a039db3a35245d37d539390ba2d90e75.ppt
- Количество слайдов: 66
Amenorrhea Amy Byerly, D. O. Contemporary OB/GYN
Definition l No period by age 14 in the absence of growth or development of secondary sexual characteristics l No period by the age of 16 regardless of the presence of normal growth and development with the appearance of secondary sexual characteristics l Absence of periods for a length of time equal to a total of at least 3 of the previous cycle intervals or 6 months of amenorrhea in a previously menstruating woman

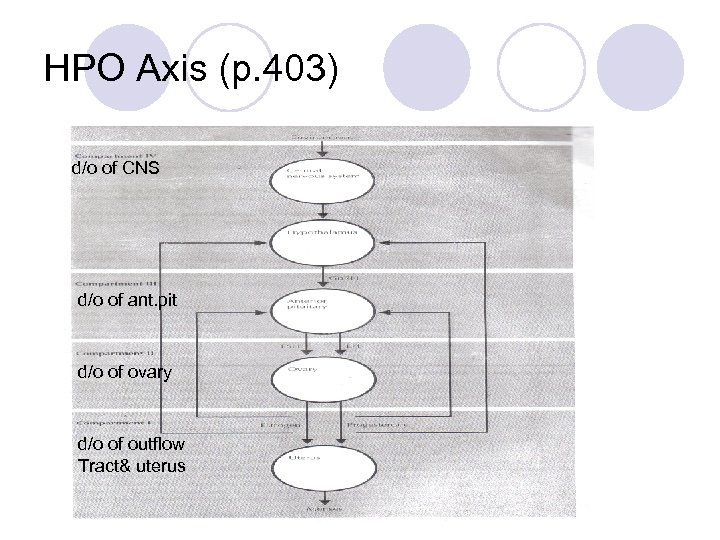
Basic Principles in Menstrual Function l Need visible external evidence of menstrual flow l Need an intact outflow tract – patency of vagina and the endocervix with the uterine cavity l Presence of an endometrial lining l Intact HPO axis (Gn. RH stimulating LH and FSH stimulating estrogen and progesterone) l Maximal number of eggs at 16 -20 weeks (6 -7 million)
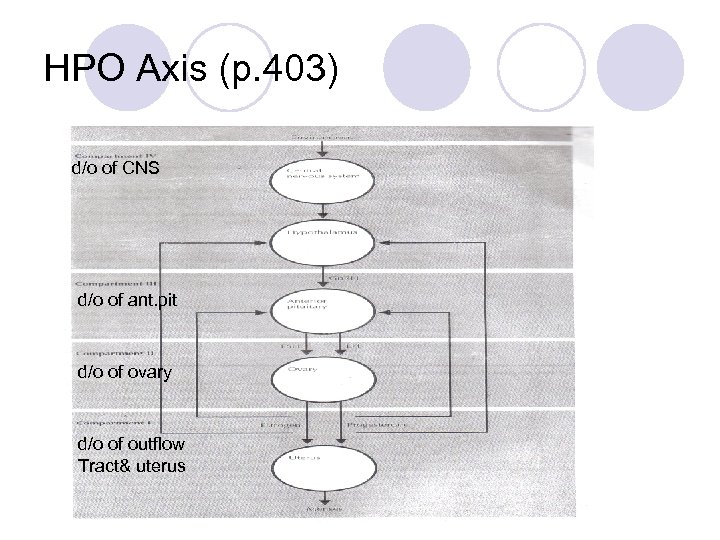
HPO Axis (p. 403) d/o of CNS d/o of ant. pit d/o of ovary d/o of outflow Tract& uterus
Evaluation of Amenorrhea l H&P ¡Emotional stress, apparent genetic anomalies, nutritional status, abnormal growth and development, presence of a normal reproductive tract, evidence for CNS disease ¡Galactorrhea – nonpuerperal breast secretion; spontaneous or present only with expression; unilateral or bilateral, persistent or intermittent l Followed by a lab work-up

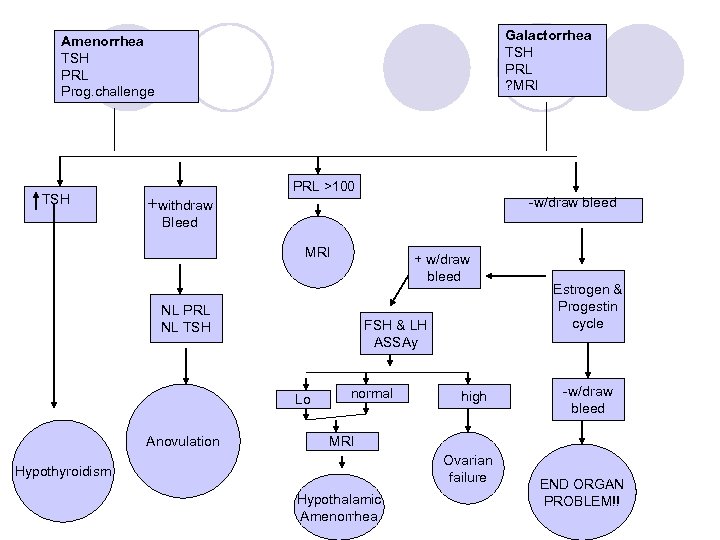
Step 1: Initial Evaluation l HCG – to rule out pregnancy l TSH – hypothyroidism; the longer the duration of hypothyroidism, the higher the incidence of galactorrhea l PRL ¡ If associated with hypothyroidism, the value is <100 l Progestational challenge – to assess the level of endogenous estrogen and the competence of the outflow tract l Lateral X-ray view of the sella turcica (for galactorrhea associated with amenorrhea)

Progestational Challenge l 3 choices: ¡Parenteral progesterone oil (200 mg) ¡Oral micronized progesterone (300 mg) take hs to avoid S. E. ¡Oral medroxyprogesterone acetate (10 mg) daily for 5 days l OCP not appropriate – not purely progesteronal l Within 2 -7 days – bleed or not bleed l If bleeds – diagnosis is anovulation; intact outflow tract; estrogen present; means minimal function of the ovary, pituitary and CNS

