ae20855823b23f66f127ef994586c9d2.ppt
- Количество слайдов: 70

AMINO ACID BIOSYNTHESIS NON-ESSENTIAL AMINO ACIDS SINGLE CARBON TRANSFERS WITH THF PHYSIOLOGIC AMINES
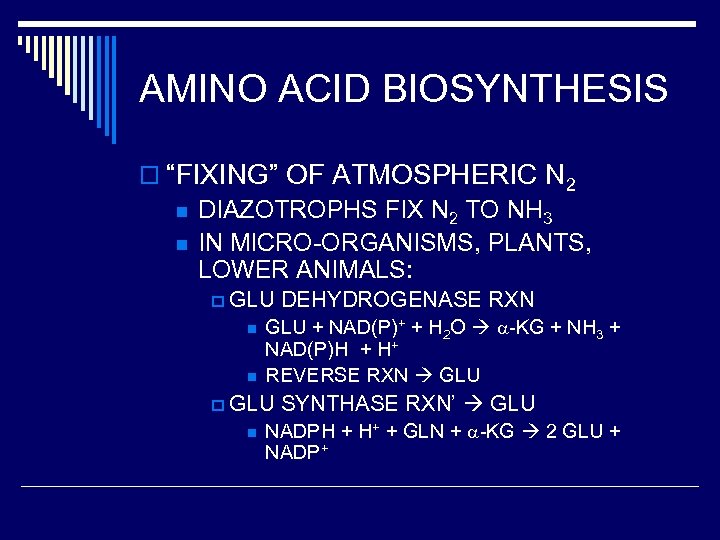
AMINO ACID BIOSYNTHESIS o “FIXING” OF ATMOSPHERIC N 2 n DIAZOTROPHS FIX N 2 TO NH 3 n IN MICRO-ORGANISMS, PLANTS, LOWER ANIMALS: p GLU n n GLU + NAD(P)+ + H 2 O -KG + NH 3 + NAD(P)H + H+ REVERSE RXN GLU p GLU n DEHYDROGENASE RXN SYNTHASE RXN’ GLU NADPH + H+ + GLN + -KG 2 GLU + NADP+
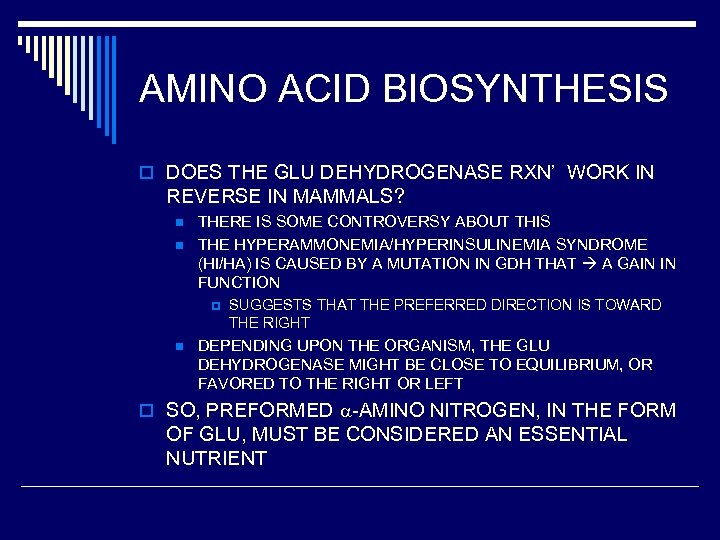
AMINO ACID BIOSYNTHESIS o DOES THE GLU DEHYDROGENASE RXN’ WORK IN REVERSE IN MAMMALS? n n THERE IS SOME CONTROVERSY ABOUT THIS THE HYPERAMMONEMIA/HYPERINSULINEMIA SYNDROME (HI/HA) IS CAUSED BY A MUTATION IN GDH THAT A GAIN IN FUNCTION p n SUGGESTS THAT THE PREFERRED DIRECTION IS TOWARD THE RIGHT DEPENDING UPON THE ORGANISM, THE GLU DEHYDROGENASE MIGHT BE CLOSE TO EQUILIBRIUM, OR FAVORED TO THE RIGHT OR LEFT o SO, PREFORMED -AMINO NITROGEN, IN THE FORM OF GLU, MUST BE CONSIDERED AN ESSENTIAL NUTRIENT

AMINO ACID BIOSYNTHESIS o ESSENTIAL AMINO ACIDS *ARGININE HISTIDINE ISOLEUCINE LYSINE METHIONINE PHENYLALANINE THREONINE TRYPTOPHAN VALINE o NOTE n ARG IS ESSENTIAL IN INFANTS AND CHILDREN n MOST SYNTHESIZED ARG ORNITHINE AND UREA VIA THE UREA CYCLE

