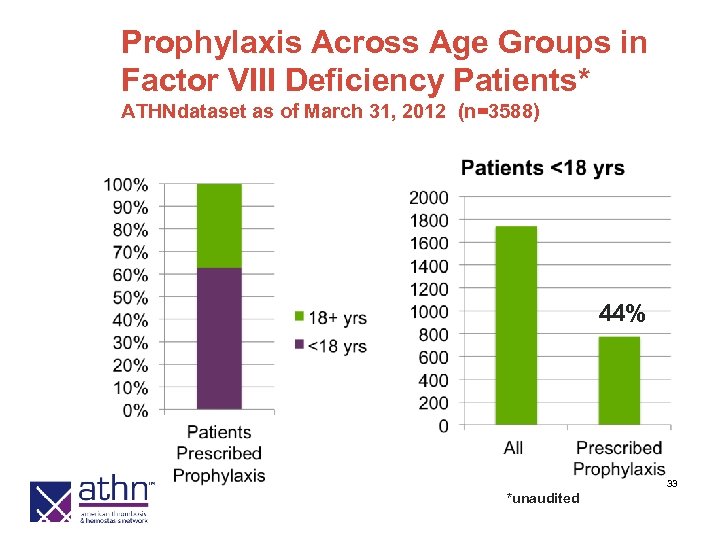
Prophylaxis Across Age Groups in Factor VIII Deficiency Patients* ATHNdataset as of March 31, 2012 (n=3588) 44% 33 *unaudited
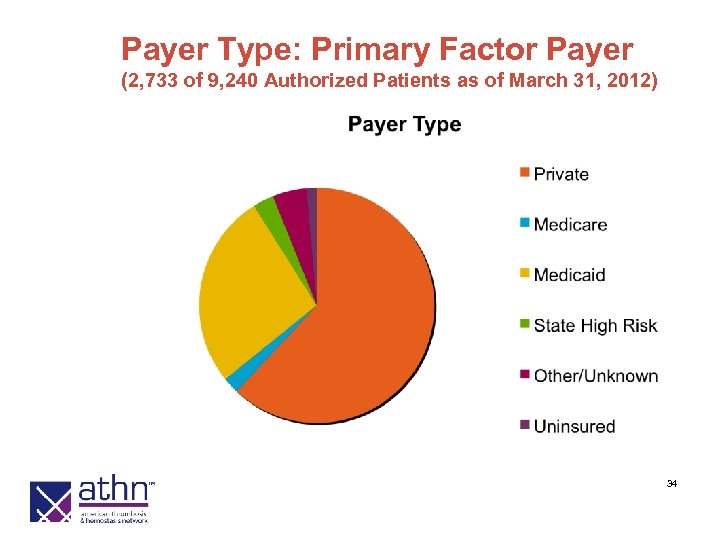
Payer Type: Primary Factor Payer (2, 733 of 9, 240 Authorized Patients as of March 31, 2012) 34
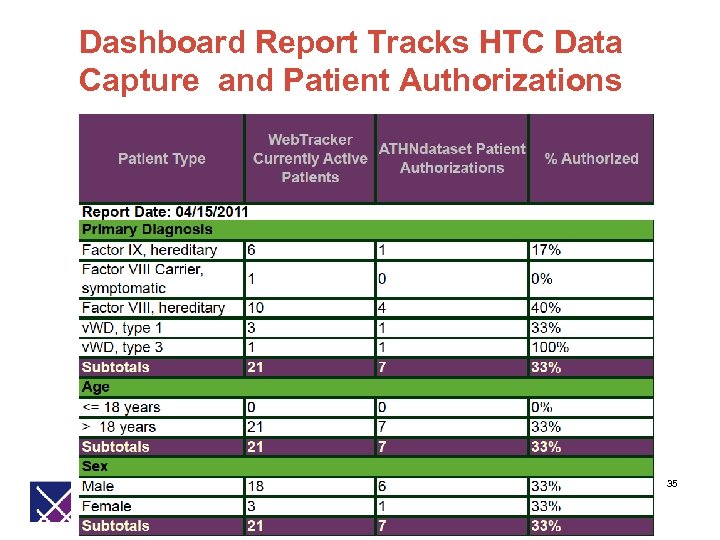
Dashboard Report Tracks HTC Data Capture and Patient Authorizations 35
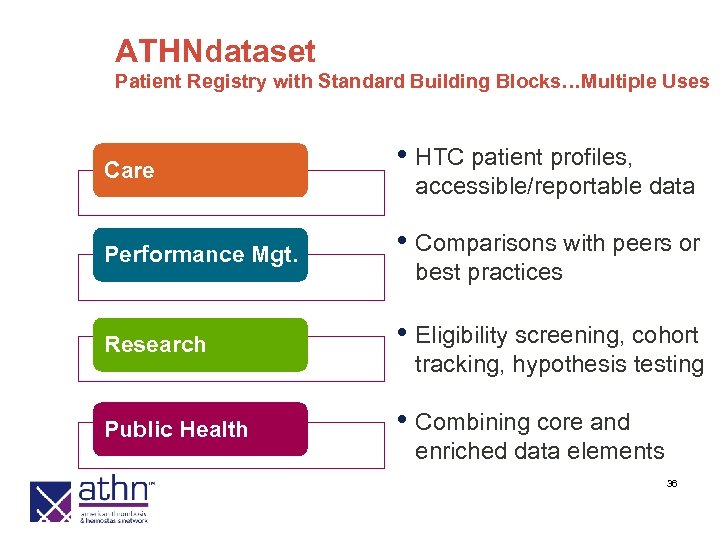
ATHNdataset Patient Registry with Standard Building Blocks…Multiple Uses Care • HTC patient profiles, accessible/reportable data Performance Mgt. • Comparisons with peers or Research • Eligibility screening, cohort Public Health • Combining core and best practices tracking, hypothesis testing enriched data elements 36
5b5c4d5a0662add20b147037e27d77a0.ppt