Anovulatory Patients l Require treatment; if untreated, unopposed estrogen can eventually lead to endometrial cancer l Minimal therapy: ¡Provera 10 mg po for the first 10 days of each month ¡Can use OCPs if contraception is also desired ¡If patient fails to have a withdrawal bleed after progesterone, further work-up is needed

Polycystic Ovarian Syndrome l Insulin insensitivity l Increased risk for endometrial hyperplasia l Diagnosis ¡Glucose to insulin ratio 4: 1 ¡Testosterone >40 ¡US not considered diagnostic

Step 2: Estrogen and Progesterone l If no withdrawal bleeding after progesterone only: ¡ Target organ outflow tract is not working ¡ Preliminary estrogen proliferation of the endometrium has not occurred l Give estrogen and progesterone ¡ Premarin 0. 3 mg 30 d ¡ Repeat Proesterone challenge l No bleeding: endometrium or outflow tract problem (due to aggressive curettage, infection, genetic anomaly); Compartment I d/o are rare l If bleeding: outflow tract and endometrium are working properly
Galactorrhea TSH PRL ? MRI Amenorrhea TSH PRL Prog. challenge TSH +withdraw PRL >100 -w/draw bleed Bleed MRI + w/draw bleed NL PRL NL TSH FSH & LH ASSAy Lo Anovulation normal high Estrogen & Progestin cycle -w/draw bleed MRI Ovarian failure Hypothyroidism Hypothalamic Amenorrhea END ORGAN PROBLEM!!