AMINO ACID BIOSYNTHESIS o NONESSENTIAL AMINO ACIDS ALANINE ASPARAGINE ASPARTATE *CYSTEINE GLUTAMATE GLUTAMINE GLYCINE PROLINE SERINE *TYROSINE o NOTE: n CYS GETS ITS SULFUR ATOM FROM MET n TYR IS HYDROXYLATED PHE p SO IT’S NOT REALLY NONESSENTIAL
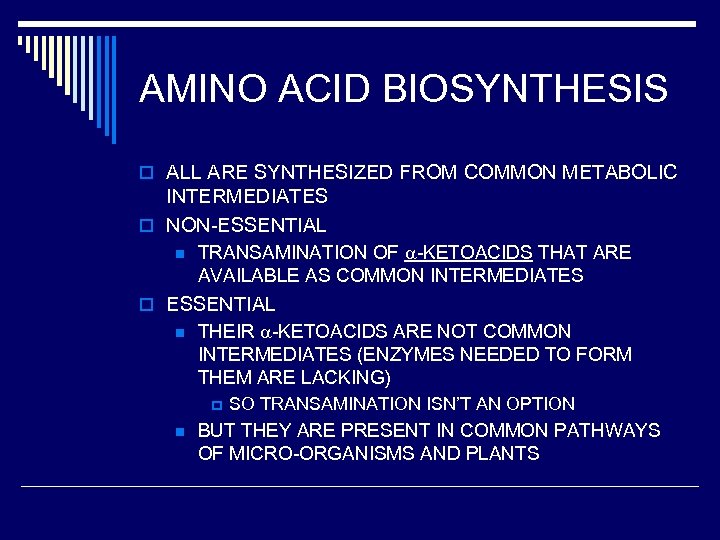
AMINO ACID BIOSYNTHESIS o ALL ARE SYNTHESIZED FROM COMMON METABOLIC INTERMEDIATES o NON-ESSENTIAL n TRANSAMINATION OF -KETOACIDS THAT ARE AVAILABLE AS COMMON INTERMEDIATES o ESSENTIAL n THEIR -KETOACIDS ARE NOT COMMON INTERMEDIATES (ENZYMES NEEDED TO FORM THEM ARE LACKING) p n SO TRANSAMINATION ISN’T AN OPTION BUT THEY ARE PRESENT IN COMMON PATHWAYS OF MICRO-ORGANISMS AND PLANTS
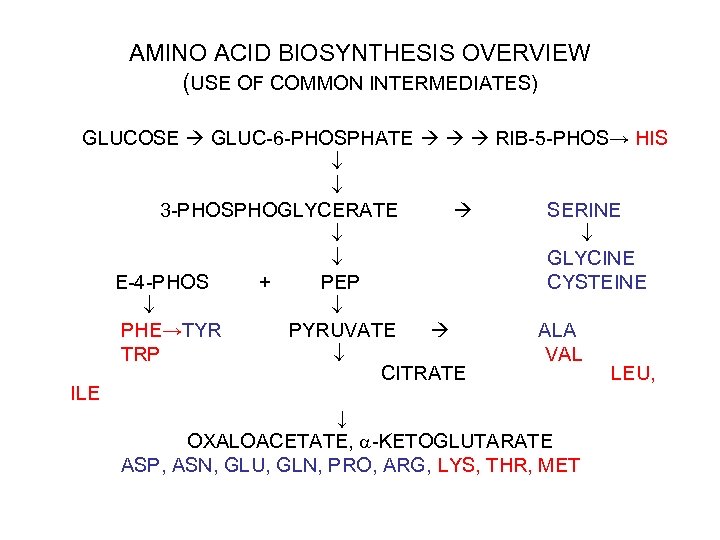
AMINO ACID BIOSYNTHESIS OVERVIEW (USE OF COMMON INTERMEDIATES) GLUCOSE GLUC-6 -PHOSPHATE RIB-5 -PHOS→ HIS 3 -PHOSPHOGLYCERATE SERINE GLYCINE E-4 -PHOS + PEP CYSTEINE PHE→TYR PYRUVATE ALA TRP VAL CITRATE LEU, ILE ↓ OXALOACETATE, -KETOGLUTARATE ASP, ASN, GLU, GLN, PRO, ARG, LYS, THR, MET

SYNTHESIS OF NON-ESSENTIAL AMINO ACIDS o ALL (EXCEPT TYR) SYNTHESIZED FROM COMMON INTERMEDIATES SYNTHESIZED IN CELL n n PYRUVATE OXALOACETATE -KETOGLUTARATE 3 -PHOSPHOGLYCERATE

SYNTHESIS OF NON-ESSENTIAL AMINO ACIDS o TRANSAMINATION REACTIONS: ONE STEP n n n PYRUVATE + AA ALANINE + -KETOACID OXALOACETATE + AA ASPARTATE + KETOACID -KETOGLUTARATE + AA GLUTAMATE + KETOACID o TRANSAMINASES: EQUILIBRATE AMINO GROUPS n n REQUIRE PYRIDOXAL PHOSPHATE (PLP) ALL AAs, EXCEPT LYS, CAN BE TRANSAMINATED MOST TRANSAMINASES GENERATE GLU OR ASP p WHY? o LOOK AT MECHANISM OF PLP (PAGE 987 IN TEXT)
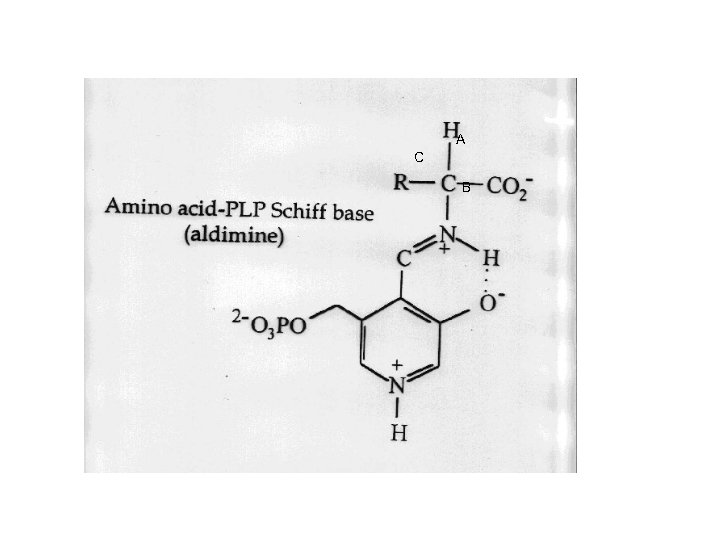
A C B