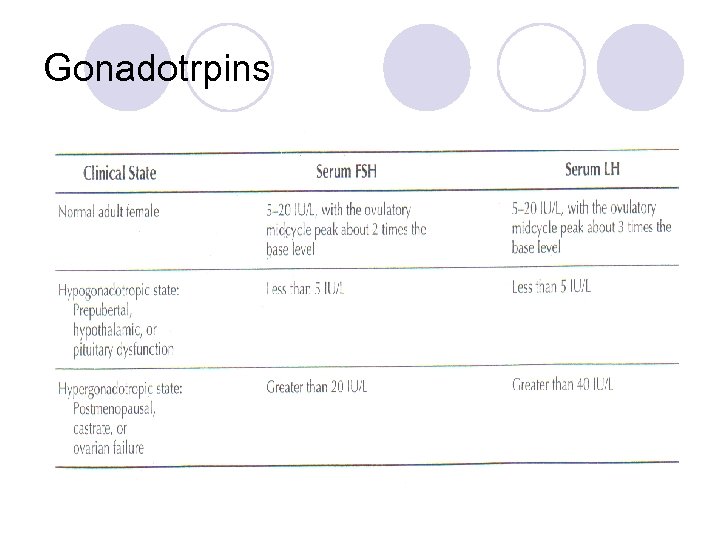
Step 3: Gonadotropin Assay l If bleeding occurred with estrogen and progesterone together, then there is a problem with the stimulation of estrogen production: follicular activity or gonadotropins l Assay the level of gonadotropins (must do this 2 weeks after the E/P challenge) – draw LH and FSH levels l Step 3 designed to determine if the lack of estrogen is a compartment II (follicle) or compartment III/IV (CNS-pituitary axis) issue
HPO Axis (p. 403) d/o of CNS d/o of ant. pit d/o of ovary d/o of outflow Tract& uterus
Gonadotrpins
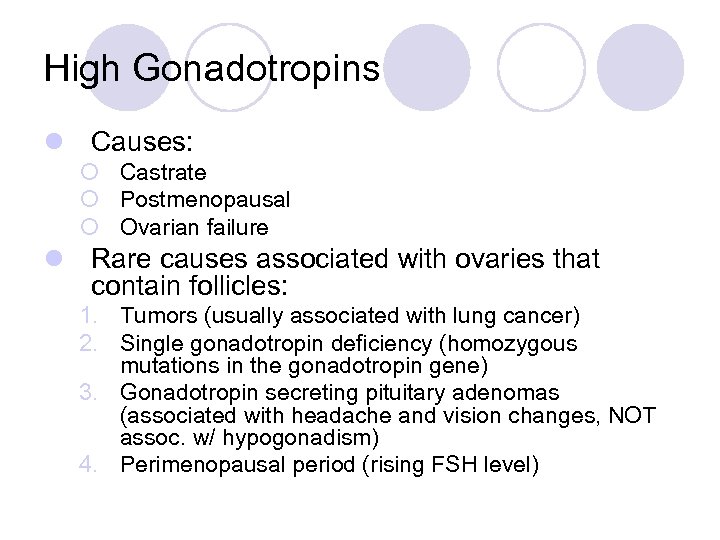
High Gonadotropins l Causes: ¡ Castrate ¡ Postmenopausal ¡ Ovarian failure l Rare causes associated with ovaries that contain follicles: 1. Tumors (usually associated with lung cancer) 2. Single gonadotropin deficiency (homozygous mutations in the gonadotropin gene) 3. Gonadotropin secreting pituitary adenomas (associated with headache and vision changes, NOT assoc. w/ hypogonadism) 4. Perimenopausal period (rising FSH level)
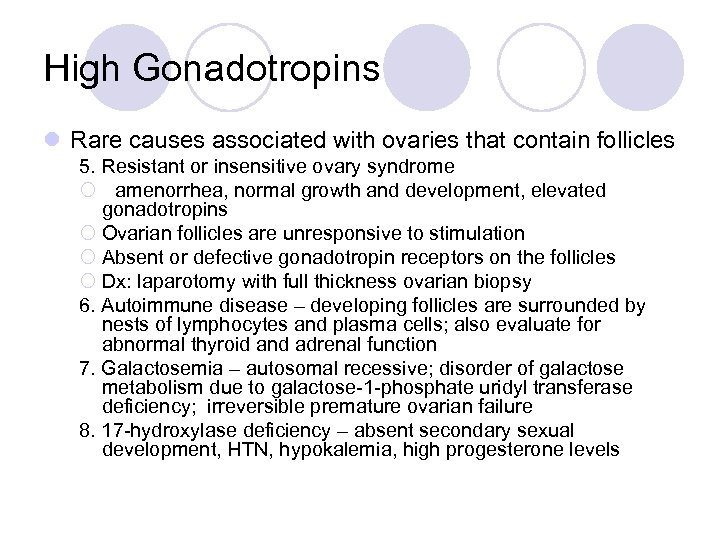
High Gonadotropins l Rare causes associated with ovaries that contain follicles 5. Resistant or insensitive ovary syndrome ¡ amenorrhea, normal growth and development, elevated gonadotropins ¡ Ovarian follicles are unresponsive to stimulation ¡ Absent or defective gonadotropin receptors on the follicles ¡ Dx: laparotomy with full thickness ovarian biopsy 6. Autoimmune disease – developing follicles are surrounded by nests of lymphocytes and plasma cells; also evaluate for abnormal thyroid and adrenal function 7. Galactosemia – autosomal recessive; disorder of galactose metabolism due to galactose-1 -phosphate uridyl transferase deficiency; irreversible premature ovarian failure 8. 17 -hydroxylase deficiency – absent secondary sexual development, HTN, hypokalemia, high progesterone levels
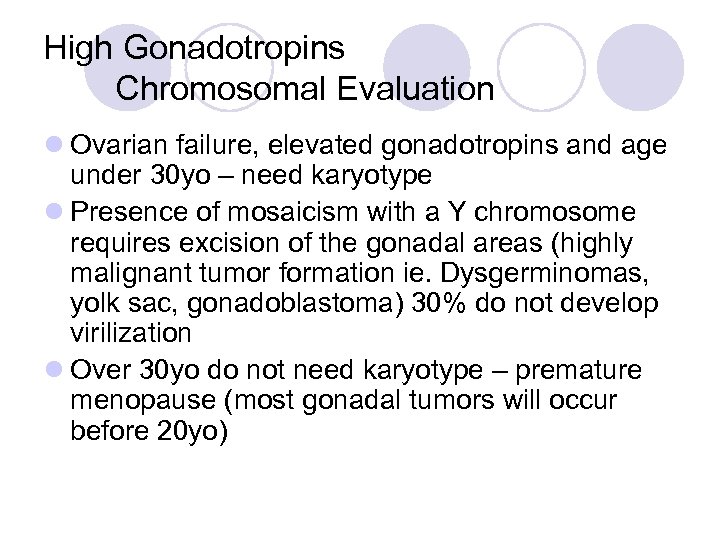
High Gonadotropins Chromosomal Evaluation l Ovarian failure, elevated gonadotropins and age under 30 yo – need karyotype l Presence of mosaicism with a Y chromosome requires excision of the gonadal areas (highly malignant tumor formation ie. Dysgerminomas, yolk sac, gonadoblastoma) 30% do not develop virilization l Over 30 yo do not need karyotype – premature menopause (most gonadal tumors will occur before 20 yo)
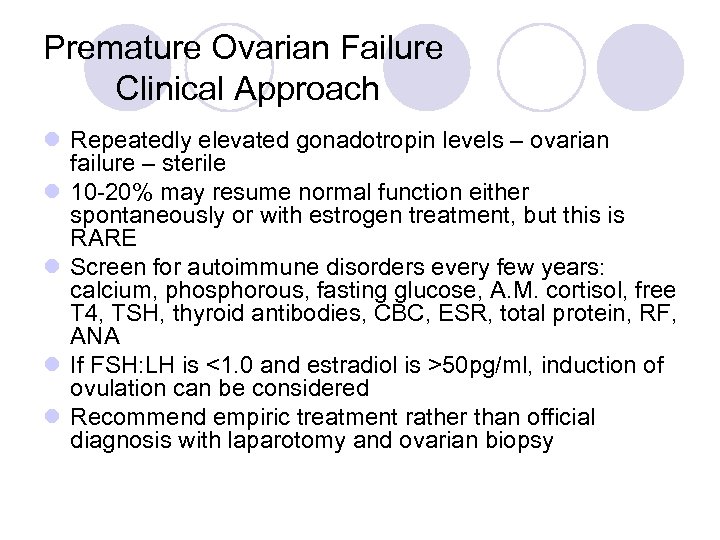
Premature Ovarian Failure Clinical Approach l Repeatedly elevated gonadotropin levels – ovarian failure – sterile l 10 -20% may resume normal function either spontaneously or with estrogen treatment, but this is RARE l Screen for autoimmune disorders every few years: calcium, phosphorous, fasting glucose, A. M. cortisol, free T 4, TSH, thyroid antibodies, CBC, ESR, total protein, RF, ANA l If FSH: LH is <1. 0 and estradiol is >50 pg/ml, induction of ovulation can be considered l Recommend empiric treatment rather than official diagnosis with laparotomy and ovarian biopsy
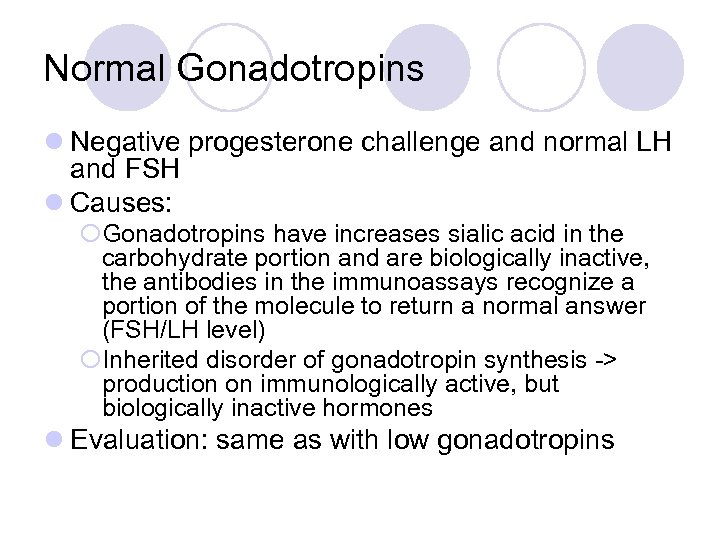
Normal Gonadotropins l Negative progesterone challenge and normal LH and FSH l Causes: ¡Gonadotropins have increases sialic acid in the carbohydrate portion and are biologically inactive, the antibodies in the immunoassays recognize a portion of the molecule to return a normal answer (FSH/LH level) ¡Inherited disorder of gonadotropin synthesis -> production on immunologically active, but biologically inactive hormones l Evaluation: same as with low gonadotropins
Low Gonadotropins l Causes: ¡Prepubertal ¡Hypothalamic dysfunction ¡Pituitary dysfunction l Evaluation: in the past, coned down view of sella turcica via x-ray was done but now MRI is imaging of choice; diagnostic modality of choice is either thin section CT with IV contrast or MRI with gadolinium (more accurate)
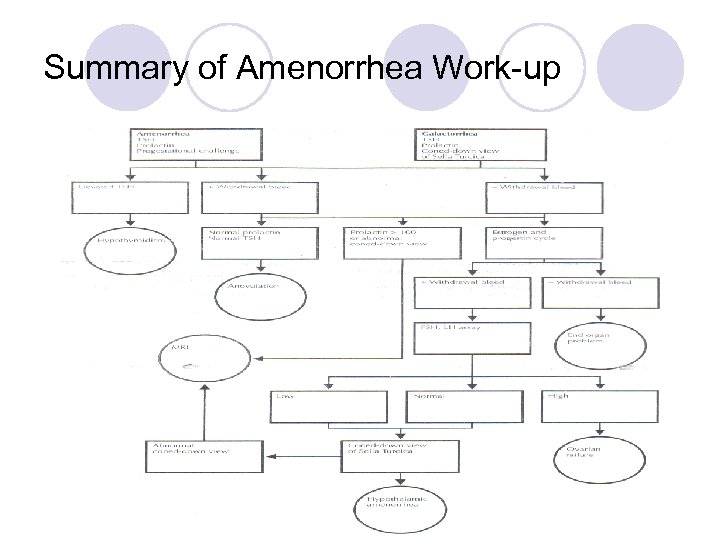
Summary of Amenorrhea Work-up

Imaging of the Sella Turcica l Reasons to use CT or MRI ¡ Abnormal cone-down ¡ Headache, visual disturbances ¡ PRL >100 (associated with large tumors) l Reasons microadenomas are not significant ¡ Microadenomas are very common ¡ They do not grow very rapidly during pregnancy ¡ Rarely progress to macroadenomas ¡ High recurrence rate after surgery ¡ Natural course is unaffected by dopamine agonist treatment ¡ No contraindication to hormone therapy or OCPs
Evaluation of abnormal sella turcica and/or high prolactin l Refer to endocrinologist

Hypogonadotropic hypogonadism l + amenorrhea l - galactorrhea l NL imaging of the pituitary Mechanism of the amenorrhea is suppression of the pulsatile Gn. RH secretion below it’s critical range. This is diagnosis of exclusion. Identifiable causes are ie anorexia, stress, wght loss. However, there is NO test to manipulate or measure the hypothalamus to prove the dxn.
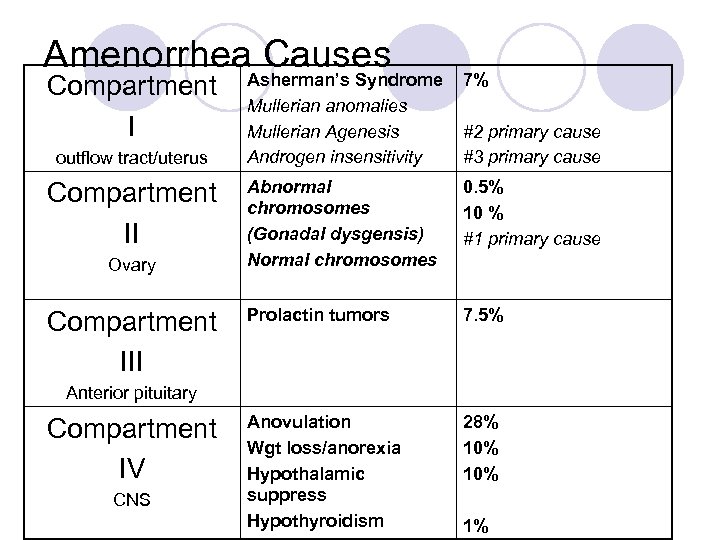
Amenorrhea. Asherman’s Syndrome Causes Compartment I outflow tract/uterus Compartment II Ovary Compartment III Mullerian anomalies Mullerian Agenesis Androgen insensitivity 7% #2 primary cause #3 primary cause Abnormal chromosomes (Gonadal dysgensis) Normal chromosomes 0. 5% 10 % #1 primary cause Prolactin tumors 7. 5% Anovulation Wgt loss/anorexia Hypothalamic suppress Hypothyroidism 28% 10% Anterior pituitary Compartment IV CNS 1%
Disorders of the Outflow Tract or Uterus (compartment I) l Asherman’s Syndrome l Mullerian Anomalies l Mullerian Agenesis l Androgen Insensitivity (Testicular Feminization)
Asherman’s Syndrome l Result of overzealous post partum curettage -> intrauterine scarring ¡Can also occur after uterine surgery, IUD infections, severe pelvic infections, tuberculosis in the uterus, uterine schistosomiasis l Diagnosis by Hysterogram or hysteroscopy: adhesions l Tx: hysteroscopic lysis of adhesions ¡Followed by pediatric Foley catheter, antibiotics l Complications: infertility, miscarriages, dysmenorrhea

Mullerian Anomalies l Imperforate hymen, obliteration of the vaginal orifice, lapses in the vaginal continuity, presence or absence of uterus or cervix l Reestablishment of mullerian duct continuity usually can be achieved surgically l Dx: MRI delineate the anatomic abnormality