SYNTHESIS OF NONESSENTIAL AMINO ACIDS o ATP-DEPENDENT AMIDATION OF ASP, GLU n ASN, GLN n GLU + ATP + NH 3 GLN + ADP + Pi p GLUTAMINE SYNTHETASE p NH 3 IS TOXIC; IT’S STORED AS GLN p GLN DONATES AMINO GPS IN MANY REACTIONS n ASP + ATP + GLN ASN + AMP + PPi + GLU p ASPARAGINE SYNTHETASE
SYNTHESIS OF NONESSENTIAL AMINO ACIDS o NITROGEN METABOLISM IS CONTROLLED BY REGULATION OF GLUTAMINE SYNTHETASE n n IN MAMMALS, GLN SYNTHETASES ACTIVATED BY -KG EXCESS AAs TRANSAMINATED TO GLU p OXIDATIVE DEAMINATION OF GLU -KG + NH 3 p NH 3 UREA OR GLN (STORAGE) o -KG IS A SIGNAL THAT ACTIVATES GLN SYNTHETASE

BACTERIAL GLUTAMINE SYNTHETASE o VERY DETAILED CONTROL SYSTEM o 12 IDENTICAL SUBUNITS (HEX PRISM) n ALLOSTERIC CONTROL n 9 FEEDBACK INHIBITORS (CUMULATIVE INH) p INDIVIDUAL BINDING SITES n 6 ARE END-PRODS OF PATHWAYS FROM GLN p HIS, TRP, CARBAMOYL PHOSPHATE, AMP, CTP, GLUCOSAMINE-6 -PHOSPHATE n 3 REFLECT CELL’S N LEVEL (ALA, SER, GLY) o ALSO COVALENTLY MODIFIED BY ADENYLYLATION

BACTERIAL GLUTAMINE SYNTHETASE o BRIEF REVIEW: REGULATING ENZYME ACTIVITY n NEAR-EQUILIBRIUM (REVERSIBLE) p p p n REACTANTS, PRODUCTS ~ EQUIL. VALUES ENZYMES ACT QUICKLY TO RESTORE EQUIL. RATES REGULATED BY [REACT], [PROD] FAR FROM EQUILIBRIUM (IRREVERSIBLE) p p p ENZYME SATURATED NOT ENOUGH ACTIVITY TO ALLOW EQUIL. RATE INSENSITIVE TO [REACT], [PROD] “STEADY STATE” (CONSTANT FLUX) “RATE-DETERMINING STEP”
BACTERIAL GLUTAMINE SYNTHETASE o BRIEF REVIEW: REGULATING ENZYME ACTIVITY CONTROL OF ENZYME ACTIVITY p p p ALLOSTERIC REGULATION COVALENT MODIFICATION GENETIC CONTROL n AT LEVEL OF TRANSCRIPTION
BACTERIAL GLUTAMINE SYNTHETASE o SEE REGULATORY DIAGRAM (PAGE 1035) n ADENYLYLATION OF A SPECIFIC TYR RESIDUE p LESS ACTIVITY OF THE ENZYME p ENZYME IS ADENYLYLTRANSFERASE IN A COMPLEX WITH A TETRAMERIC REGULATORY PROTEIN, PII n URIDYLYLATION OF PII (AT A TYR) DEADENYLYLATION n A URIDYL-REMOVING ENZYME RESULTS IN ADENYLYLTRANSFERASE CATALYZING ADENYLYLATION OF GLN SYNTHETASE
BACTERIAL GLUTAMINE SYNTHETASE o SEE REGULATORY DIAGRAM (PAGE 1035) o WHAT CONTROLS ACTIVITY OF URIDYLYL TRANSFERASE? n ACTIVATED BY -KG AND ATP n DEACTIVATED BY GLN AND Pi o URIDYL-REMOVING ENZYME INSENSITIVE TO THESE


BACTERIAL GLUTAMINE SYNTHETASE o IN-CLASS EXERCISE EXPLAIN THE SIGNIFICANCE OF -KG AS AN ACTIVATOR OF GLUTAMINE SYNTHETASE SHOW, IN DETAIL, THE EFFECT OF LEVEL OF -KG ON THIS ENZYME. DO THE SAME FOR ATP, GLN AND Pi

NONESSENTIAL AMINO ACID SYNTHESIS o PRO, ORNITHINE, ARG ARE DERIVED FROM GLUTAMATE n NOTE: 7 OF THE 10 “NONESSENTIALS” ARE ULTIMATELY DERIVED FROM PYR, -KG AND OXALOACETATE o SEE PATHWAYS ON PAGE 1036 o HIGHLIGHTS: n n STEP 1: ACTIVATE GLU; A KINASE GLUTAMATE-5 -SEMIALDEHYDE BRANCH POINT p p SPONTANEOUS CYCLIZATION TO AN INTERNAL SCHIFF BASE PRO TRANSAMINATION TO ORNITHINE ARG IN UREA CYCLE o SCHIFF BASE: AMINE + (ALDEHYDE OR KETONE) IMINE (CONTAINS A C=N BOND)
NONESSENTIAL AMINO ACID SYNTHESIS o 3 -PHOSPHOGLYCERATE IS PRECURSOR OF n SER (A 3 -STEP PATHWAY) (1) 3 -PG + NAD+ 3 -PHOSPHOHYDROXYPYRUVATE + NADH + H+ (2) 3 -PHP + GLU 3 -PHOSERINE + -KG (3) 3 -PHOSERINE + H 2 O SER + Pi n GLY (2 DIFFERENT WAYS) (1) SER + THF GLY + N 5, N 10 – METHYLENE-THF (DIRECT) (2) N 5, N 10 – METHYLENE-THF + CO 2 + NH 4+ GLY + THF (CONDENSATION)


NONESSENTIAL AMINO ACID SYNTHESIS o CYSTEINE n SER + HOMOCYSTEINE CYSTATHIONINE p HOMOCYSTEINE IS A BREAKDOWN PRODUCT OF METHIONINE CYSTATHIONINE -KETOBUTYRATE + CYS o NOTE: -SH GROUP COMES FROM MET n n SO CYS IS ACTUALLY AN ESSENTIAL AMINO ACID