Mullerian Agenesis l Lack of Mullerian development: ¡ Mayer-Rokitansky-Kuster-Hauser Syndrome l Absence or hypoplasia of the internal vagina; may have absence of uterus and fallopian tubes l Female karyotype, normal ovarian function, normal growth and development l Ovaries are NOT mullerian structures l Tx: vaginal dilators (Frank) or surgical (Vecchietti procedure), neovagina w/ Creatsas modification of Willimas vaginoplasty l Additional studies: pelvic u/s or MRI assess anatomic structures l Look for other associated problems: urinary/renal tract abnormalities, skeletal (spine) anomalies
Androgen Insensitivity Testicular Feminization l Complete androgen insensitivity – no vagina, no uterus l Incomplete androgen insensivity- clitoral enlargement/phallus & axillary/pubic hair present l present, Male karyotype, female appearance l X-linked recessive transmission l Normal or elevated testosterone levels l Male pseudohermaphrodite (gonads opposite of genitalia) l Suspect if: female child w/ inguinal hernias, primary amenorrhea, no uterus, & absent body hair l May defer gonadectomy until after puberty 16 -18 for complete form (only exception to the rule that gonads w/ Y chromosome should be removed as soon as dxn is made)
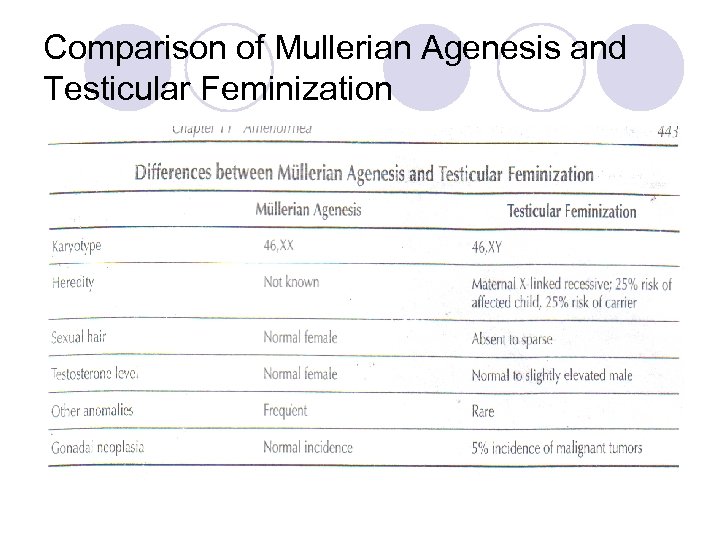
Comparison of Mullerian Agenesis and Testicular Feminization
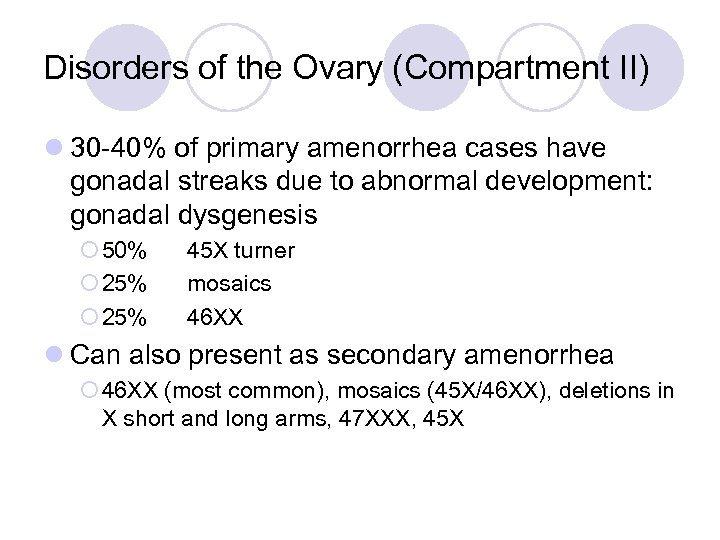
Disorders of the Ovary (Compartment II) l 30 -40% of primary amenorrhea cases have gonadal streaks due to abnormal development: gonadal dysgenesis ¡ 50% ¡ 25% 45 X turner mosaics 46 XX l Can also present as secondary amenorrhea ¡ 46 XX (most common), mosaics (45 X/46 XX), deletions in X short and long arms, 47 XXX, 45 X
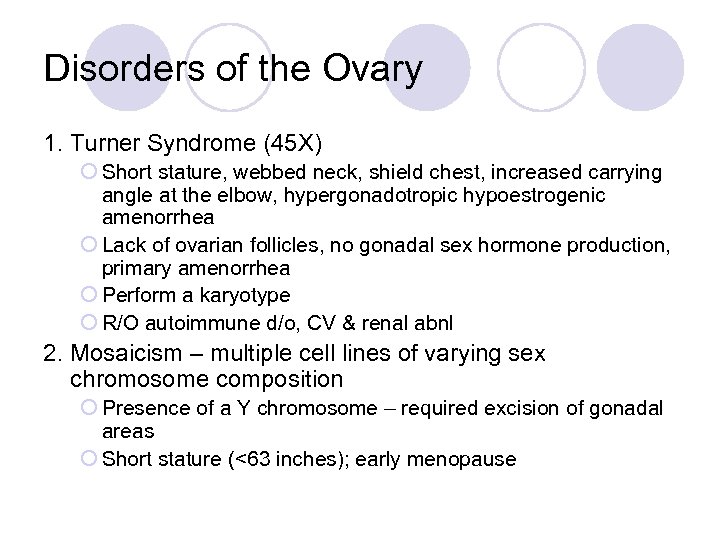
Disorders of the Ovary 1. Turner Syndrome (45 X) ¡ Short stature, webbed neck, shield chest, increased carrying angle at the elbow, hypergonadotropic hypoestrogenic amenorrhea ¡ Lack of ovarian follicles, no gonadal sex hormone production, primary amenorrhea ¡ Perform a karyotype ¡ R/O autoimmune d/o, CV & renal abnl 2. Mosaicism – multiple cell lines of varying sex chromosome composition ¡ Presence of a Y chromosome – required excision of gonadal areas ¡ Short stature (<63 inches); early menopause
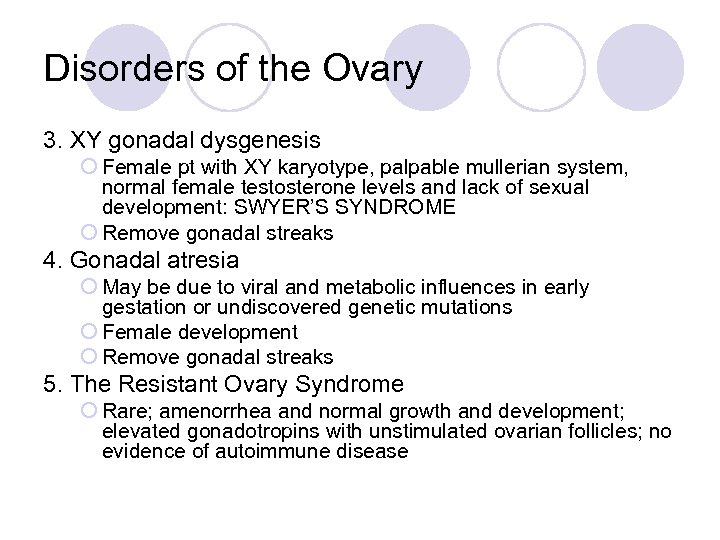
Disorders of the Ovary 3. XY gonadal dysgenesis ¡ Female pt with XY karyotype, palpable mullerian system, normal female testosterone levels and lack of sexual development: SWYER’S SYNDROME ¡ Remove gonadal streaks 4. Gonadal atresia ¡ May be due to viral and metabolic influences in early gestation or undiscovered genetic mutations ¡ Female development ¡ Remove gonadal streaks 5. The Resistant Ovary Syndrome ¡ Rare; amenorrhea and normal growth and development; elevated gonadotropins with unstimulated ovarian follicles; no evidence of autoimmune disease

Disorders of the Ovary 6. Premature Ovarian Failure ¡ Early depletion of ovarian follicles ¡ 1% of women will experience this before 40 yo ¡ Etiology is unknown: l Genetic disorders l Chromosome anomalies l Autoimmune disease l Infection: mumps l Physical assault: chemotherapy or radiation ¡ Age of presentation depends on how fast the follicles are lost
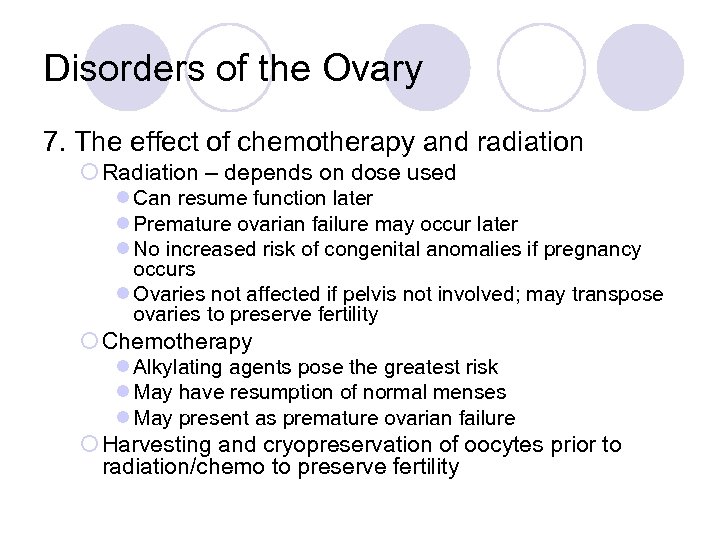
Disorders of the Ovary 7. The effect of chemotherapy and radiation ¡ Radiation – depends on dose used l Can resume function later l Premature ovarian failure may occur later l No increased risk of congenital anomalies if pregnancy occurs l Ovaries not affected if pelvis not involved; may transpose ovaries to preserve fertility ¡ Chemotherapy l Alkylating agents pose the greatest risk l May have resumption of normal menses l May present as premature ovarian failure ¡ Harvesting and cryopreservation of oocytes prior to radiation/chemo to preserve fertility
Disorders of the Anterior Pituitary (Compartment III) l Pituitary tumors: most are benign; can grow and cause compression of the optic chiasm leading to visual changes ¡Other rare causes: craniopharyngioma, meningiomas, gliomas, metastatic tumors l Nonfunctioning adenomas (30 -40% of pituitary tumors) – of gonadotroph origin ¡Secrete FSH, free alpha-subunit and rarely LH ¡Have elevated PRL
Disorders of the Anterior Pituitary l Other causes of pituitary compression ¡ Cysts ¡ Tuberculosis ¡ Sarcoidosis ¡ Fat deposits ¡ Lymphocytic hypophysitis – autoimmune infiltration of the pituitary; often occurs after pregnancy ¡ Internal carotid artery aneurysm ¡ Obstruction of the aqueduct of Sylvius ¡ Ischemia and infarction secondary to obstetrical hemorrhage – Sheehan’s syndrome
Treatment of Nonfunctioning Adenomas l Microadenoma (<10 mm) ¡ No treatment needed ¡ F/U imaging every 1 -2 years l Macroadenoma (>10 mm) ¡ If symptomatic – surgical resection ¡ High recurrence rate so radiation therapy is needed ¡ F/U imaging q 6 mos for 1 year, then yearly for 3 -5 years ¡ Dopamine agonists and octreotide treatment has been disappointing, but there have been some reductions in size, so keep this option open
Pituitary Prolactin-Secreting Adenomas l Most common pituitary tumors l Account for 50% of all pituitary adenomas l Microadenomas range form 9 -27% of pituitary tumors found at autopsy l Age 2 -86 yo; highest incidence 6 th decade l 1/3 rd of women with amenorrhea have elevated prolactin level l PRL >1000 = invasive tumor; treated with dopamine agonists l Amenorrhea associated with high PRL levels is due to prolactin inhibition of the pulsatile secretion of Gn. RH l Treatment that lowers the circulating levels of prolactin restores ovarian responsiveness and menstrual function

Pituitary Prolactin-Secreting Adenomas Results with Surgery l Transsphenoidal neurosurgery – immediate resolution of hyperprolactinemia with resumption of cyclic menses in 30% of patients with macroadenomas and 70% with microadenomas l Recurrence rate high; long term cure rate 50% l Complications: CSF leaks, meningitis, diabetes insipidus (for <6 months) l Best results when PRL level is 150 -500 ng (the higher the PRL the lower the cure rate) l Explanations for recurrence ¡ Difficult to completely resect ¡ Tumor may be multifocal in origin ¡ Continuing abnormality of the hypothalamus -> chronic stimulation of the lactotrophs