NONESSENTIAL AMINO ACID SYNTHESIS o SUMMARY POINT: n ALL NONESSENTIALS (EXCEPT TYR) ARE DERIVED FROM ONE OF THE FOLLOWING COMMON INTERMEDIATES: p PYRUVATE p OXALOACETATE p -KG p 3 -PHOSPHOGLYCERATE

IN-CLASS EXERCISE o WHICH OF THE 4 AMINO ACID INTERMEDIATES OF THE UREA CYCLE IS ESSENTIAL IN CHILDREN? o OUTLINE A PATHWAY BY WHICH ADULTS CAN SYNTHESIZE THIS AA FROM 1 GLUCOSE MOLECULE. n HINTS: YOU WILL NEED TO CONSIDER THE FOLLOWING METABOLIC PATHWAYS: p GLYCOLYTIC p GLUCONEOGENIC p CITRIC ACID CYCLE p GLUTAMATE DEHYDROGENASE REACTION n p p ASSUME IT CAN GO IN REVERSE DIRECTION ORNITHINE PRODUCTION UREA CYCLE
TRANSFER OF C 1 UNITS TO METABOLIC PRECURSORS o MOST CARBOXYLATION REACTIONS USE A BIOTIN COFACTOR n EXAMPLE: PYRUVATE CARBOXYLASE REACTION o S-ADENOSYLMETHIONINE (SAM) AS A METHYLATING AGENT n CYTOSINE METHYLATION OF Cp. Gs IN GENE PROMOTER REGIONS o TETRAHYDROFOLATES n CAN TRANSFER SINGLE C UNITS IN A NUMBER OF DIFFERENT OXIDATION STATES
TETRAHYDROFOLATES o REVIEW STRUCTURE (PAGE 1028 OF TEXT) n FOCUS ON HETEROCYCLIC RING STRUCTURE p 2 -AMINO-4 -OXO-6 -METHYLPTERIN p NOTICE THE NUMBERING OF THE ATOMS p LOOK AT N 5 n PABA JOINS TO 2 -AMINO-4 -OXO-6 METHYLPTERIN TO FORM PTEROIC ACID p FIND N 10 o COVALENT ATTACHMENT OF C 1 UNITS AT n N 5 n N 10 n BOTH

TETRAHYDROFOLATE o THREE DIFFERENT OXIDATION STATES n METHANOL p n METHYL (-CH 3) FORMALDEHYDE AT N 5, N 10 p n AT N 5 METHYLENE (-CH 2 -) FORMATE p p p FORMYL (-CH=O) FORMIMINO (-CH=NH) METHENYL ( -CH=) AT N 5 OR N 10 AT N 5, N 10 o LOOK AGAIN AT THE 2 REACTIONS FOR SYNTHESIS OF GLY n n SERINE HYDROXYMETHYLTRANSFERASE GLYCINE SYNTHASE o THF IS INVOLVED IN EACH

TETRAHYDROFOLATE o C 1 UNITS ENTER THE THF POOL MAINLY FROM THESE TWO REACTIONS n AS N 5, N 10 –METHYLENE-THF OXIDATION STATES OF C 1 UNITS ATTACHED TO THF ARE INTERCONVERTIBLE VIA ENZYMATIC REDOX REACTIONS o WE WILL SEE THF AGAIN n METHIONINE SYNTHESIS n HIS SYNTHESIS n PURINE SYNTHESIS n d. TMP (THYMIDYLATE) SYNTHESIS

TETRAHYDROFOLATE o THF IS DERIVED FROM FOLIC ACID n MAMMALS CANNOT SYNTHESIZE IT n DEFICIENCY DURING EARLY PREGNANCY CAN LEAD TO NEURAL TUBE DEFECTS p ANENCEPHALY SPINA BIFIDA o BACTERIA SYNTHESIZE FOLIC ACID n SULFONAMIDES COMPETITIVELY INHIBIT p STRUCTURAL ANALOGS OF PABA p GOOD ANTIBACTERIAL AGENTS p WHY ARE MAMMALS UNAFFECTED?

TETRAHYDROFOLATE o STUDY QUESTION: IF I GIVE YOU THE STRUCTURE OF THF, NUMBERING THE ATOMS ACCORDINGLY, BE ABLE TO SHOW WHERE TO ATTACH THE 5 DIFFERENT C 1 GROUPS.
TRANSAMINATION REACTIONS IN-CLASS STUDY QUESTION o DRAW THE STRUCTURES OF THE KETO- ACID PRODUCTS OF THE REACTIONS OF THE FOLLOWING AMINO ACIDS WITH -KG. n n n GLY ARG SER o DRAW THE STRUCTURE OF THE AMINO ACID PRODUCT COMMON TO ALL 3 RXNS’

REFERENCES o HERE ARE TWO ARTICLES THAT MIGHT HELP YOU TO ORGANIZE YOUR THINKING ABOUT AMINO ACID METABOLISM: (1) “Glutamate and Glutamine, at the Interface between Amino Acid and Carbohydrate Metabolism” (Brosnan JT, The Journal of Nutrition, Apr 2000, 130, 4 S: 988 S – 990 S) (2) “Disorders of Glutamate Metabolism” (Kelly A, Stanley CA, 2001. Mental Retardation and Developmental Disabilities Research Reviews, 7: 287 -295

SYNTHESIS OF ESSENTIAL AMINO ACIDS o ALL SYNTHESIZED FROM COMMON METABOLIC PRECURSORS n n n ASPARTATE PYRUVATE PHOSPHOENOLPYRUVATE ERYTHROSE-4 -PHOSPHATE PURINE + ATP (HISTIDINE) o PATHWAYS ONLY IN MICRO-ORGANISMS AND PLANTS n n n PROBABLE EVOLUTIONARY LOSS IN MAMMALS PATHWAYS ARE VERY COMPLICATED ACTUAL PATHWAYS VARY ACROSS SPECIES! p IN CONTRAST TO LIPID AND CARBOHYDRATE PATHWAYS, WHICH ARE ALMOST UNIVERSAL