Pituitary Prolactin-Secreting Adenomas Results with Surgery l Management for patients who have had surgery ¡If cyclic menses returns: periodic evaluation for the problem of anovulation ¡If amenorrhea or oligomenorrhea and hyperprolactinemia persist or recur: PRL level q 6 mos and imaging yearly for 2 years l. Then image every 2 years l. If tumor growth is evident – control of growth with a dopamine agonist

Pituitary Prolactin-Secreting Adenomas Results with Radiation l Less satisfactory than with surgery; slow response l PRL levels take years to fall l Panhypopituitarism can occur for 10 years l Focused irradiation is better for small tumors or a small residual tumor after surgery l Can be an adjunctive therapy for shrinking larger tumors or for tumors unresponsive to medical therapy l Only a small number of women return to normal hormonal function

Pituitary Prolactin-Secreting Adenomas Dopamine Agonist Treatment l Bromocriptine – dopamine agonist. Binds to dopamine R therefore mimicking dopamaine inhibition of pituitary prolactin secretion l Metabolized by liver; 28% absorbed by the GI tract l Dose: start at 2. 5 mg daily to a maximum of 10 mg daily l Oral, IM and vaginal preparations; vaginal preparation has fewer side effects and complete absorptio`n l SE: nausea, headache, faintness (due to orthostatic hypotension), dizziness, fatigue, nasal congestion, vomiting and abdominal cramps; 10% discontinue due to SE l For patients seeking pregnancy: ¡ 2. 5 mg BID until patient is pregnant ¡ 2. 5 mg BID during follicular phase, discontinue when temperature indicates ovulation, resume when menses occurs
Pituitary Prolactin-Secreting Adenomas Dopamine Agonist Treatment l Tx purpose: pregnancy, suppression of bothersome galactorrhea, and reduction of tumor mass l Results of Treatment ¡ 80% have restored menses (average time 5. 7 weeks) ¡ 50 -60% have cessation of galactorrhea (12. 7 wks) ¡ 75% have regression of breast secretions (6. 4 wks) ¡ Cessation of galactorrhea is slower than restoration of menses ¡ Discontinuation of bromocriptine l Amenorrhea recurs in 41% (4. 4. weeks) l Galactorrhea recurs in 69% (6. 0 weeks)

Pituitary Prolactin-Secreting Adenomas Dopamine Agonist Treatment l Regression of Tumors with Bromocriptine ¡ Macroadenomas will regress with treatment ¡ Shrinkage occurs promptly (usually with 5 -7 mg) ¡ If fails to shrink with 10 mg – do not go higher – no better results ¡ Reduction can take days to weeks ¡ The most rapid reduction occurs during the first 3 months ¡ Even tumors with PRL >1000 have a good response to bromocriptine ¡ Must be taken indefinitely ¡ PRL levels will increase again with discontinuation

Pituitary Prolactin-Secreting Adenomas Other Dopamine Agonists l Pergolide: more potent, longer lasting, better tolerated ¡ Single daily dose: 50 -150 mg l Lysuride, terguride, metergoline l Quinagolide – 75 -300 mg qhs; good for tumors resistant to bromocriptine; also has antidepressive effect l Cabergoline – weekly dose of 0. 5 -3 mg ¡ Low rate of side effects ¡ Unsure of fetal safety in patients trying to conceive ¡ Can be given intravaginally
Summary: Treatment of Pituitary Prolactin -Secreting Macroadenomas l Dopamine agonist treatment l Follow PRL levels q 3 mos l Withdrawal of drug -> regrowth or reexpansion of the tumor l Long term treatment required l MRI 1 year after treatment began l Transsphenoidal surgery if visual changes persist l Can use short term treatment with a dopamine agonist before surgery to help shrink the tumor l 10% do not respond to dopamine agonists; early surgery is indicated

Summary: Treatment of Pituitary Prolactin -Secreting Microadenomas l Treat if patient has breast discomfort or infertility problems l Dopamine agonist – treatment of choice l Hypoestrogenic amenorrhea – estrogen therapy for bones; can use low dose OCPs if contraception is also needed
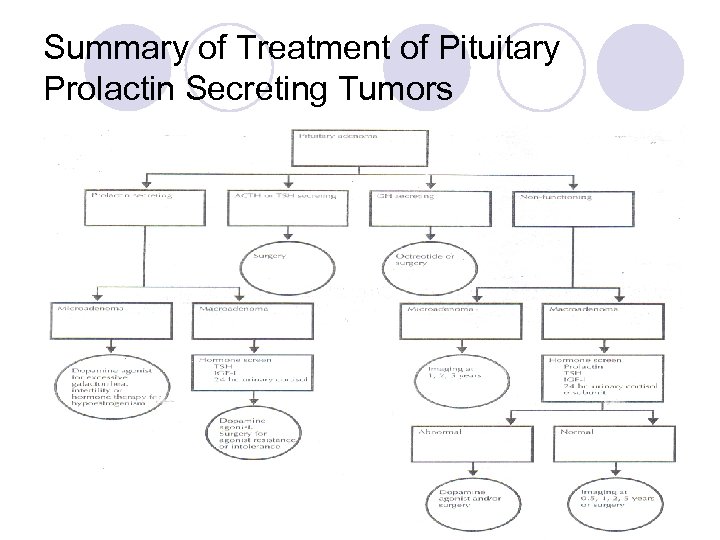
Summary of Treatment of Pituitary Prolactin Secreting Tumors

Pregnancy and Prolactin Adenomas l 80% achieve pregnancy with dopamine agonist treatment l Breastfeeding can be done l Some women resume cyclic menses after pregnancy (due to tumor infarction? ) l <2% have tumor growth evident by HA, bitemporal hemianopsia l No increase in miscarriage, perinatal M&M l Surveillance as needed l Can use bromocriptine during pregnancy
Empty Sella Syndrome l Congenital incompleteness of the sellar diaphragm; allows an extension of the subarachnoid space into the pituitary fossa l Coned down view is similar to a tumor l Found in 5% of autopsies (Women>men) l Elevated PRL, galactorrhea; annual surveillance required l Benign; does not progress to pituitary failure