ESSENTIAL AMINO ACID SYNTHESIS o FOUR “FAMILIES” n ASPARTATE p LYS p MET p THR n PYRUVATE p LEU, ILE, VAL (THE “BRANCHED CHAIN” AMINO ACIDS) n AROMATIC p PHE p TYR p TRP n HISTIDINE

THE ASPARTATE FAMILY o FIRST COMMITTED STEP IS n ASP + ATP ASPARTYL-βPHOSPHATE + ADP p ENZYME: n n ASPARTOKINASE 3 ISOZYMES IN E. coli EACH RESPONDS DIFFERENTLY AS FAR AS FEEDBACK INHIBITION AND REPRESSION OF ENZYME SYNTHESIS p THR, LYS, MET PATHWAYS INDEPENDENTLY CONTROLLED
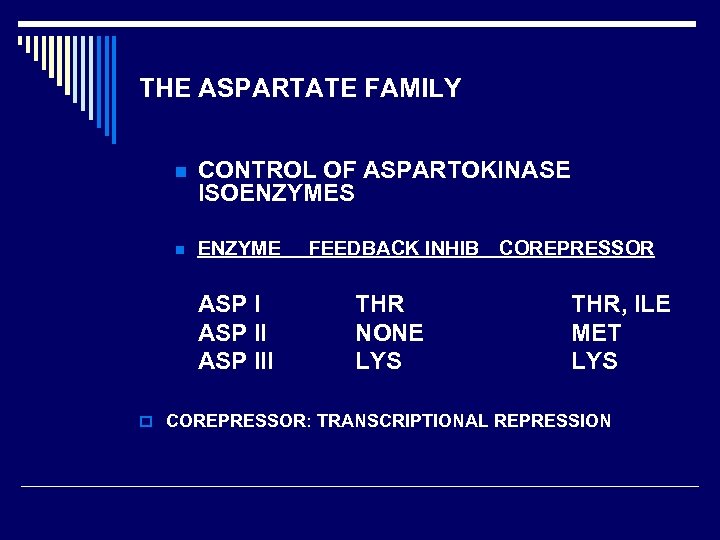
THE ASPARTATE FAMILY n CONTROL OF ASPARTOKINASE ISOENZYMES n ENZYME ASP III FEEDBACK INHIB COREPRESSOR THR NONE LYS THR, ILE MET LYS o COREPRESSOR: TRANSCRIPTIONAL REPRESSION
ASPARTATE FAMILY o ALSO CONTROL AT BRANCH POINTS o NOTE THE FOLLOWING REACTION: n HOMOCYSTEINE + N 5 -METHYL-THF MET + THF p ENZYME: METHIONINE SYNTHASE (? ) HOMOCYSTEINE CV DISEASE RISK FACTOR n EAT FOODS CONTAINING FOLATE o RECALL: SER + HOMOCYSTEINE CYSTATHIONINE o ENZYME DEFECTS IN REMETHYLATION OF HOMOCYSTEINE TO MET OR IN RXN’ FROM CYSTATHIONINE CYS HOMOCYSTEINE n DEFECT IN SYNTHESIS OF CYSTATHIONE-β-SYNTHASE p p HYPER HOMOCYSTENEMIA HOMOCYSTEINURIA SYMPTOMS: n n n PREMATURE ATHEROSCLEROSIS THROMBOEMBOLIC COMPLICATIONS SKELETAL ABNORMALITIES ECTOPIA LENTIS MENTAL RETARDATION
THE PYRUVATE FAMILY o “BRANCHED CHAIN AMINO ACIDS” n LEU n ILE n VAL o VAL, ILE: SAME PATHWAY AFTER 1 st STEP o LEU PATHWAY BRANCHES FROM VAL PATHWAY o FINAL STEPS ALL CATALYZED BY AMINOTRANSFERASES n GLU IS THE AMINO DONOR

THE PYRUVATE FAMILY o THE FIRST STEP: n PYR + TPP HYDROXYETHYL-TPP p FIRST PYR AND TPP FORM AN ADDUCT p THEN DECARBOXYLATED TO HE-TPP p A RESONANCE-STABILIZED CARBANION n n A STRONG NUCLEOPHILE ADDS TO KETO GROUP OF o PYRUVATE VAL, LEU o -KETOBUTYRATE ILE
THE PYRUVATE FAMILY o LOOK AT THE REACTION MECHANISM OF PYRUVATE DECARBOXYLASE (PAGE 605) n n THIS SHOWS THE FORMATION OF THE HYDROXYETHYL-TPP ADDUCT THIAMINE (VIT B 1) o SOME INTERESTING CHEMISTRY n THIAZOLIUM RING p p n n n ACIDIC HYDROGEN “ELECTRON SINK” TRANSITION STATE STABILIZATION MECH. YLIDS RESONANCE

THE AROMATIC FAMILY o IN PLANTS AND MICRORGANISMS n PHE n TYR n TRP o PECURSORS ARE: n PEP n ERYTHROSE-4 -PHOSPHATE n THESE CONDENSE WITH ULTIMATE CONVERSION TO CHORISMATE
THE AROMATIC FAMILY o CHORISMATE n BRANCH POINT FOR TRP SYNTHESIS n CHORISMATE ANTHRANILATE TRP n CHORISMATE PREPHENATE o PREPHENATE n BRANCH POINT FOR PHE, TYR SYNTH p AMINOTRANSFERASES IN EACH FINAL STEP n IN MAMMALS, TYR IS A PRODUCT OF: p PHE HYDROXYLATION
THE TRP PATHWAY o TRYPTOPHAN SYNTHASE n CATALYZES FINAL 2 STEPS INDOLE-3 -GLYCEROL PHOS INDOLE + GLYC-3 -P INDOLE + SER H 2 O + TRP n 2β 2 BIFUNCTIONAL ENZYME n WHAT ENZYME CLASS?