Sheehan’s Syndrome l Acute infarction and necrosis of the pituitary gland due to post partum hemorrhage and shock l See symptoms of hypopituitarism in the postpartum period l Failure of lactation, loss of pubic and axillary hair l Deficiencies of GH, LH and FSH are most common

CNS Disorders (Compartment IV) l Hypothalamic Amenorrhea l Weight loss, anorexia and bulimia l Exercise and Amenorrhea l Eating Disorders and Pregnancy l Inherited Genetic Defects l Postpill Amenorrhea l Hormone Therapy

Hypothalamic Amenorrhea l Deficiency in Gn. RH pulsatile secretion l Often a psychobiologic response to life events – stressful situations l Mild suppression: marginal effect on reproduction l Moderate suppression: anovulation with menstrual irregularity l Profound suppression: hypothalamic amenorrhea l Low/normal LH and FSH, nl PRL, nl sella turcica; evaluate annually l Stress increases secretion of CRH which can inhibit gonadotropin secretion l Can use induction of ovulation to achieve pregnancy

Weight loss, Anorexia, Bulimia l Acute weight loss can lead to the hypogonadotropic state l Diagnosis of anorexia nervosa ¡ Onset age 10 -30 yo ¡ Weight loss of 25% or weight 15% below normal ¡ Denial, distorted body image, unusual handling of food ¡ Lanugo, bradycardia, overactivity, overeating and vomiting (bulimia) ¡ Amenorrhea ¡ No medical or psychiatric illness ¡ Constipation, low BP, hypercarotenemia, diabetes insipidus

Weight loss, Anorexia, Bulimia l Occurs in 1% of women l Usually starts with a diet which brings a sense of power and control l Excessive physical activity l Overachievers and strivers l Judgmental; few friends due to high expectations l Delayed psychosexual development l May having binging and purging - bulimia

Weight loss, Anorexia, Bulimia l Dysfunction of body mechanisms regulated by the hypothalamus: appetite, thirst and water conservation, temperature, sleep, autonomic balance and endocrine secretion l Low FSH and LH; elevated cortisol; normal PRL, TSH and T 4; low T 3 and high reverse T 3 (relative hypothyroidism) l Response of Gn. RH is regained at 15% below the ideal weight -> resumption of normal menses l No specific therapy
Exercise and Amenorrhea l 2 major influences on normal menses ¡ Critical level of body fat ¡ Effect of stress l Women who weigh less than 115 pounds and lose more than 10 pounds while exercising are the women who develop problems l Critical weight hypothesis: the onset and regularity of menstrual function necessitate maintaining weight above a critical level and therefore above a critical amount of body fat l 10 th %ile at 16 yo ~ 22% body fat – minimum for maintaining menstruation l 10 th %ile at 13 yo ~ 17% body fat – minimum for menarche l A loss of body weight of 10 -15% of normal may result in abnormal menstrual function

Exercise and Amenorrhea l Stress and energy expenditure appear to play an independent role l Ovarian activity can also be affected by seasonal variation (more problems in the fall and winter) l Acute exercise decreases gonadotropins and increases PRL, GH, testosterone, ACTH, adrenal steroids and endorphins l Endogenous opiates inhibit gonadotropin secretion by suppressing Gn. RH l Naltrexone (opioid receptor blocker) restores menstrual function when given to women with amenorrhea associated with weight loss l CRH also inhibits Gn. RH secretion l Prognosis: excellent with weight gain and decrease in exercise ¡ Can give hormone therapy for bone protection in women who do not quit exercising ¡ Can use ovulation induction in women who want to become pregnant ¡ Recommend weight gain and decrease in exercise in women desiring conception

Eating Disorders and Pregnancy l l Typical pregnancy requires 300 extra calories a day Weight gain in an average weight person: 22 -26 pounds Weight gain in an underweight person: 26 -33 pounds Linear relationship between birth weight and maternal weight gain l As prepregnancy weight increases, the importance of maternal weight gain diminishes l Weight gain during pregnancy in underweight women can bring an infant into the normal range for birth weight l Underweight status before pregnancy and inadequate weight gain during the second half of pregnancy increase the risk of preterm birth

Eating Disorders and Pregnancy l Women who have been treated for anorexia or bulimia and are in remission gained more weight and had higher birth weights l Women with active disease had worsening symptoms and psychological problems during pregnancy and smaller birth weights l Rate of preterm labor and delivery in patients with eating disorders is twice the normal incidence l Recommend treatment for eating disorders before getting pregnant l Expert consultation for pregnant women with a current or previous history of an eating disorder l Careful monitoring of maternal and fetal growth
Inherited Genetic Defects l Kallmann’s Syndrome ¡ Congenital hypogonadotropic hypogonadism due to deficient secretion of Gn. RH; anosmia ¡ Primary amenorrhea, infantile sexual development, low gonadotropins, normal female karyotype, inability to perceive odors ¡ X linked, autosomal dominant, autosomal recessive l Molecular Explanations – isolated deficiency of Gn. RH secretion ¡ Autosomal mode of transmission; only pursue this in patients with a family history l Adrenal Hypoplasia – X-linked inherited disorder that results in adrenal insufficiency ¡ Hypogonadotropic hypogonadism ¡ Mutation in DAX-1 gene

Postpill Amenorrhea l Investigation should be pursued if patient is amenorrheic: ¡ 6 months after discontinuing OCPs ¡ 12 months after last injection of Depo-Provera

Hormonal Therapy and Amenorrhea l Any patient who is hypoestrogenic needs hormone therapy to maintain bone density l Standard therapy: ¡ 0. 625 mg conjugated estrogens or 1 mg estradiol daily with 5 mg medroxyprogesterone acetate for 2 weeks every month ¡ Menstruation generally occurs 3 days after last progestin medication l For patients not wanting menstruation ¡ 0. 625 mg conjugated estrogens and 2. 5 mg medroxyprogesterone acetate given daily without a break l For patients refusing hormone therapy: 1000 -1500 mg calcium daily
The end
a039db3a35245d37d539390ba2d90e75.ppt