THE TRP PATHWAY o “CHANNELING” n INDOLE IS SEQUESTERED BETWEEN THE TWO ACTIVE SITES n DIFFUSES BETWEEN TWO SITES n IT’S NONPOLAR o STUDY QUESTION: n WHAT ARE THE BENEFITS OF CHANNELING? o SEE RIBBON DIAGRAM OF TRP SYNTHASE ON PAGE 1044 n MECHANISM?

PHENYLKETONURIA (PKU) o DEFECTIVE OR ABSENT PHENYLALANINE HYDROXYLASE CANNOT FORM TYROSINE PHE BUILDS UP o PHE IS TRANSAMINATED TO PHENYL-PYRUVATE o SEVERE MR IF NOT TREATED SOON AFTER BIRTH WITH LOW PHE DIET n UNIVERSAL NEWBORN SCREENING

PHENYLKETONURIA IN-CLASS STUDY QUESTION o WRITE OUT THE REACTION IN WHICH PHE IS TRANSAMINATED TO PHENYLPYRUVATE, SHOWING STRUCTURES o EXPLAIN WHY CHILDREN WITH A TETRAHYDROBIOPTERIN DEFICIENCY EXCRETE LARGE AMOUNTS OF PHE o WHY DO PEOPLE WITH PKU HAVE BLOND HAIR, BLUE EYES AND VERY LIGHT SKIN? o WHY DO PEOPLE ON A LOW PHE-DIET NEED TO INCREASE THEIR TYR INTAKE?

HISTIDINE BIOSYNTHESIS o ATOMS DERIVED FROM: n 5 -PHOSPHORIBOSYL- -PYROPHOSPHATE p p p n PROVIDES 5 C-ATOMS PRPP INVOLVED IN PURINE SYNTHESIS PRPP INVOLVED IN PYRIMIDINE SYNTHESIS PURINE SALVAGE PATHWAY AN INTERMEDIATE IN TRP SYNTHESIS ATP PROVIDES THE 6 th C-ATOM o ATP + -D-RIBOSE-5 -PHOSPHATE PRPP + AMP n -D-RIBOSE-5 -PHOSPHATE FROM H-M SHUNT

HISTIDINE BIOSYNTHESIS o NOTICE THE PRODUCTS OF THE AMIDO- TRANSFERASE STEP: n n AICAR p AN INTERMEDIATE IN PURINE BIOSYNTHESIS IMIDAZOLE GLYCEROL PHOSPHATE o THERE IS AN APPARENT EVOLUTIONARY OVERLAP OF PURINE AND HIS SYNTHESIS n THE FIRST STEP IN HIS SYNTHESIS INVOLVES FORMATION OF A PURINE!

HISTIDINE BIOSYNTHESIS o IS THE HIS PATHWAY A RELIC OF THE TRANSITION FROM RNA-BASED TO PROTEIN-BASED LIFE FORMS? n HIS IS FREQUENTLY FOUND IN p ENZYME ACTIVE SITES n n n NUCLEOPHILES GENERAL ACID/BASE CATALYSIS RNA HAS CATALYTIC PROPERTIES p IMIDAZOLE GROUP PROBABLY PLAYS A SIMILAR ROLE

PHYSIOLOGICALLY ACTIVE AMINES o THESE ARE DERIVED FROM AMINO ACIDS o THEY INCLUDE n EPINEPHRINE (ADRENALINE) n NOREPINEPHRINE n DOPAMINE n SEROTONIN n -AMINOBUTYRIC ACID (GABA) o HORMONES o NEUROTRANSMITTERS

PHYSIOLOGICALLY ACTIVE AMINES o DECARBOXYLATION OF PRECURSOR AMINO ACID n PLP-DEPENDENT, AA DECARBOXYLASES o TYR DOPAMINE, EPI, NOREPINEPHRINE o GLUTAMATE GABA o HISTIDINE HISTAMINE o TRP SEROTONIN
DECARBOXYLATION REACTION o PLP FORMS A SCHIFF BASE WITH AA n RESULTS IN FORMATION OF C CARBANION p UNSTABLE CHARGE BUILDUP ON C WHEN CO 2 SPLITS OFF p PLP IS AN “ELECTRON SINK” o IN-CLASS EXERCISE: USING THE STRUCTURE OF THE AMINO-ACID-PLP SCHIFF BASE AS SHOWN IN CLASS, SHOW (USING ARROWS TO SHOW FLOW OF ELECTRONS) HOW THE C CARBANION FORMED AFTER CO 2 SPLITS OFF IS STABILIZED.

GABA o GLUTAMATE GABA + CO 2 n GLU DECARBOXYLASE o GABA IS THE MAJOR INHIBITORY NEURO- TRANSMITTER IN BRAIN n GLU IS THE MAJOR EXCITATORY NEUROTRANSMITTER o STIMULATION OF NEURONS BY GABA n PERMEABILITY TO CHLORIDE IONS p p BENZODIAZEPINES (VALIUM) ENHANCE MEMBRANE PERMEABILITY OF Cl IONS BY GABAPENTIN PROTECTS AGAINST GLU EXCITOTOXICITY

HISTAMINE o HISTIDINE HISTAMINE + CO 2 n HIS DECARBOXYLASE o HISTAMINES INVOLVED IN n ALLERGIC RESPONSE p H 1 n n RECEPTORS IN GUT, BRONCHI STIMULATION SMOOTH MUSCLE CONTRN’ H 1 RECEPTOR ANTAGONISTS o CLARITIN, ZYRTEC, ETC
HISTAMINE o HISTAMINES INVOLVED IN n CONTROL OF ACID SECRETION IN STOMACH p H 2 RECEPTORS n STIMULATION HCl SECRETION n H 2 ANTAGONISTS o CIMETIDINE o RANITIDINE o H 2 RECEPTORS IN HEART n STIMULATION HEART RATE

SEROTONIN o TRP 5 -HYDROXYTRYPTOPHAN n TRP HYDROXYLASE n REQUIRES 5, 6, 7, 8 TETRAHYDROBIOPTERIN o 5 -HT SEROTONIN + CO 2 n AROMATIC ACID DECARBOXYLASE o SEROTONIN CAUSES n SMOOTH MUSCLE CONTRACTION n BRAIN NEUROTRANSMITTER n MELATONIN SYNTHESIZED IN PINEAL GLAND
CATECHOLAMINES o EPI, NOREPINEPHRINE, DOPAMINE o AMINE DERIVATIVES OF CATECHOL o REACTIONS: n TYR L- DOPA p n L-DOPA DOPAMINE + CO 2 p n AROMATIC ACID DECARBOXYLASE DOPAMINE NOREPINEPHRINE p n TYR HYDROXYLASE DOPAMINE β-HYDROXYLASE NOREPINEPHRINE p REQUIRES SAM
L-DOPA AND DOPAMINE o IN SUBSTANTIA NIGRA, CATECHOLAMINE PRODUCTION STOPS AT DOPAMINE n n PARKINSON’S DISEASE: DEGENERATION OF SUBSTANTIA NIGRA DOPAMINE TREAT BY GIVING PRECURSOR, L-DOPAMINE CANNOT CROSS BLOOD/BRAIN BARRIER TRANSPLANTATION OF ADR. MEDULLA CELLS TO BRAIN o L-DOPA A PRECURSOR OF MELANIN PRODUCTION

IN-CLASS EXERCISE o IN KWASHIORKOR, A DIETARY PROTEIN DEFICIENCY DISEASE IN CHILDREN, DEPIGMENTATION OF HAIR AND SKIN IS SEEN. EXPLAIN THE BIOCHEMICAL BASIS FOR THIS.
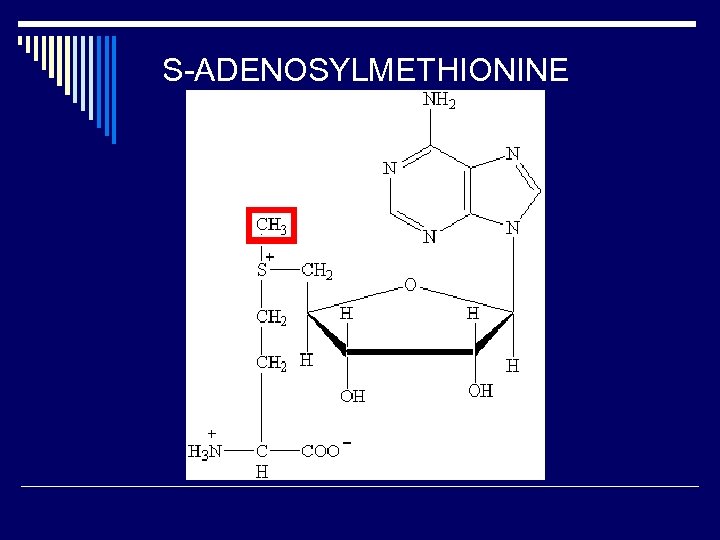
S-ADENOSYLMETHIONINE

ACTIONS OF NOREPINEPHRINE o NOT NEARLY AS ACTIVE AS EPINEPHRINE n DURING EXTREME STRESS o CIRCULATORY SYSTEM n CONSTRICTS GREAT VEINS ( 2) n VASOCONSTRICTIVE TO SKIN ( 1) n VASOCONSTRICTION ( 1) EFFECTS ON p GI TRACT p SPLEEN p PANCREAS p KIDNEYS o NEUROTRANSMITTER IN THE BRAIN

ACTIONS OF EPINEPHRINE o AS AN INSULIN ANTAGONIST n ACTIVATES MUSCLE GLYCOGEN PHOSPHORYLASE p n TRIGGERS PHOSPHORYLATION (ACTIVATION) OF HORMONE-SENSITIVE LIPASE IN FAT CELLS p n n n GLUCOSE-6 -P USED IN GLYCOLYSIS MOBILIZES FAT BY HYDROLYZING TGs GLYCOGEN BREAKDOWN IN LIVER ACTIVATES GLUCONEOGENESIS IN LIVER INHIBITS FATTY ACID SYNTHESIS

ACTIONS OF EPINEPHRINE o ON CARDIAC MUSCLE n β 1 -ADRENERGIC RECEPTOR STIMULATION p HEART RATE AND CARDIAC OUTPUT n p β-BLOCKERS BLOOD PRESSURE DILATES CORONARY ARTERIES (β 2) o ON SMOOTH MUSCLE (β 2 -ADRENERGIC) n IN BRONCHIOLES, FOR EXAMPLE n MUSCLE RELAXATION p ACTIVATION OF G-PROTEINS n p c. AMP , ETC ASTHMA MEDICATIONS
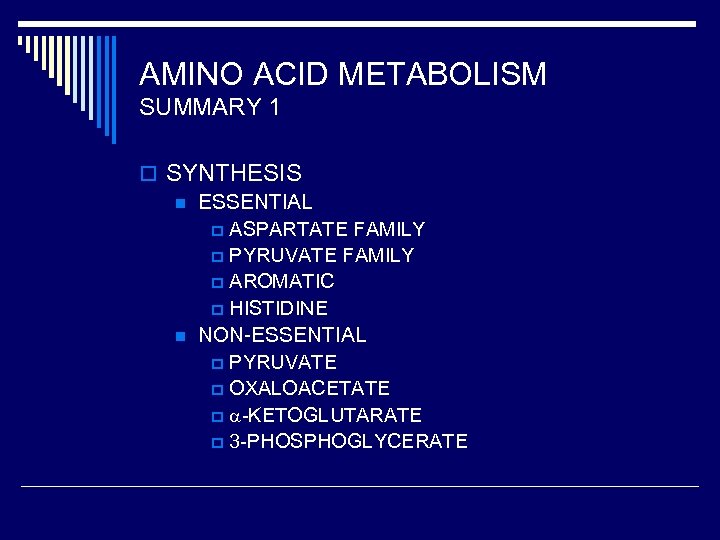
AMINO ACID METABOLISM SUMMARY 1 o SYNTHESIS n ESSENTIAL p ASPARTATE FAMILY p PYRUVATE FAMILY p AROMATIC p HISTIDINE n NON-ESSENTIAL p PYRUVATE p OXALOACETATE p -KETOGLUTARATE p 3 -PHOSPHOGLYCERATE
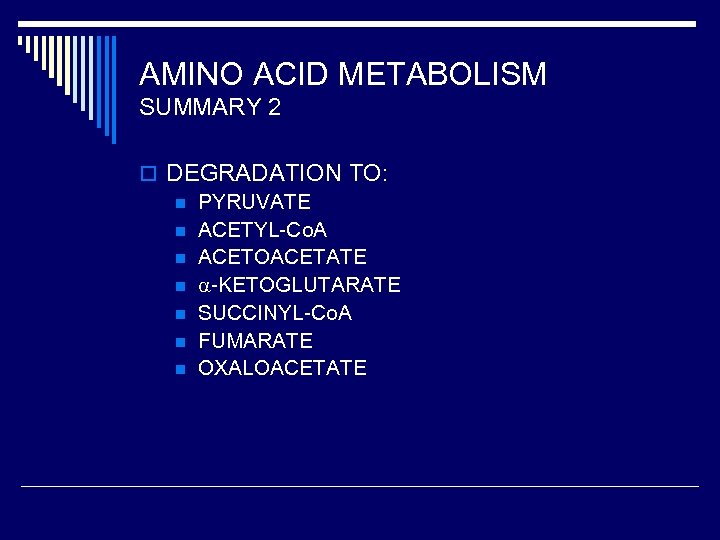
AMINO ACID METABOLISM SUMMARY 2 o DEGRADATION TO: n PYRUVATE n ACETYL-Co. A n ACETOACETATE n -KETOGLUTARATE n SUCCINYL-Co. A n FUMARATE n OXALOACETATE
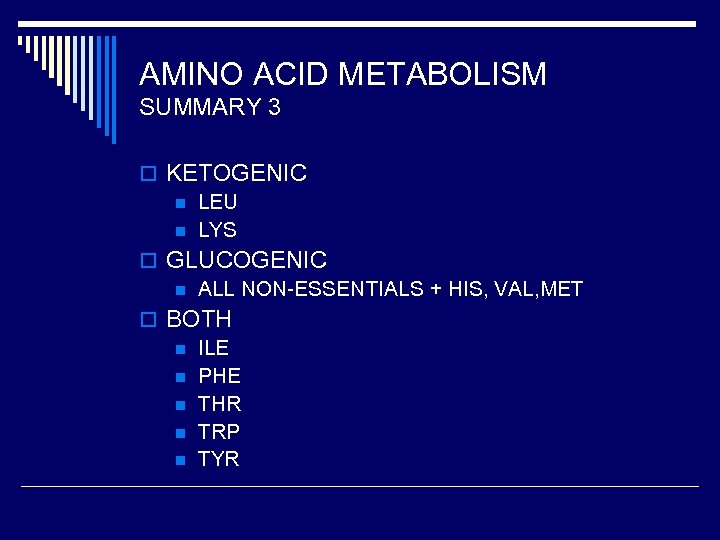
AMINO ACID METABOLISM SUMMARY 3 o KETOGENIC n LEU n LYS o GLUCOGENIC n ALL NON-ESSENTIALS + HIS, VAL, MET o BOTH n ILE n PHE n THR n TRP n TYR

IN-CLASS STUDY QUESTION o EXPLAIN WHY IT IS POSSIBLE FOR THE CARBON SKELETON OF EACH AMINO ACID TO BE BROKEN DOWN TO ACETYL-Co. A.
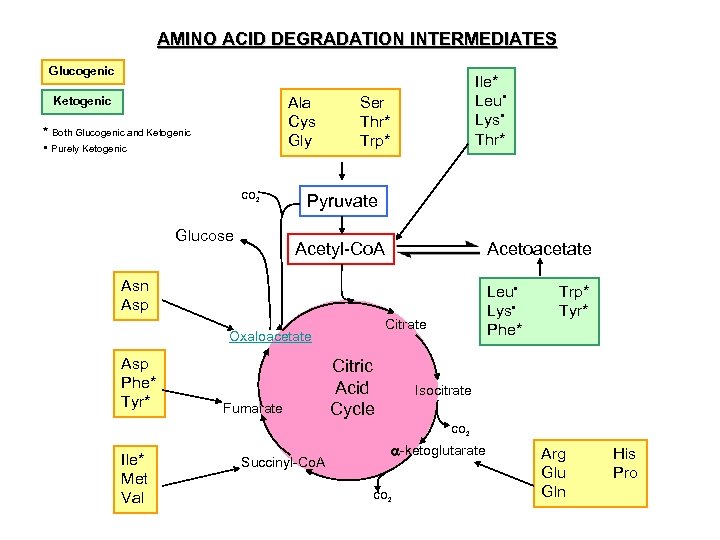
AMINO ACID DEGRADATION INTERMEDIATES Glucogenic Ala Cys Gly Ketogenic * Both Glucogenic and Ketogenic • Purely Ketogenic CO 2 Glucose Ile* Leu • Lys • Thr* Ser Thr* Trp* Pyruvate Acetyl-Co. A Acetoacetate Asn Asp Citrate Oxaloacetate Asp Phe* Tyr* Fumarate Leu • Lys • Phe* Citric Acid Cycle Trp* Tyr* Isocitrate CO 2 Ile* Met Val Succinyl-Co. A -ketoglutarate CO 2 Arg Glu Gln His Pro
ae20855823b23f66f127ef994586c9d2.ppt